Abstract
Hydroxydihydrocarvone (HC) is a monoterpene analog prepared as a semisynthetic intermediate by hydration of the R-(–)-carvone monoterpene. Recent reports from studies carried out on HC have demonstrated its antinociceptive effect. However, no complete toxicological evaluation has yet been carried out with HC. The objective of the present work was to investigate a possible pharmacological tolerance in the hot plate test and the expression of toxic effects of HC (200 mg/kg, i.p.) in mice. During 28 days of treatment, the compound caused an increase in the reaction time of mice in the hot plate test and failed to produce catalepsy. HC caused a reduction in the rectal temperature of these animals and had no effect on water and food consumption or on their body weight compared to the control group. An increase in blood glucose levels was found during the second observation period; nevertheless, no biochemical abnormalities were encountered at the end of the HC treatment. In the hematological analysis, HC affected only neutrophil counts; however, all changes remained within the acceptable range for neutrophils in mice. In addition, no change was found in the weight of the liver, heart, or kidneys of the animals treated with HC compared to controls. Therefore, based on the results obtained, it is reasonable to assume that when administered for 28 days, HC exerts a central antinociceptive effect without causing pharmacological tolerance, and no significant toxicological alterations were observed during treatment.
Introduction
Essential oils have been widely used to prevent and treat human disease since ancient times. These oils are natural, complex, multicomponent systems, composed mainly of terpenes in addition to some other non-terpene components. The pharmaceutical properties of essential oils are partially attributed to their volatile constituents, for example the monoterpenes present in many essential oils (CitationEdris, 2007). Monoterpenes have been shown to exert chemopreventive as well as chemotherapeutic effects in rodents, and may therefore represent a new range of therapeutic agents. Many studies have shown that some monoterpenes exert effects on the central nervous system (CNS) that include anxiolytic (CitationSilva et al., 2007), sedative (CitationDe Sousa et al., 2007a), antinociceptive (CitationAmaral et al., 2007; CitationGonçalves et al., 2008), and anticonvulsant (CitationDe Sousa et al., 2007b, Citation2007c) effects in several animal models. Monoterpene derivatives have also been shown to have several psychopharmacological properties, exerting antinociceptive (CitationDe Sousa et al., 2004; CitationAlmeida et al., 1996) and antidepressant (CitationDe Sousa et al., 2006a) effects.
On the other hand, phytotherapeutic products and other similar substances are often mistakenly regarded as safe because they are “natural” (CitationGesler, 1992). Nevertheless, these products contain bioactive principles with the potential to cause a wide spectrum of adverse effects (CitationBent & Ko, 2004). In addition, the inadequate control exerted by the regulatory agencies in this area makes it difficult to establish the frequency of adverse effects caused by the use of phytotherapeutic products and their derivatives (CitationEisenberg et al., 1998). Therefore, all “natural” products and similar substances used therapeutically should be submitted to the same tests of efficacy and safety as used for evaluating new synthetic drugs (CitationTalalay & Talalay, 2001).
Despite the various pharmacological studies carried out with monoterpenes, few studies have evaluated the toxicity and long-term effects of these substances, mainly with respect to their possible pharmacological tolerance. Hydroxydihydrocarvone (HC) is a monoterpene analog prepared as a semisynthetic intermediate by hydration of the R-(–)-carvone monoterpene. This compound possesses structural and functional groups similar to those of some monoterpenes that have an effect on the CNS (CitationGaleotti et al., 2002; CitationPeana et al., 2003).
Recent reports of studies carried out on HC have demonstrated the psychopharmacological activity of this compound (CitationDe Sousa et al., 2006b) as well as the antinociceptive effects (CitationOliveira et al., 2008). Therefore, the following experiments were designed to evaluate the long-term safety of HC with respect to pharmacological tolerance and subacute toxicity, including possible biochemical and hematological alterations, as well as the occurrence of side effects during a 28-day treatment with HC in mice.
Materials and methods
Preparation of hydroxydihydrocarvone
HC was prepared in this laboratory as previously described by CitationBüchi and Wüest (1979). Next, it was dissolved in 5% Tween 80, which was used as an emulsion.
Animals
Albino male Swiss mice (25–35 g) were obtained from the Prof. Dr. Thomas George Laboratory of the Federal University of Paraíba, and were subsequently separated in cages to form three groups of four mice each for treatment (n = 12). The animals were kept under standard environmental temperature conditions (21 ± 1°C) with 12 h light/dark periods, light beginning at 06:00 h. Food and water were provided ad libitum until 1 h prior to the experimental procedures. The animals were acclimatized to the laboratory for 1 h prior to the experiments and the studies were conducted between 12:00 and 17:00 h. The animals were submitted to tests 30 min after the treatments. All experimental protocols were approved by the institution’s Ethics Committee for the Care and Use of Animals (approval #1206/06). illustrates the time course of the experiment and the tests used in the experimental protocol.
Hot plate test
This test was used to measure response latencies according to the method previously described by CitationEddy and Leimbach (1953), a modification of the original method of CitationWoolfe and MacDonald (1944). Animals were placed on a hot plate maintained at 47 ± 0.5°C. The time elapsed between placing the animal on the hot plate and the animal either licking its fore or hind paws or jumping on the surface was considered the response latency. Mice with baseline latencies of more than 15 s were excluded from the study. Response latency testing was measured prior to intraperitoneal administration (baseline) of HC (200 mg/kg), vehicle (saline solution 0.9% and 5% Tween 80: control), and morphine (10 mg/kg) and at the 1st, 7th, 14th, 21st, and 28th days of treatment. The cut-off time for hot-plate test latency was set at 30 s (CitationSilva et al., 2005; CitationAlmeida & Oliveira, 2006).
Catalepsy test
For evaluation in a horizontal bar test, fore paws were placed on a 6 cm high horizontal bar, while the hind paws remained on the floor. Latency to step down was recorded before the intraperitoneal administration of HC (200 mg/kg), vehicle (control), and haloperidol (5 mg/kg) and at the 3rd, 10th, 17th, and 24th days of treatment. Animals were placed on the bar for a series of three tests (CitationCarlini, 1973).
Rectal temperature
Rectal temperature was measured using a digital thermometer connected to a thermoelectric probe, which was carefully inserted into the rectum of an animal using Vaseline. Rectal temperature was recorded prior to the administration of treatment (day 0) and on the 5th, 12th, 19th, and 26th days of treatment.
Food and water intake and body weight gain
The amounts of food and water ingested were calculated daily from the quantity of food and water supplied and the amount remaining after 24 h. Body weight was also evaluated every day.
Blood glucose evaluation
Blood glucose levels were measured at the beginning of the experiment and on the 9th and 23th days of treatment. Animals were fasted for 6 h prior to the experiment. Blood samples were obtained from a small cut in the tail vein. Blood glucose levels were measured using a glucometer (Optium™; Abbott Laboratories).
Blood analyses
At the end of the treatment period, the mice were fasted for 6 h, then anesthetized using ether and euthanized by drawing blood samples for hematological and biochemical analyses. Biochemical analyses were performed using an automatic chemistry analyzer (Cobas Mira Roche®) with blood serum obtained after centrifugation of total blood in microtubes of 0.8 mL with separate gel (Minicollect®; Greiner Bio-one) and without anticoagulants at 3500 rpm for 5 min. Standardized diagnostic kits manufactured by Labtest® were used to perform spectrophotometrical evaluation of the following biochemical parameters: glucose, urea, uric acid, creatinine, cholesterol, triglyceride, total protein, albumin, globulin, aspartate aminotransferase (AST, EC 2.6.1.1), alanine aminotransferase (ALT, EC 2.6.1.2), amylase (EC 3.2.1.1), lactate dehydrogenase (EC 1.1.1.27), creatine kinase (EC 2.7.3.2), and alkaline phosphatase (EC 3.1.3.1.). Hematological analyses were performed using an automatic hematological analyzer (Vet abc™; Animal Blood Counter) with whole blood samples collected in microtubes of 0.5 mL with ethylenediaminetetraacetic acid (EDTA) (Minicollect®; Greiner Bio-one). The following parameters were evaluated: red blood cell count (RBC), hemoglobin, hematocrit, mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBC), neutrophils, lymphocytes, monocytes, eosinophils, and platelet count.
Organ weight
Following blood sampling, the abdominal cavity of each animal was opened and the organs, specifically the heart, liver, and kidneys, were quickly removed, cleaned, and weighed for the purpose of recording the weight of each individual organ.
Statistical analysis
The results are presented as mean ± SEM. Statistical significance between control and other experimental groups was evaluated using Student’s t-test. The results obtained were considered significant when p < 0.05.
Results and discussion
The present work shows the effect of subacute treatment with HC. In the hot plate test, 28 day treatment with HC was found to have an antinociceptive effect throughout the duration of treatment without causing tolerance, in comparison with the control group, while the effect of morphine was observed only up to the 14th day of treatment (). Repeated use of opioids induces tolerance that cancels the effect of the drug or requires an increase in dose to relieve pain. The neurobiological mechanisms of the development of opioid tolerance are multifaceted and only partially understood (CitationGabra et al., 2007). In the catalepsy test, HC did not induce catalepsy in mice throughout the entire observation. This behavior pattern is observed with many depressants of the CNS for which there have been reports of adverse effects. This result shows that, unlike opioids and antipsychotics, the antinociceptive effect of HC does not include catalepsy. On the other hand, haloperidol induced catalepsy throughout the entire treatment period ().
Figure 2. Effect of HC in the hot plate test in mice treated for 28 days. Values represent mean ± SEM (n = 12); *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from control.
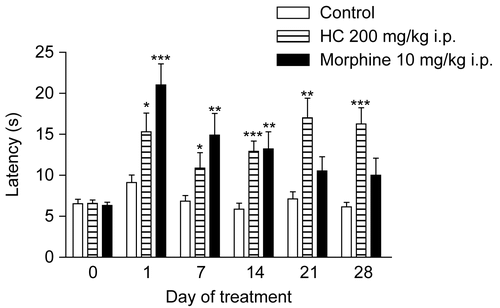
Table 1. Effect of hydroxydihydrocarvone (HC) in the catalepsy test in mice treated for 28 days.
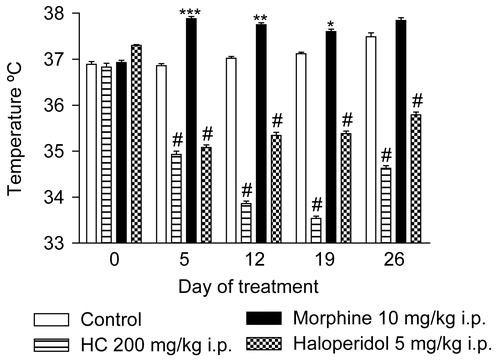
Anatomically, A-delta and C nerve fibers present in the nerve roots and afferent peripheral nerves are known to conduct pain and temperature impulses through the dorsal horn and dorsal root (CitationCarpenter & Mackey, 1992). Change in body temperature is one of the principal pharmacological effects of opioid agonists such as morphine and other drugs such as haloperidol. It is thought that opioids act directly on the temperature control center in the hypothalamus to produce this change (CitationSmith et al., 1995). The hypothermic effect of HC provides evidence of the involvement of the activity of HC on the temperature/pain pathways ().
Figure 3. Effect of HC in the rectal temperature test in mice treated for 28 days. Values represent mean ± SEM (n = 12); *p < 0.05, **p < 0.01, ***p < 0.001, #p < 0.001 significantly different from control.
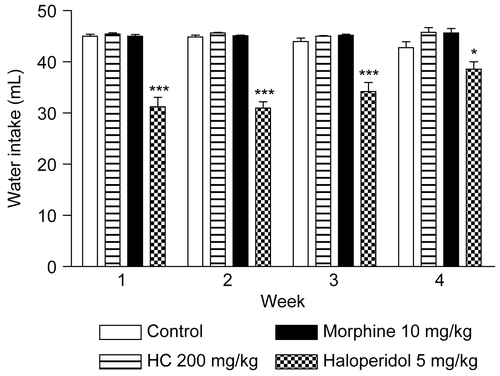
In addition, changes in body weight are indicative of adverse side effects, since the animals that survive cannot lose more than 10% of their initial body weight (CitationRaza et al., 2002; CitationTeo et al., 2002). In the present study, no significant difference was found with respect to the evaluation of body weight in animals treated with HC; however, in the morphine and haloperidol groups decreases of body weight were observed (). Moreover, no changes were found in water and food intake in mice treated with HC. The results of these tests also showed that morphine induces a decrease of food intake, and in the haloperidol group there was a decrease of food and water intake ( and ). Defining these parameters is an essential component of the safety studies that have to be carried out on any product intended for therapeutic purposes. Appropriate nutrient and water intake is essential to the physiological status of the animal and for ensuring that the response to the drug tested is reliable and not a “false” response due to inadequate nutritional conditions (CitationStevens & Mylecraine, 1994; CitationFéres et al., 2006).
Figure 4. Effect of HC on body weight gain in mice treated for 28 days. Values represent mean ± SEM (n = 12); *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from control.
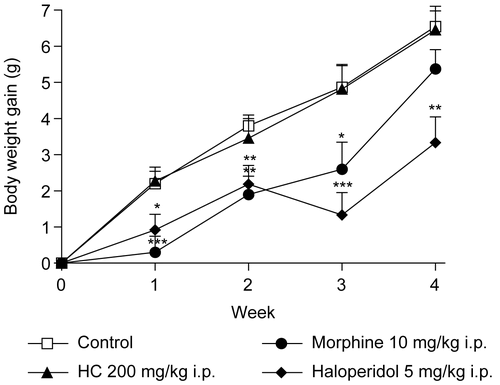
Figure 5. Effect of HC on food intake in mice treated for 28 days. Values represent mean ± SEM (n = 12); *p < 0.05, **p < 0.01, ***p < 0.001 significantly different from control.
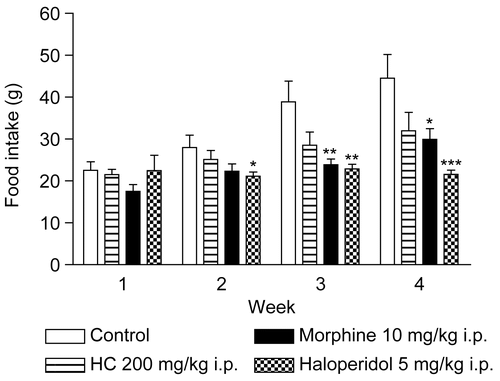
Figure 6. Effect of HC on water intake in mice treated for 28 days. Values represent mean ± SEM (n = 12); *p < 0.05, ***p < 0.001 significantly different from control.
Administration of HC led to an increase in glucose levels at the second observation (); however, at the end of treatment, no statistically significant abnormalities were found in the biochemical parameters of the mice treated with HC compared to the control group. On the other hand, morphine altered ALT levels, while haloperidol affected various biochemical parameters (). With respect to the hematological study, no change occurred in the parameters analyzed in the mice treated with HC. The differential leukocyte counts revealed a slight alteration; however, levels remained within normal reference values and were not considered to be clinically significant. In the group treated with morphine, no hematological abnormalities were found. Treatment with haloperidol induced small fluctuations in hemoglobin levels and in MCH and MCHC (). Analysis of the weight of the liver, heart, and kidneys of the mice showed that HC had no effect on the weight of these organs, whereas haloperidol reduced the weight of the liver, the principal organ involved in drug metabolism (). Based on these results, we may conclude that the subacute administration of HC to mice did not lead to pharmacological tolerance in the hot plate test, induced alterations in temperature similar to those found with other drugs that act on the CNS, and produced no significant toxic effects for the route and dose tested; however, there are many more subtle end points that remain to be investigated regarding the safe use of HC (e.g., reproductive, genotoxic, etc.). These results may serve to stimulate further promising investigations, which could lead to the development of a new therapeutic agent.
Figure 7. Effect of HC in the glycemia test in mice treated for 28 days. Values represent mean ± SEM (n = 12); ***p < 0.001 significantly different from control.
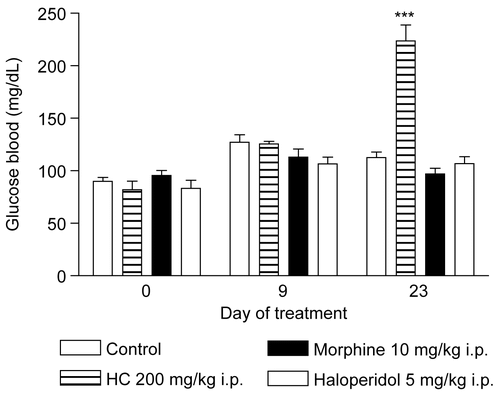
Figure 8. Effect of HC on weight of organs of mice treated for 28 days. Values represent mean ± SEM (n = 12); **p < 0.01 significantly different from control.
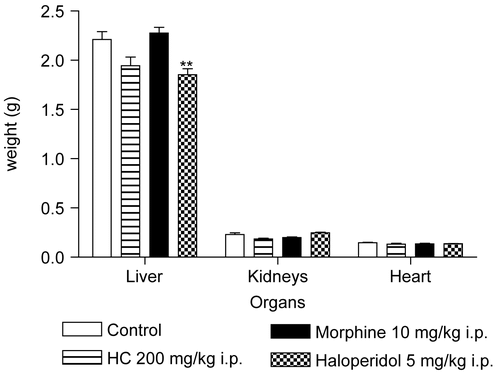
Table 2. Biochemical parameters of mice after treatment for 28 days with HC.
Table 3. Hematological parameters of mice after treatment for 28 days with HC.
Acknowledgements
The authors are grateful to José Crispim Duarte for technical support.
Declaration of interest: The authors wish to thank CNPq for providing financial support.
References
- Almeida FRC, Oliveira FS (2006): Avaliação de drogas analgésicas de ação central. In: Almeida RN, ed., Psicofarmacologia: Fundamentos Práticos. Rio de Janeiro, Guanabara Koogan, pp.179–;188.
- Almeida RN, Hiruma CA, Barbosa-Filho JM (1996): Analgesic effect of rotundifolone in rodents. Fitoterapia 67: 334–338.
- Amaral JF, Silva MIG, Neto MRA, Neto PFT, Moura BA, Melo CTV, Araújo FLO, De Sousa DP, Vasconcelos PF, Vasconcelos SM, Sousa FCF (2007): Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol Pharm Bull 30: 1217–1220.
- Bent S, Ko R (2004): Commonly used herbal medicines in the United States: A review. Am J Med 116: 478–485.
- Büchi G, Wüest HJ (1979): New synthesis of β-agarofuran and of dihydroagarofuran. J Org Chem 44: 546–549.
- Carlini EA (1973): Farmacologia prática sem aparelhagem. São Paulo, Sarvier, p. 44.
- Carpenter RL, Mackey DC (1992): Local anaesthetic. In: Barash PG, Cullen BF, Stoelting RR, eds., Clinical Anaesthesia. Philadelphia, JB Lippincott, pp. 509–541.
- De Sousa DP, Nobrega FFF, Almeida RN (2007c): Influence of the chirality of (R)-(-)- and (S)-(+)-carvone in the central nervous system: A comparative study. Chirality 19: 264–268.
- De Sousa DP, Oliveira FS, Almeida RN (2006b): Evaluation of the central activity of hydroxydihydrocarvone. Biol Pharm Bull 29: 811–812.
- De Sousa DP, Quintans-Júnior JL, Almeida RN (2007b): Evalution of the anticonvulsant activity of α-terpineol. Pharm Biol 45: 69–70.
- De Sousa DP, Raphael E, Brocksom U, Brocksom TJ (2004): Antinociceptive profile of 2-phenylselenennyl-1,8-cineole in mice. Biol Pharm Bull 27: 910–911.
- De Sousa DP, Raphael E, Brocksom U, Brocksom TJ (2007a): Sedative effect of monoterpene alcohols in mice: A preliminary screening. Z Naturforsch 62C: 563–566.
- De Sousa DP, Schefer RR, Brocksom U, Brocksom TJ (2006a): Synthesis and antidepressant evaluation of three para-benzoquinone mono-oximes and their oxy derivatives. Molecules 11: 148–155.
- Eddy NB, Leimbach DJ (1953): Synthetic analgesics II. Dithienylbutenyl and diethienylbutylamines. Pharmacol Exp Ther 107: 385–393.
- Edris AE (2007): Pharmaceutical and therapeutical potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21: 308–323.
- Eisenberg DM, Davis RB, Ettener SL (1998): Trends in alternative medicine use in the United States, 1990–1997: Results of a follow-up national survey. J Am Med Assoc 280: 1569–1575.
- Féres CAO, Madalosso RC, Rocha OA, Leite JPV, Guimarães TMDP, Toledo VPP, Tagliati CA (2006): Acute and chronic toxicological studies of Dimorphandra mollis in experimental animals. J Ethnopharmacol 108: 450–456.
- Gabra BH, Bailey CP, Kelly E, Sanders AV, Henderson G, Smith FL, Dewey WL (2007): Evidence for an important role of protein phosphatases in the mechanism of morphine tolerance. Brain Res 1159: 86–93.
- Galeotti N, Mannelli LDC, Mazzanti G, Bartolini A, Ghelardini C (2002): Menthol: A natural analgesic compound. Neurosci Lett 322: 145–148.
- Gesler WM (1992): Therapeutic landscapes: Medical issues in light of the new cultural geography. Soc Sci Med 34: 735–746.
- Gonçalves JCR, Oliveira FS, Benedito RB, De Sousa DP, Almeida RN, Araújo DAM (2008): Antinociceptive activity of (–)-carvone: Evidences of association with the peripheral nerve excitability decreasing. Biol Pharm Bull 31: 1017–1020.
- Oliveira FS, De Sousa DP, Almeida RN (2008): Antinociceptive effect of hydroxydihydrocarvone. Biol Pharm Bull 31: 588–591.
- Peana AT, D’aquila PS, Chessa ML, Moretti MDL, Serra G, Pippia P (2003): (-)-Linalool produces antinociception in two experimental models of pain. Eur J Pharmacol 460: 37–41.
- Raza M, Al-Shabanah OA, El-Hadiyah TM, Al-Majed AA (2002): Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm 70: 135–145.
- Silva MIG, Neto MRA, Neto PFT, Moura BA, Amaral JF, De Sousa DP, Vasconcelos SMM, Sousa FCF (2007): Central nervous system activity of acute administration of isopulegol. Pharmacol Biochem Behav 88: 141–147.
- Silva MG, Oliveira FS, Quintans-Júnior LJ, Oliveira TML, Diniz MFFM (2005): Investigação do efeito analgésico central e antiinflamatório de Conocliniopsis prasiifolia (DC) R.M. King & H. Robinson em roedores. Acta Farm Bonaerense 24: 533–537.
- Smith AP, Lee NM, Loh HH (1995): Opioid analgesic and antagonists. In: Paul M, Robert AM, George RB, eds., Principles of Pharmacology: Basic Concepts and Clinical Application. New York, Chapman and Hall, pp. 402–406.
- Stevens KR, Mylecraine L (1994): Issues in chronic toxicology. In: Hayes AW, ed., Principles and Methods of Toxicology. New York, Raven Press, p. 673.
- Talalay P, Talalay P (2001): The importance of using scientific principles in the development of medicinal agents from plants. Acad Med 76: 238–247.
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V (2002): A 90-day oral gavage toxicity study of d-methylphenidate and d,l-methylphenidate in Sprague Dawley rats. Toxicology 179: 183–196.
- Woolfe G, MacDonald AD (1944): The evaluation of the analgesic action of pethidine hydrochloride (demerol). J Pharmacol Exp Ther 80: 300–307.