Abstract
Inflammatory processes are characterized by the stimulation of humoral and cellular mediator systems resulting in increased levels of mediators of inflammation such as interleukins (ILs) and prostaglandins (PGs), which play central roles in regulating the inflammatory response and inflammation-mediated damage. The aim of the study was to develop natural therapeutics for the interventions and/or prevention of inflammatory disorders. A methanol plant extract of Centaurea ainetensis Boiss. (Asteraceae) was tested for its anti-inflammatory potential activity in two cell models of inflammation, which served as the basis for the bio-guided fractionation of the extract and the identification of its active ingredient salograviolide A. The guaianolide salograviolide inhibited endotoxin (ET)-induced IL-6 levels in SCp2 mammary epithelial cells and decreased the levels of IL-1-induced cyclooxygenase enzyme levels in intestinal epithelial cells. The results provide evidence for the anti-inflammatory activities of the sesquiterpene agent salograviolide A and the biochemical basis for further studies on salograviolide A mechanism of action.
Introduction
Centaurea ainetensis Boiss., known in Lebanon as “Chaouk El Dardar” or “Qanturyun aynata”, belongs to one of the largest genera in the Asteraceae family, and is used in Lebanese folk medicine (CitationTalhouk et al., 2007). It grows in stony, sterile, or bushy places, and the flowering period extends from May to June (CitationPost & Dinsmore, 1932; CitationTalhouk et al. 2008). Many species of the genus Centaurea (C. chilensis Bert. Ex Bull., C. cyanus L., and C. scabiosa L.) have long been used in folk medicine for the treatment of several ailments (CitationKumarasamy et al., 2003) including rheumatism (CitationHelal et al., 1997; CitationNegrete et al., 1993), malaria, hypertension, diarrhea (CitationKumarasamy et al., 2002), cough (CitationFlamini et al., 2001), and diabetes (CitationHelal et al., 1997; CitationKumarasamy et al., 2002).
Extensive chemical investigations of Centaurea species led to the isolation and identification of various types of compounds, including alkaloids (CitationSarker et al., 2001), lignans (CitationAslan & Oksuz, 1999; CitationKumarasamy et al., 2003), acetylenes, flavonoids (CitationAkkal et al., 1997, Citation1999, Citation2003; CitationAslan & Oksuz, 1999; Citationde Almeida et al., 1998; CitationFlamini et al., 2001, Citation2002), and sesquiterpene lactones (CitationAslan & Oksuz, 1999; CitationBruno et al., 2002; CitationChaves & De Oliviera, 2003; CitationFortuna et al., 2001, Citation2002; CitationHelal et al., 1997; CitationMedjroubi et al., 1997, Citation1998; CitationOksuz et al., 1994; CitationSkaltsa et al., 2000; CitationVajs et al., 1999; CitationYoussef, 1998). It has been reported that the most characteristic constituents of the genus Centaurea are sesquiterpene lactones, which are classified into guaianolides, the most abundant in this genus, followed by germacranolides, elemenolides, and finally eudesmanolides (CitationSkaltsa et al., 2000).
Recently, we have reported that crude water extracts from C. ainetensis possessed anti-inflammatory bioactivity that reduced interleukin-6 (IL-6) and gelatinases A and B expression by endotoxin (ET)-treated mammary epithelial cells. The extract also reduced paw edema and thermal hyperalgesia in rats receiving intraplantar injections of ET (CitationTalhouk et al., 2008). This study focuses on the isolation and structure identification of an anti-inflammatory agent from the aerial parts of C. ainetensis following a bioassay-guided fractionation procedure. Detailed description of the fractionation and identification procedures in parallel with the anti-inflammatory assays is presented.
Materials and methods
General experimental procedure
All deuterated and non-deuterated solvents were purchased from Acros Organics, Belgium, and the preparative thin layer chromatography (TLC) plates, silica cartridges, and silica gel were obtained from Alltech Associates, Inc., Pennsylvania, USA. Dulbecco’s modified Eagle’s medium nutrient mixture F12 Ham (DMEM/F12), phosphate-buffered saline (PBS), and heat inactivated fetal bovine serum (FBS), glucose, sodium pyruvate, trypsin, Trypan blue dye, and non-essential amino acids were obtained from Gibco, Paisley, Scotland. Insulin, gentamycin, hydrocortisone, and ovine prolactin were obtained from Sigma, St. Louis, USA; Escherichia coli, strain 055:B5, endotoxin (ET; lipopolysaccharide) was purchased from Difco Laboratories (Detroit, MI, USA). Complete Protease Inhibitor Cocktail was obtained from Roche Diagnostics GmbH. IL-1 was obtained from US Biological, Swampscott, MA, USA. Tris-HCl, sodium dodecyl sulfate (SDS), glycerol, polyacrylamide, bisacrylamide, glycine, Tween 20, polyvinylidene fluoride (PVDF) membranes, and bromophenol blue were obtained from Amersham BioSciences through Numelab (Beirut, Lebanon).
Plant material
The aerial parts of Lebanese C. ainetensis (= C. eryngioides Lam. var. ainetensis Bois.) (CitationPost & Dinsmore, 1932) were collected during the flowering season of the plant in the months of May and June 2002–2006. The plant material was identified by Dr. Stephen Jury at the University of Reading, UK. A voucher specimen has been deposited in the Post Herbarium (BEI), at the American University of Beirut. The aerial parts were dried by leaving the plant sample in the shade for 2 weeks before grinding it into around 10 mm pieces using a blender.
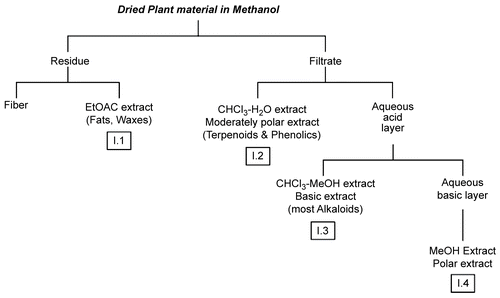
Extraction and purification
The ground plant material (250 g) was soaked in 2500 mL of methanol (MeOH) (1:10 w/v) for 16 h, during which the mixture was incubated on a shaker at 20°C. The extraction scheme was adopted from CitationHarborne (1998) and is shown in . The methanol extract was filtered to give a residue labeled as “R-I” and a filtrate labeled as “I” and named as “methanol crude extract”. R-I was then soaked in ethyl acetate (EtOAc) (10:1 w/v) and filtered to give a filtrate, labeled “I.1”. To the crude methanol extract “I”, concentrated sulfuric acid solution was added dropwise untill the pH reached 2. Following acidification, a mixture of chloroform (CHCl3):water (2:1 v/v) was added. The chloroform phase was collected and labeled as “I.2”. The aqueous layer was then basified to pH 10 by adding concentrated ammonium hydroxide solution dropwise and then resuspended in CHCl3:MeOH mixture (3:1 v/v). It was later separated into organic and aqueous layers labeled “I.3” and “I.4”, respectively. All these fractions were concentrated in vacuo at 40°C; residues were weighed and then dissolved in a minimal amount of ethanol or dimethylsulfoxide (DMSO) and were assayed for their anti-inflammatory bioactivity against ET-treated SCp2 cells. Only fraction I.2, which exhibited anti-inflammatory activity, was concentrated in vacuo at 40°C, and the obtained residue (0.48 g) was applied to a chromatographic column consisting of 150 g of silica gel (0.035–0. 07 mm and 6 nm pore diameter). A gradient elution was performed using petroleum ether:CHCl3:EtOAc (2:2:1) (3000 mL), followed by petroleum ether:CHCl3:EtOAc (1:3:1) (500 mL), CHCl3:EtOAc (3:2) (250 mL), CHCl3:EtOAc:MeOH (3:3:1) (525 mL), CHCl3:EtOAC:MeOH (3:3:2) (900 mL), CHCL3:MeOH (3:2) (750 mL), and MeOH (500 mL) successively. The anti-inflammatory bioactivity was found to be present in sub-fraction I.2-2, a fraction eluted with CHCl3:EtOAC:MeOH (3:3:1). The I.2-2 sub-fraction was purified using a conditioned normal phase SPE (solid phase extraction) cartridge (Alltech; silica, 200 mg/4.0 mL, 50/P; particle size 50 μm and average pore size 60 å). Gradient elution consisting of CH2Cl2 (20 mL), CHCl3 (20 mL), and MeOH (10 mL) was applied. The pure bioactive compound was eluted with CHCl3 (20 mL).
Figure 1. Acid–base extraction procedure adopted from CitationHarborne (1998) to fractionate the methanol crude extract of the aerial parts of C. ainetensis.
Spectroscopic measurements
One-dimensional (1H and 13C nuclear magnetic resonance (NMR) as well as DEPT (distortionless enhancement by polarization transfer) spectra and 2D NMR experiments were recorded using a Bruker 300 MHz spectrometer in deuterated chloroform (CDCl3). Chemical shifts were reported in δ (ppm) values relative to tetramethylsilane (TMS). Gas chromatography (GC) analysis was performed using a Hewlett-Packard 6890 gas chromatograph equipped with HP-5 capillary column (30 m long, 250 μm i.d, and 0.25 μm film thickness). Helium was used as a carrier gas at a flow rate of 1 mL/min. The maximum temperature was 350°C, and the column was heated from 35°C to 290°C. The injector temperature was set at 300°C in a splitless mode. Results were recorded as percent of total peak areas. The mass spectrometer employed in the GC-mass spectroscopy (MS) analysis was a Hewlett-Packard 7972 series mass selective detector in the electron impact (EI) ionization mode (70 eV). Fourier transform infrared (FTIR) spectra were recorded on a Nicolet AVATAR 360 FTIR spectrometer. Ultraviolet-visible (UV-Vis) spectra were measured in methanol using a Jasco V-570 UV/VIS/NIR spectrophotometer.
SCp2 cell culture and treatment
SCp2 mouse mammary epithelial cells, sub-clones of CID-9 cells (CitationDesprez et al., 1993), were seeded at 1 × 106 cells/well (six-well tissue culture plates) in DMEM/F12 with 5% heat inactivated FBS, insulin (5 μg/mL), and gentamycin (50 μg/mL) in a humidified incubator (95% air, 5% CO2) at 37°C. Twenty-four hours after plating, cells were washed three times with PBS and FBS-free DMEM/F12 medium, containing insulin (5 μg/mL), hydrocortisone (1 μg/mL), ovine prolactin (3 μg/mL), and gentamycin (50 μg/mL), and cells were incubated until confluent for 2–3 days. The medium was then supplemented with 1% FBS, and cells were treated with whole plant extracts or with I.1, I.2, I.3, I.4, and the resulting fractions from I.2, namely I.2-1–I.2-6, in the absence or presence of ET. Endotoxin, at 10 μg/mL, was added to the cells in culture 30 min post-treatment with plant extracts, while control cells were not treated with ET and/or plant extract as indicated. All treatments were performed in triplicate. Samples were collected 9 h post-ET treatment in the presence of “complete” protease inhibitors (final concentration of 40 μL of one tablet dissolved in 2 mL water per 1 mL of sample) and were stored at −80°C for further use.
Enzyme-linked immunosorbent assay (ELISA)
Interleukin-6 (IL-6) levels in the medium samples were measured using two-site (sandwich) ELISA CytoSets (BioSource International, Inc., USA) as described before (CitationSafieh-Garabedian et al., 2004) and according to the manufacturer’s instructions. Enhanced binding 96-well plates (Cliniplate EB; ThermoLabsystems, Helsinki, Finland) were used in all ELISA assays. Samples were assayed in triplicate from duplicate experiments.
Mode-K cell culture and treatments
Murine intestinal epithelial cells, Mode-K cells, were maintained in DMEM (low glucose) containing 10% FBS, 1000 mg/L glucose, 10 mM sodium pyruvate, and non-essential amino acids. At 70−80% confluency, cells were detached by trypsinization and replated on tissue culture plastic flasks for maintenance (1:6 or 1:8 ratio of transfer) or were used for different experiments. For IL-1 treatment, cells were plated in growth media. Twenty-four hours later, the growth medium was replaced by an incomplete medium without FBS, and on the third day, cells were treated with 10 ng/mL IL-1 with or without C. ainetensis pure compound at different concentrations and for different time periods.
Trypan blue exclusion assay
To test for toxicity of the pure compound, Mode-K cells were grown in 12-well plates until 70–80% confluency. They were then treated with different concentrations of the C. ainetensis pure compound at different time points and different concentrations. At the time of harvesting, cells were washed and then trypsinized and added back to the wash suspension. Aliquots of 50 μL of the suspension were added to 50 μL of Trypan blue dye, and cells that did not take up the dye were counted as live cells and the percentage calculated as a percentage of the total number of cells present.
Preparation of homogenate and Western blotting
The effect of IL-1 and/or C. ainetensis pure compound treatment on the protein level of the enzyme cyclooxygenase was studied using Western blotting. Cells were washed twice in PBS, scraped in 2 × electrophoresis sample buffer (SB) containing Complete Protease Inhibitor Cocktail (40 μL/mL), 0.25 M Tris-HCl (pH 6.8), 4% w/v SDS, 20% w/w glycerol, and 0.1% bromophenol blue. Samples were boiled for 5 min and centrifuged, and the supernatant collected and stored at −80°C until further use. Total protein extracts were run on a 12% SDS-polyacrylamide gel at room temperature. After electrophoresis, proteins were transferred at 4°C overnight to PVDF membranes in a transfer buffer containing glycine, Tris-base, 10% SDS, and methanol (Tris-buffered saline (TBS) buffer). Following transfer, membranes were washed once with TTBS wash buffer (1 × TBS buffer containing 0.1% Tween 20) and then blocked for 1 h in 5% non-fat dry milk at room temperature. Antibodies were then added in TTBS and incubated for 2 h at room temperature. Unbound antibodies were washed three times with TTBS, after which horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG; at 1:5000 dilution) was added and membrane incubated for 60 min. Membranes were washed and incubated with luminal reagents at room temperature for 1 min and directly exposed to autoradiography.
Results and discussion
Structure determination of the isolated guaianolide
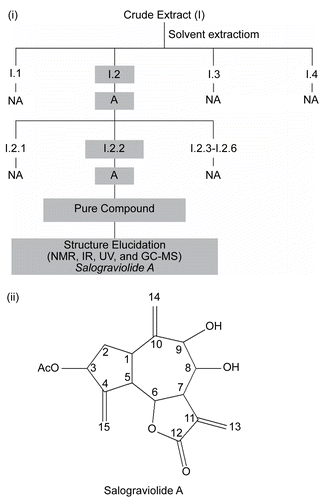
The bio-guided fractionation procedure illustrated in was followed in order to isolate and identify the guaianolide, salograviolide A (). Spectroscopic measurements confirmed the reported data on salograviolide A, which was previously isolated and identified in C. salonitana Vis. (CitationDaniewski et al., 1992) and C. nicolai Baldacci (CitationVajs et al., 1999). In addition, the position and stereochemistry of the hydroxyl groups were in agreement with the values of the coupling constants (J8,9 = 8.06 Hz trans coupling; J8,7 = 10.38 Hz; J6,7 = 9.37 Hz) determined by CitationDaniewski et al. (1992).
Figure 2. (i) Diagram summarizing the fractionation of C. ainetensis extract. “A” indicates suppression of interleukin-6 (IL-6) production and “NA” indicates marginal or no suppression of IL-6 production by endotoxin (ET)-treated SCp2 cells. (ii) The suggested structure of the guaianolide, salograviolide A.
Anti-inflammatory properties of isolated guaianolide
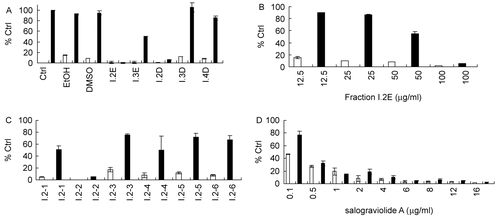
ET-treated SCp2 cells, derived from mammary heterocellular CID-9, were used as a cell model of mastisis in vitro (CitationDesprez et al., 1993; CitationSchmidhauser et al., 1990). Interleukin-6 (IL-6), an inflammatory cytokine (CitationKariko et al., 2004) whose levels are increased significantly in ET-treated SCp2 in a concentration- and time-dependent manner, has been used previously in our laboratory as a marker for ET-induced inflammation both in vivo (CitationSafieh-Garabedian et al., 2004) and in vitro (CitationSafieh-Garabedian et al., 2004). Prior to methanol extract studies, crude water extracts from C. ainetensis were shown to downregulate, in vitro, ET-induced IL-6 and gelatinase levels in mammary CID-9 cells (parent strain of the SCp2 cells) and SCp2 cells, as well as suppress, in vivo, ET-induced paw edema and hyperalgesia (CitationTalhouk et al., 2008). In this work, the ability of the methanol extract and subsequent fractions of C. ainetensis fractions to modulate IL-6 levels produced by ET-treated SCp2 cells was assessed by measuring the levels of IL-6 at 9 h following ET treatment in the presence of different concentrations of plant fractions (100 μg/mL for fractions I.1–I.4 and 16 μg/mL for fractions I.2-1–I.2-6). Fraction I.1, consisting mostly of waxes and fat residue, was not soluble in solvents compatible with cell culture media and was not assessed for its bioactivity. Out of fractions I.2, I.3, and I.4 that were dried and then reconstituted in either ethanol (EtOH) or diemthylsulfoxide (DMSO), only fraction I.2 suppressed ET-induced IL-6 levels () at 100 μg/mL. This suppression was found to be concentration dependent with approximately 40% suppression at 50 μg/mL and no observed effect at 25 and 12.5 μg/mL (). Further fractionation of I.2 yielded subfractions I.2-1–I.2-6 which were also bioassayed for their anti-inflammatory activities. Out of these six sub-fractions, only I.2-2 showed anti-inflammatory effects (), an activity which was maintained when salograviolide A (isolate I.2-2) was purified and identified. This effect was concentration dependent and was observed at a concentration as low as 1 μg/mL ().
Figure 3. Suppression of IL-6 production in ET-treated SCp2 cells by bioactive fractions of C. ainetensis: (A) Effect of I.2, I.3, and I.4 fractions (100 μg/mL) of C. ainetensis dissolved in ethanol (EtOH) or dimethylsulfoxide (DMSO) on IL-6 production (E, EtOH; D, DMSO). (B) Dose-dependent inhibition of IL-6 production by fraction I.2 at 100, 50, 25, and 12.5 μg/mL. (C) Effect of sub-fractions (I.2-2–I.2-6) at 16 μg/mL on IL-6 production. (D) Concentration-dependent effect of salograviolide A, purified fraction I.2-2, at 0.1, 0.5, 1, 2, 4, 6, and 8 μg/mL on IL-6 production. □ ▪ −ET, +ET, respectively. 100% Ctrl in (A) corresponds to 565 pg/mL of IL-6. Values in (A)–(D) were normalized to their own control values expressed as % control (Ctrl). Error bars show standard deviation of duplicate experiments.
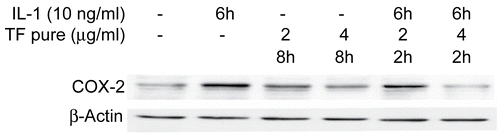
Salograviolide A (isolate I.2-2) was tested on IL-1-induced cyclooxygenase-2 (COX-2) synthesis in intestinal epithelial cells. Interleukin-1 (IL-1), a proinflammatory cytokine, is produced by a variety of cell types in response to inflammatory stimulus or injury. In the intestine, increased IL-1 production has been reported in the mucosa of patients with inflammatory bowel disease (IBD) and in intestinal tissue of animal models of IBD, suggesting that IL-1 plays a role in the pathophysiology of IBD (CitationCominelli et al., 1989; CitationMahida et al., 1989). IL-1 is a major player in many inflammatory responses, including the induction of arachidonic acid mobilization and metabolism to prostaglandins (PGs) and other eicosanoids, a hallmark of inflammatory response. The cyclooxygenase (COX) enzyme converts arachidonic acid to prostaglandins and thromboxanes and exists in at least two isoforms, a constitutive isoform COX-1 and an inducible form COX-2. COX-1 represents most of the activity under basal conditions while COX-2 appears to be the predominant isoform present under stimulatory conditions, as in the case of inflammation. We have previously shown that IL-1 treatment of murine intestinal epithelial cells, Mode-K cells, induces COX-2 expression to maximal levels at 4–6 h following IL-1 treatment (CitationHomaidan et al., 2001, Citation2002) without affecting COX-1 levels, suggesting the use of this cell system as a model of inflammation in vitro. Salograviolide A added 2 h prior to the addition of IL-1 to Mode-K cells caused a significant decrease in COX-2 levels in a concentration-dependent manner (), while COX-1 levels, as expected, were not affected by such treatment (data not shown). All working concentrations of the plant extract and its sub-fractions did not affect cell viability.
Figure 4. Modulation of cyclooxygenase-2 (COX-2) expression by salograviolide A. Western blot analysis demonstrates that 4 and 8 μg/mL of salograviolide A applied for 2 h to IL-1-treated Mode-K cells decreases COX-2 expression. β-Actin demonstrates equal loading (TF: salograviolide A).
In general, the structural requirement of α-methylidene-γ-butyrolactone to mediate its anti-inflammatory effects has been proposed to be the conjugated O=C—C=CH2. This structural moiety acts as an alkylating agent by Michael-type addition with biological nucleophiles (CitationRüngeler et al., 1999) such as thiols or amines, which react by nucleophilic addition to the carbonyl group. As a result of this chemical reaction, the α-methylidene-γ-butyrolactone moiety tends to react non-specifically, especially at high concentrations. Furthermore, it has been shown that some sesquiterpene lactones have maintained activity after reducing their unsaturated γ-lactone moiety due to their selective non-covalent inhibition of proteins (CitationBlanco et al., 2001), while others have lost their activity upon reduction (CitationKwok et al., 2001) and some have increased their activity by adding more than one Michael acceptor site (CitationLyss et al., 1998). In reference to salograviolide A, the presence of three exocyclic methylene groups, in addition to the unsaturated γ-lactone, could increase its reactivity and selectivity toward nucleophiles in proteins. Hence, derivatives to reduce the exomethylene functional groups situated at C4, C10, and C11 and their biological effects are under study.
Conclusion
Secondary metabolites of the genus Centaurea have been reported to have a multitude of biological activities including antioxidant, antifungal, antimicrobial, hypoglycemic, and anti-tumor activities. After following an anti-inflammatory guided fractionation method of extraction and isolation, salograviolide A from C. ainetensis was identified. This is the first report that describes the anti-inflammatory activity of salograviolide A. The anti-inflammatory activity noted in ET-treated SCp2 cells was concentration dependent, and suppression of IL-6 production was observed at concentrations of 1 μg/mL. In IL-1-treated Mode-K cells, salograviolide A (at 2 μg/mL) downregulated IL-1-induced COX-2 levels. The results of this study serve to define the composition of the bioactive extracts from C. ainetensis, and establish the preliminary steps in order to investigate the mechanism of the anti-inflammatory actions of salograviolide A in cell and animal models of inflammation.
Declaration of interest: This work was supported by grants from HITECH, FZE, Dubai, UAE, and the research was conducted at the IBSAR Center.
References
- Akkal S, Benayache F, Benayache S, Jay M (1997): Flavonoids from Centaurea incana (Asteraceae). Biochem Syst Ecol 25: 361–362.
- Akkal S, Benayache F, Benayache S, Medjroubi K, Jay M, Tillequin F, Seguin E (1999): A new flavone glycoside from Centaurea furfuracea. Fitoterapia 70: 368–370.
- Akkal S, Benayache F, Medjroubi K, Tillequin F, Seguin E (2003): Flavonoids from Centaurea furfuracea (Asteraceae). Biochem Syst Ecol 31: 641–643.
- Aslan U, Oksuz S (1999): Chemical constituents of Centaurea cuneifolia. Turk J Chem 23: 15–20.
- Blanco JG, Gil RR, Bocco JL, Meragelman TL, Genti-Raimondi S, Flury A (2001): Aromatase inhibition by an 11,13-dihydroderivative of a sesquiterpene lactone. J Pharmacol Exp Ther 297: 1099–1105.
- Bruno M, Maggio A, Rosseli S, Gedris T, Herz W (2002): Sesquiterpene lactones and other constituents of Centaurea paniculata ssp. castellana. Biochem Syst Ecol 379–381.
- Chaves JS, De Oliviera DCR (2003): Sesquiterpene lactones and other chemical constituents of Mikania hoehnei R. J Braz Chem Soc 14: 734–737.
- Cominelli F, Nast CC, Dinarello DA, Gentilini P, Zisper RD (1989): Regulation of eicosanoid production by rabbit colon in interleukin-1. Gastroenterolgy 97: 1400–1405.
- Daniewski WM, Nowak G, Routsi E, Rychlewska U, Szczepanska B, Skibicki P (1992): Salograviolide A, a sesquiterpene from Centaurea salonitana. Phytochemistry 31: 2891–2893.
- de Almeida AP, Miranda MMFS, Simoni IC, Wigg MD, Lagrota MHC, Costa SS (1998): Flavonol monoglycosides isolated from the antiviral fractions of Persea americana (Lauraceae) leaf infusion. Phytother Res 12: 562–567.
- Desprez PY, Roskelley C, Campisi J, Bissell MJ (1993): Isolation of functional cell lines from a mouse mammary epithelial cell strain: The importance of basement membrane and cell-cell interaction. Mol Cell Diff 1: 99–110.
- Flamini G, Antognoli E, Morelli I (2001): Two flavonoids and other components from the aerial parts of Centaurea bracteata from Italy. Phytochemistry 57: 559–564.
- Flamini G, Pardini M, Morelli I, Ertugrul K, Dural H, Bagci Y, Kargioglu M (2002): Flavonoid glycosides from Centaurea pseudoscabiosa subsp. pseudoscabiosa from Turkey. Phytochemistry 61: 433–437.
- Fortuna AM, de Riscala EC, Catalan CAN, Gedris TE, Herz W (2001): Sesquiterpene lactones from Centaurea tweediei. Biochem Syst Ecol 29: 967–971.
- Fortuna AM, de Riscala EC, Catalan CAN, Gedris TE, Herz W (2002): Sesquiterpene lactones and other constituents of Centaurea diffusa. Biochem Syst Ecol 30: 805–808.
- Harborne JB (1998): Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed. London, Chapman & Hall, pp. 302.
- Helal AM, Nakamura N, Meselhy MH, El-Fishawy A, Hattori M, Mahran GH (1997): Guaianoldes from Centaurea scoparia. Phytochemistry 45: 551–554.
- Homaidan FR, Chakroun I, Dbaibo GS, El-Assaad W, El-Sabban ME (2001): IL-1 activates two phospholipid signaling pathways in intestinal epithelial cells. Inflam Res 50: 375–381.
- Homaidan FR, Chakroun I, El-Sibai M, Dbaibo GS, El-Sabban ME (2002): IL-1 stimulates ceramide accumulation without inducing apoptosis in intestinal epithelial cells. Mediat Inflam 11: 39–45.
- Kariko K, Weissman D, Welsh FA (2004): Inhibition of toll-like receptor and cytokine signalling – a unifying theme in ischemic tolerance. J Cerebr Blood F Met 24: 1288–1304.
- Kumarasamy Y, Fergusson ME, Nahar L, Sarker SD (2002): Bioactivity of moschamindole from Centaurea moschata. Pharm Biol 40: 307–310.
- Kumarasamy Y, Middleton M, Reid RG, Nahar L, Sarker SD (2003): Biological activity of serotonin conjugates from the seeds of Centaurea nigra. Fitoterapia 74: 609–612.
- Kwok BHB, Koh B, Ndubuisi MI, Elofsson M, Crews CM (2001): The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkB Kinase. Chem Biol 8: 759–766.
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I (1998): The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kB by directly targeting p65. J Biol Chem 273: 33508–33516.
- Mahida YR, Wu K, Jewell DP (1989): Enhanced production of interleukin 1- by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn’s disease. Gut 30: 835–838.
- Medjroubi K, Benayache F, Benayache S, Akkal S, Khalfallah N, Aclinou P (1997): Guaianolides from Centaurea musimomum. Phytochemistry 45: 1449–1451.
- Medjroubi K, Benayache F, Benayache S, Akkal S, Kaabeche M, Tillequin F, Seguin E (1998): Eudesmanolide from Centaurea granata. Phytochemistry 49: 2425–2427.
- Negrete RE, Backhouse N, Cajigal I, Delporte C, Cassels BK, Breitmaier E, Eckhardt G (1993): Two new antiinflammatory elemanolides from Centaurea chilensis. J Ethnopharmacol 40: 149–153.
- Oksuz S, Serin S, Topcu G (1994): Sesquiterpene lactones from Centaurea hermannii. Phytochemistry 35: 435–438.
- Post G, Dinsmore J (1932). Flora of Syria, Palestine and Sinai, 2nd ed, Vol. 2. Beirut, American Press.
- Rüngeler P, Castro V, Mora G, Gören N, Vichnewski W, Pahl HL, Merfort I, Schmidt TJ (1999): Inhibition of transcription factor NF-kB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem 7:2343–2352.
- Safieh-Garabedian B, Mouneimne GM, El-Jouni W, Khattar M, Talhouk R (2004): The effect of endotoxin (ET) on functional parameters of mammary CID-9 cells in culture. Reproduction 127: 397–406.
- Sarker SD, Laird A, Nahar L, Kumarasamy Y, Jaspars M (2001): Indole alkaloids from the seeds of Centaurea cyanus (Asteraceae). Phytochemistry 57: 1273–1276.
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF (1990): Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5’ sequences in stably transfected mouse mammary cells. Proc Natl Acad Sci USA 87: 9118–9122.
- Skaltsa H, Lazari D, Panagouleas C, Georgiadou E, Garcia B, Sokovic M (2000): Sesquiterpene lactones from Centaurea thessala and Centaurea attica. Antifungal activity. Phytochemistry 55: 903–908.
- Talhouk RS, Karam C, Fostok S, El-Jouni W, Barbour EK (2007): Anti-inflammatory bioactivities in plant extracts. J Med Food 10(1): 1–10.
- Talhouk RS, El-Jouni W, Kogan J, Baalbaki R, Mohtaseb H, Talhouk S, Barbour EK (2008): Anti-inflammatory bio-activities in water extract of Centaurea ainetensis. J Med Plant Res 2: 24–33.
- Vajs V, Todorovic N, Ristic M, Tesevic V, Todorovic B, Janackovic P, Marin P, Milosavljevic S (1999): Guaianolides from Centaurea nicolai: Antifungal activity. Phytochemistry 52: 383–386.
- Youssef DTA (1998): Sesquiterpene lactones of Centaurea scoparia. Phytochemistry 49: 1733–1737.