Abstract
The present study explored the protective effect of Embelia ribes Burm. (Myrsinaceae) fruit ethanol extract on isoproterenol (ISO)-induced cardiomyopathy in streptozotocin (STZ)-induced diabetic rats. STZ (40 mg/kg, intravenously, single-injection)-induced diabetic rats had significantly (p < 0.01) increased heart rate (HR), systolic blood pressure (SBP), blood glucose, HbA1C, serum LDH, serum CK, and myocardial TBARS levels, and decreased blood glutathione and myocardial endogenous antioxidants, viz. SOD, CAT, and GSH levels in comparison to group I rats. ISO (5.25 and 8. 5 mg/kg, s.c., for two consecutive days) administration produced myocardial necrosis as evidenced by a significant (p < 0.01) increase in HR, SBP, serum LDH, serum CK, and myocardial TBARS levels and a significant (p < 0.01) decrease in blood glutathione, and myocardial endogenous antioxidant levels in comparison to group I rats. Further, ISO administration to diabetic rats showed a significant (p < 0.01) increase in SBP, blood glucose, and HbA1C levels along with a significant (p < 0.01) decrease in HR, blood glutathione, serum LDH, serum CK, myocardial TBARS, SOD, CAT, and GSH levels as compared to ISO-only treated (i.e. group IV) rats. Forty days treatment of Embelia ribes ethanol extract (200 mg/kg) to pathogenic (STZ + ISO treated) rats resulted in a significant (p < 0.01) increase in HR, blood glutathione, serum LDH, and myocardial endogenous antioxidant levels with a significant (p < 0.01) decrease in SBP, blood glucose, HbA1C, serum CK, and myocardial TBARS levels as compared to pathogenic (STZ + ISO treated) rats. The study demonstrates the ability of Embelia ribes extract to attenuate ISO-induced oxidative stress in diabetic rats, enhancing cellular antioxidant defense.
Introduction
Diabetes mellitus is one of the most challenging diseases facing health care professionals today (CitationLeroith & Smith, 2005). According to the CitationWorld Health Organization (2006), more than 176 million patients are affected by this disease worldwide. Altered cardiovascular function is a major complication of diabetes mellitus (CitationRegan et al., 1977). The incidence of myocardial infarction and subsequent heart failure and death is significantly greater in patients with diabetes mellitus than in non-diabetics (CitationPartamian & Bradley, 1965). There are conflicting reports regarding the susceptibility of the diabetic myocardial cell to ischemia injury. While some investigators have reported that diabetic hearts are more sensitive than normal hearts to ischemia (CitationHearse et al., 1975), others have shown either that the severity of injury after ischemia with reperfusion is normal for diabetic hearts (CitationVogela & Apstein, 1988) or that diabetic hearts are more resistant to ischemia (CitationTani & Neely, 1988).
In traditional medicine, several Indian medicinal plants or their extracts have been used to treat diabetes (CitationAkhtar & Ali, 1984). A scientific investigation of traditional herbal remedies for diabetes may provide valuable leads for the development of alternative drugs and strategies. Embelia ribes Burm. (Myrsinaceae), commonly known as Vidanga, is widely distributed in India, Sri Lanka, Malaysia, and South China (CitationGuhabakshi et al., 2001). Several studies have demonstrated the beneficial effects of Embelia ribes, which include anti-inflammatory (CitationKapoor et al., 1983), antibacterial (CitationChitra et al., 2003), antimicrobial (CitationRani & Khullar, 2004), and anthelmintic activity (CitationHordegen et al., 2006). CitationBhandari et al. (2002, Citation2007) have reported the diabetic dyslipidemic and antioxidant activity of the ethanol extract of Embelia ribes in streptozotocin-induced diabetes in rats using gliclazide as the positive control drug.
Experimental evidence on the biochemical role of Embelia ribes in diabetic cardiomyopathy is lacking. This is the first study to investigate the response of the diabetic heart to ischemic assault by isoproterenol, and to evaluate the cardioprotective and antioxidant effectiveness of the ethanol extract of Embelia ribes in streptozotocin-induced diabetes in rats.
Materials and methods
Chemicals
Isoproterenol (ISO) and streptozotocin (STZ) were obtained from Sigma Chemicals (St. Louis, MO, USA). All other chemicals used were of analytical grade. Double-distilled water was used for all biochemical assays.
Plant material
The dried fruits of Embelia ribes were purchased from the local market, New Delhi, India in October 2005 and authenticated by Dr. M. P. Sharma, Taxonomist, Department of Botany, Faculty of Science, Hamdard University, New Delhi, India, where a voucher sample has been deposited (voucher specimen no. UB 2).
The dried and coarsely powdered drug (100 g) was packed in a Soxhlet apparatus and was subjected to extraction with ethanol over 72 h. The filtrate was evaporated under a vacuum drier and the brown mass residue obtained was stored at 4°C for further use. The average yield of the Embelia ribes ethanol extract was approximately 7.9%. For experimental study, a weighed amount of the Embelia ribes ethanol extract (200 mg/kg) was dissolved in 1% Tween 80 in normal saline and administered to albino rats by the oral route.
Animals
The study was approved by the Institutional Animal Ethics Committee (IAEC) (registration no. and date of registration: 173/CPCSEA) (Committee for the Purpose of Control and Supervision of Experiments on Animals), Jamia Hamdard, dated 28 January 2000). Wistar rats of either sex (200–250 g weight, aged 10 weeks) were obtained from the Central Animal House Facility of Hamdard University, New Delhi. They were maintained under standard laboratory conditions at 25 ± 2°C and relative humidity 50 ± 15%, and the normal photoperiod (12 h light–dark cycle) was used for the experiment. Commercial pellet diet (MFD; Nav Maharastra Chakan Oil Mills Ltd., New Delhi, India) and water were provided ad libitum.
Experimental induction of diabetes
After fasting for 18 h, the rats were injected intravenously through the tail vein with a single dose of 40 mg/kg STZ, freshly dissolved in citrate buffer (pH 4.5). After injection, the rats had free access to food and water and were given 5% glucose solution to drink overnight to counter hypoglycemic shock. Diabetes in rats was observed by moderate polydipsia and marked polyuria.
After 3 days, fasting blood glucose levels were determined by the orthotoluidine method (CitationHyavarina & Nikkita, 1962). Rats showing fasting blood glucose more than 200 mg/dL were considered diabetic and were selected for experimentation (CitationZhang & Tan, 2000).
Experimental design
Normal and diabetic albino rats of either sex were randomly divided into six groups of 10 rats each and treated as follows:
Group I, normal healthy control – rats received 1 mL/kg of vehicle (1% Tween 80) alone for 40 days;
Group II, STZ treated – rats received only STZ (40 mg/kg, i.v. single injection) through the tail vein;
Group III, STZ + Embelia ribes ethanol extract treated – rats received STZ followed by Embelia ribes ethanol extract (200 mg/kg, p.o.) for 40 days;
Group IV, ISO treated – rats received ISO (5.25 and 8. 5 mg/kg) for two consecutive days;
Group V, STZ + ISO treated – rats received STZ followed by ISO administration on 41st and 42nd days;
Group VI, STZ + Embelia ribes ethanol extract + ISO treated – rats received STZ + Embelia ribes ethanol extract (200 mg/kg, p.o.) for 40 days followed by ISO (5.25 and 8. 5 mg/kg) for two consecutive days on 41st and 42nd days.
Groups I, II, IV, and V rats received 1% Tween 80 solution orally once a day for 40 days. The experiment was terminated at the end of 42 days and the animals were fasted overnight.
In all the animals, the hemodynamic parameters, viz., heart rate and systolic blood pressure (BP), were recorded on day 43, as described previously, by means of a non-invasive method of rat tail cuff plethysmography using an LE 5001 pressuremeter (LETICA Scientific Instruments, USA) (CitationTomlinson et al., 1991).
After hemodynamic measurement, fasting blood samples were collected from the tail vein of all groups of rats. Whole blood was collected for estimation of blood glucose (CitationHyavarina & Nikkita, 1962), glycosylated hemoglobin (HbA1C) (CitationTrivalli et al., 1971), and glutathione (CitationBeutler et al., 1963) levels.
Serum was separated for the estimation of specific serum marker enzymes, namely, lactate dehydrogenase (LDH) (CitationLum & Gambino, 1974) and creatine kinase (CK) (CitationCristopher et al., 2001). STZ-induced oxidative stress in diabetes is also a predictor of cardiac damage. Since LDH and CK are specific cardiac marker enzymes, increased serum LDH and CK levels were considered as markers of oxidative stress-induced cardiac damage.
After blood collection, all the animals were sacrificed and hearts were dissected out and washed with ice cold saline, weighed, and minced; 10% homogenate was prepared in 0.15 M ice-cold KCl for TBARS (thiobarbituric acid-reactive substances), a marker for lipid peroxidation (CitationOhkawa et al., 1979), and protein estimation (CitationLowry et al., 1951); in 0.02 M EDTA (ethylenediaminetetraacetic acid) for glutathione estimation (CitationSedlak & Lindsay, 1968); and in phosphate buffer (pH 7.4) for superoxide dismutase (SOD) (CitationMarklund, 1985) and catalase estimations (CitationClairborne, 1985) using a Teflon tissue homogenizer. A decrease in levels of endogenous antioxidants with a rise in TBARS levels was considered as oxidative stress.
Heart tissue was fixed in 10% formalin, routinely processed, and embedded in paraffin wax. Paraffin sections (5 μm) were cut, mounted on glass slides, and stained with hematoxylin and eosin (H&E) and examined under a light microscope by a pathologist blinded to the groups studied.
Statistical analysis
All data are expressed as mean ± SEM. All groups of data were analyzed by one-way analysis of variance followed by Dunnett’s t-test using GraphPad Prism 3.0 software (GraphPad, San Diego, CA). p < 0.01 values were considered to be statistically significant.
Results
The heart rate and systolic blood pressure were significantly (p < 0.01) increased in diabetic (group II) and ISO-treated (group IV) rats as compared to normal control (i.e., group I) rats. However, the heart rate was significantly (p < 0.01) decreased with a significant (p < 0.01) increase in systolic blood pressure in diabetic rats following ISO administration (group V) as compared to the ISO-only treated (i.e., group III) rats. Further, Embelia ribes ethanol extract treatment in diabetic rats following ISO administration (group VI) produced a significant (p < 0.01) increase in heart rate and decrease in systolic blood pressure as compared to group IV (STZ + ISO treated) rats ().
Table 1. Effect of Embelia ribes (ER) ethanol extract administration on heart rate, systolic blood pressure, serum CK, and serum LDH levels.
The levels of marker enzymes, that is, LDH and CK, were significantly (p < 0.01) increased in diabetic (group II) and ISO-treated (group IV) rats as compared to normal control (i.e., group I) rats. ISO administration in diabetic rats significantly (p < 0.01) decreased the serum LDH and CK levels as compared to ISO-only treated (i.e., group IV) rats. However, the test drug treatment for 40 days significantly (p < 0.01) reduced the levels of CK and increased the levels of LDH when compared to pathogenic (STZ + ISO) rats ().
STZ administration significantly (p < 0.01) increased the levels of glucose and HbA1C in blood and decreased blood glutathione levels as compared to normal control (i.e., group I) rats. ISO treatment (group IV) resulted in normal levels of glucose and HbA1C in blood and decreased levels of blood glutathione as compared to normal control (i.e., group I) rats. Further, ISO administration in diabetic rats resulted in significant (p < 0.01) increases in glucose and HbA1C levels and a decrease in glutathione levels in blood as compared to ISO-only treated (i.e., group IV) rats, while treatment with Embelia ribes ethanol extract in ISO-treated diabetic rats significantly (p < 0.01) decreased the glucose and HbA1C levels and increased the glutathione levels in blood as compared to group V (i.e., STZ + ISO treated) rats ().
Table 2. Effect of Embelia ribes (ER) ethanol extract administration on blood glucose, blood glycosylated hemoglobin (HbA1C), and blood glutathione levels.
A significant (p < 0.01) increase in TBARS levels and reduction in SOD, catalase (CAT), and glutathione (GSH) levels in hearts of diabetic (group II) and ISO-treated (group IV) rats were observed as compared to normal control rats (group I). ISO treatment in diabetic rats resulted in significant decreases in myocardial TBARS, SOD, CAT, and GSH levels as compared to ISO-only treated (i.e., group IV) rats. Further, treatment with Embelia ribes ethanol extract in diabetic rats following ISO administration showed a significant decrease in TBARS levels and increases in SOD, CAT, and GSH levels as compared to pathogenic (STZ + ISO) rats, i.e., group V ().
Table 3. Effect of Embelia ribes (ER) ethanol extract administration on myocardial TBARS, GSH, SOD, and CAT levels.
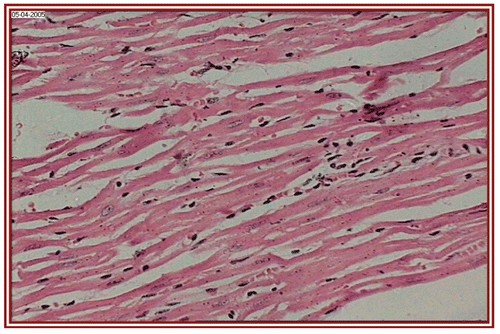
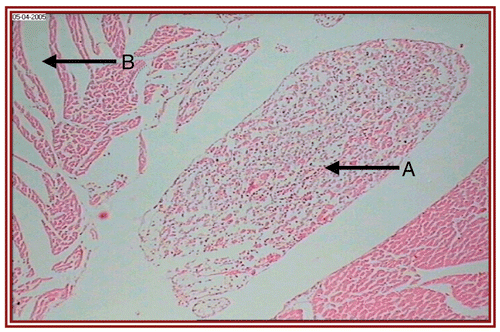
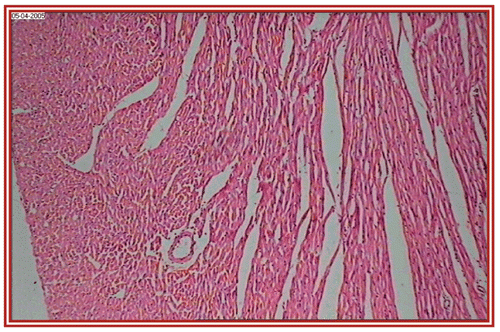
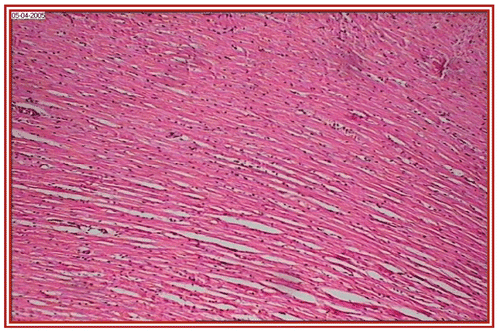
depict the cardiac muscles of the hearts of rats in different groups. A photomicrograph from the vehicle control group shows normal cardiac muscle bundles (). STZ treatment showed mild edema in group II diabetic rats (). ISO treatment in normal rats resulted in marked inflammatory infiltrate with edema formation (). Treatment of diabetic rats with ISO showed mild necrosis (). However, group VI treatment resulted in normal myocardial fibers ().
Figure 1. Normal healthy control group (i.e. group I) rat heart section, showing normal cardiac muscle bundles (×10).
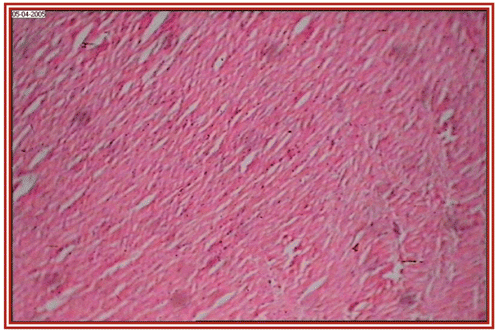
Figure 3. STZ + Embelia ribes ethanol extract treated group (i.e., group II) rat heart section, showing normal myocardial fibers (×10).
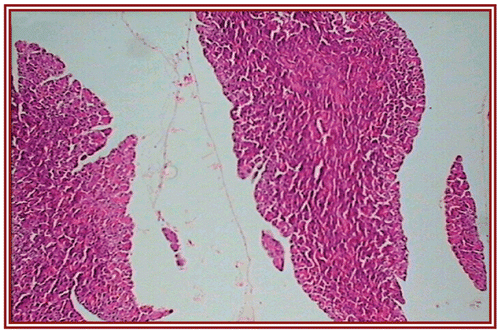
Discussion
The most convenient way of producing experimental myocardial lesions is by subcutaneous administration of isoproterenol (ISO), a strong β-adrenoceptor agonist. The mechanism is probably via myocardial calcium overload and consequent high energy phosphate depletion (CitationFlekeinstein, 1983). Myocardial exposure to high concentrations of catecholamines has been shown to reduce β-receptor density. Free radical production with altered lipid content of the membranes is one postulated mechanism. The direct effects of abnormal carbohydrate metabolism and excessive fatty acid oxidation have been implicated as an important proximate cause of diabetic cardiomyopathy in STZ-treated rats.
In the present study, ISO administration produced cardiac lesions in non-diabetic rats; however, desensitization of myocardium occurred in diabetic rats when subjected to ISO-induced cardiotoxicity, i.e., in group V rats.
Further, STZ and ISO treatment significantly increased the heart rate and systolic blood pressure in rats as compared to vehicle control rats. Furthermore, ISO administration in diabetic rats significantly decreased the heart rate and increased the systolic blood pressure as compared to ISO-only treated rats. Embelia ribes ethanol extract treatment in ISO-treated diabetic rats significantly increased the heart rate and decreased the systolic blood pressure as compared to STZ + ISO treated rats.
Glucose and glycosylated hemoglobin (HbA1C) levels in blood were significantly increased in diabetic rats but maintained normal in ISO treated rats as compared to vehicle control rats. A significant increase in glucose and HbA1C levels was observed when ISO was administered in diabetic rats as compared to ISO-only treated rats. Further, Embelia ribes ethanol extract significantly decreased the blood glucose and HbA1C levels as compared to STZ + ISO treated rats.
Serum LDH and CK levels are valuable diagnostic indicators of myocardial damage. In the present study, diabetic rats were found to have increased serum LDH, serum CK, and myocardial TBARS levels, as compared to normal rats. Further, ISO administration to control rats resulted in severe myocardial necrosis as evidenced by LDH and CK leakage into the serum and increased levels of myocardial TBARS, a marker of lipid peroxidation, as compared to normal control rats. ISO administration to diabetic rats produced a significant decrease in serum LDH, serum CK, and myocardial TBARS levels as compared to ISO-only treated rats. Further, Embelia ribes ethanol extract treatment in ISO treated diabetic rats significantly decreased serum CK and myocardial TBARS levels and increased serum LDH levels, as compared to STZ + ISO treated rats.
Myocardial endogenous antioxidants, viz., GSH, SOD, and CAT levels, were significantly decreased in diabetic, ISO treated, and ISO-administered diabetic rats, as compared to normal rats. Further, Embelia ribes ethanol extract treatment in ISO treated diabetic rats significantly increased the myocardial endogenous antioxidant levels.
The number of β-adrenergic receptors has been reported to be lower in diabetes (CitationWilliams et al., 1983), which could account for the reduced sensitivity to ISO in diabetes. However, the degree of tachycardia following ISO administration is reported to be similar in both diabetic and control groups (CitationEl-Hage et al., 1985). An altered ion permeability of the sarcolemma due to either hyperglycemia itself or lack of insulin has been suggested as a mechanism of desensitization to ISO in the diabetic state (CitationGotzsche, 1983).
Further investigations are necessary to address the significance of the structural, functional, and metabolic abnormalities of the myocardium of diabetic rats. These observations serve as a pointer for further scientific exploration, which could have crucial clinical implications.
Declaration of interest: This study was supported by a major research project grant to Dr. Uma Bhandari from the University Grants Commission, New Delhi, India.
References
- Akhtar MS, Ali MR (1984): Study of antidiabetic effect of a compound medicinal plant prescription in normal and diabetic rabbits. J Pak Med Assoc 34: 239–244.
- Beutler E, Duron O, Kelly BM (1963): Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888.
- Bhandari U, Jain N, Pillai KK (2007): Further studies on antioxidant potential and protection of pancreatic beta-cells by Embelia ribes in experimental diabetes. Exp Diabetes Res 2007: 15803.
- Bhandari U, Kanojia R, Pillai KK (2002): Effect of ethanolic extract of Embelia ribes on dyslipidaemia in diabetic rats. Int J Exp Diabetes Res 3: 159–162.
- Chitra M, Devi CS, Sukumar E (2003): Antibacterial activity of embelin. Fitoterapia 74: 401–403.
- Clairborne A (1985): Catalase activity. In: Greenwald R, ed., Handbook of Methods for Oxygen Radical Research. Boca Raton, CRC Press, pp. 283–284.
- Cristopher GG, Piersma T, Williams TD (2001): A sport physiological perspective on bird migration: Evidence for flight-induced muscle damage. J Exp Biol 204: 2683–2690.
- El-Hage AN, Herman EH, Jordan AW, Ferrans VJ (1985): Influence of the diabetic state on isoproterenol-induced cardiac necrosis. J Mol Cell Cardiol 17: 361–369.
- Flekeinstein A (1983): Calcium antagonism in heart and smooth muscle. In: Experimental Facts and Therapeutic Prospect. New York, Wiley Interscience Publication, p. 109.
- Gotzsche O (1983): Decreased myocardial calcium uptake after isoproterenol in streptozotocin-induced diabetic rats. Studies in the in vitro perfused heart. Lab Invest 48: 156–161.
- Guhabakshi DN, Sensarma P, Pal DC (2001): A Lexicon of Medicinal Plants of India. Calcutta, Naya Prakashan, p. 135.
- Hearse DJ, Stewart DA, Chain EB (1975): Diabetes and the survival and recovery of the anoxic myocardium. J Mol Cell Cardiol 7: 397–415.
- Hordegen P, Cabaret J, Hertzberg H, Langhans W, Maurer V (2006): In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methylthiazolyl-tetrazolium reduction assay. J Ethnopharmacol 108: 85–89.
- Hyavarina A, Nikkita E (1962): Specific determination of blood glucose with ortho-toluidine. Clin Chim Acta 7: 140–143.
- Kapoor VK, Chawla AS, Kumar M, Kumar P (1983): Anti-inflammatory agent in Indian laboratories. Indian Drugs 30: 481–488.
- Leroith D, Smith DO (2005): Monitoring glycemic control: The cornerstone of diabetes care. Clin Ther 27: 1489–1499.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951): Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275.
- Lum SR, Gambino G (1974): A comparision of serum as heparinised plasma for routine chemistry tests. Am J Clin Pathol 61: 108–112.
- Marklund SL (1985): Pyrogallol oxidation. In: Greenwald R, ed., Handbook of Methods for Oxygen Radical Research. Boca Raton, CRC Press, pp. 243–247.
- Ohkawa H, Ohishi N, Yagi K (1979): Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351–358.
- Partamian JO, Bradley RF (1965): Acute myocardial infarction in 258 cases of diabetes: Immediate mortality and five year survival. N Engl J Med 273: 455–461.
- Rani P, Khullar N (2004): Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytotherapy Res 18: 670–673.
- Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmed MR, Haider B (1977): Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest 60: 885–899.
- Sedlak J, Lindsay RH (1968): Estimation of total, proteinbound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25: 192–205.
- Tani M, Neely JR (1988): Hearts from diabetic rats are more resistant to in vitro ischemia: Possible role of altered Ca2+ metabolism. Circulation Res 62: 931–940.
- Tomlinson KC, Gardiner SM, Bennett T (1991): Blood pressure measurement. Am J Physiol 258: R852.
- Trivalli LA, Ranney PH, Lai HT (1971): Glycated haemoglobin estimation. N Engl J Med 284: 353–354.
- Vogela WM, Apstein CS (1988): Effects of alloxan-induced diabetes on ischemia-reperfusion injury in rabbit hearts. Circulation Res 62: 975–982.
- World Health Organization (2006): Available at http://www.who.org (accessed February, 2006).
- Williams RS, Schaible TF, Scheuer J, Kennedy R (1983): Effects of experimental diabetes on adrenergic and cholinergic receptors of rat myocardium. Diabetes 32: 881–886.
- Zhang XF, Tan BKH (2000): Antihyperglycaemic and antioxidant properties of Andrographis paniculata in normal and diabetic rats. Clin Exp Pharmacol Physiol 27: 358–363.