Abstract
The present study aimed at isolation of the flavonoid constituents of Leonotis leonurus R. Br. (Lamiaceae) flowering aerial parts, identification of the isolated compounds, and evaluation of the hepatoprotective, anti-inflammatory and cytotoxic activities of the aqueous alcoholic and chloroform extracts. Isolation of the flavonoid constituents was performed using chromatographic techniques. Ten flavonoid compounds were isolated and identified as six flavone glycosides, two methylated flavones, and two flavone aglycons. The structures were established through chemical and spectral analysis. Paracetamol-induced hepatotoxicity, carrageenan-induced hind rat paw edema and sulforhodamine B (SRB) assay were used in the evaluation of hepatoprotective, anti-inflammatory and cytotoxic activities, respectively. The 70% methanol and chloroform extracts showed strong hepatoprotective and anti-inflammatory activity; no cytotoxic activity was observed at the chosen extract concentrations and they possess promising protective activity against paracetamol-induced hepatic damage and anti-inflammatory activity in rats. The flavonoids isolated from Leonotis leonurus in this study were found to be isolated for the first time from the genus Leonotis.
Introduction
The family Lamiaceae (mint family) is widely distributed in temperate regions, comprising about 3500 species distributed among 200 genera, most of them being herbaceous, less often shrubs, and rarely trees (CitationFeinbrun-Dothan, 1978). The mint family is of great economic importance; a source of aromatic oils such as lavender and rosemary, flavor producers such as mentha and thyme, cultivated as ornamentals such as salvia, ajuga and monarda and used for a number of medicinal purposes including relief of stomach aches, gas, and diarrhea. Mints also have antibacterial and antiviral activities. Lamiaceae is rich in flavonoids and have been reported to contain flavanones (CitationUlubelen & Brieskorn, 1977), flavone-C-glycosides (CitationAbdallah et al., 1983), flavonoles and flavones (CitationWollenweber, 1974).
Many traditional uses have been recorded for Leonotis leonurus R. Br., leaves being smoked for the relief of epilepsy, and commonly made into a medicinal tea favored for its hypnotic effect. An infusion and a decoction of the leaf and stem have been used internally for cough, cold, influenza, bronchitis, high blood pressure, headaches, diabetes, viral hepatitis, dysentery, and diarrhea and as an emetic for snakebites. A tincture of the flower has also been used for the same purpose. Externally, decoctions have been applied to treat boils, eczema, skin disease, itching, hemorrhoids, and muscular cramps (CitationGerstner, 1941; CitationWatt & Breyer-Brandwijk, 1962; Citationvan Wyk et al., 2000). Preliminary phytochemical screening of the plant revealed the presence of sterols, terpenes, tannins, flavonoids, alkaloids and saponins.
In this work, the phytochemical investigation of Leonotis leonurus flavonoids from an aqueous alcoholic extract of the flowering aerial parts of the plant is described. Also, the evaluation of the hepatoprotective, anti-inflammatory, and cytotoxic activities of the aqueous alcoholic and chloroform extracts of L. leonurus was carried out.
Materials and methods
Plant material
Flowering aerial parts of L. leonurus R. Br. was collected on spring mornings from the garden of the National Research Center (NRC), Dokki, Egypt, in March 2004. The plant was identified by S. Kawashty of the Department of Phytochemistry and Plant Systematics, National Research Center. A voucher specimen was deposited in the herbarium of the National Research Center (CAIRC).
Preparation of plant extracts
The ground-dried plant was separately extracted with 70% methanol and chloroform by percolation until exhaustion, the extracts were filtered, and the solvents were evaporated under reduced pressure at low temperature until dryness (CitationMabry et al., 1970).
Flavonoid isolation and purification
To yield the fractions of the methanolic extract, the extract was defatted with petroleum ether and applied to a polyamide column using water/methanol mixtures of decreasing polarities as eluent. Each fraction was subjected separately to preparative paper chromatography (3 mm) using BAW, 15% acetic acid/water and water as eluents. The separated bands were scraped off and eluted with 70% methanol to get the pure compounds (1-10), which were further purified by rechromatography on a Sephadex LH-20 column using methanol.
Experimental animals
Male albino rats (Laboratory Animal Colonies, NRC, Cairo) weighing 150-200 g were used in this study. The animals were housed in groups of 6 in stainless steel community cages at 22° ± 2°C with a 12 h light/dark cycle and allowed to acclimatize for a period of 15 days prior to experimental use. Throughout the experiment the rats were allowed free access to feed (rats dietary pellets prepared by Cairo Company of Oil & Soap) and water. The protocol of these experiments was approved according to the National Research Center guideline for dealing with experimental animals.
Acute toxicity test
Groups of 6 animals were used for each of the 70% methanol and chloroform extract. One group was used as a control and given orally the respective volume of the vehicle (1% Tween 80 in distilled water). The tested extracts were suspended in the vehicle immediately before use and were orally administered to the groups, through increasing doses from 100 to 5000 mg/100 g body weight (BW). All groups were kept under observation for 24 h after administration of the extracts to record the percentage of mortalities (CitationLitchfield & Wilcoxon, 1949).
Paracetamol-induced hepatotoxicity
Twenty-four male rats were divided into four groups of six animals each; Group I was served as control and received vehicle for 8 days. Group II received vehicle for 7 days. Group III received 70% methanol extract (200 mg/100 g BW/day) for 7 days. Group IV received chloroform extract (200 mg/100 g BW/day) for 7 days. Paracetamol was administered to the animals of Group II, III, and IV in a single dose of paracetamol (3 g/kg BW) on the eighth day. All animals received respective treatment by forced oral administration (CitationJafri et al., 1999). After 24 h of paracetamol administration, blood samples were withdrawn from ratino-bulber venous plexus with the help of a glass capillary under light anesthesia (CitationSanford, 1954), and were kept at room temperature for 2 h, to complete the coagulation process. The blood samples were centrifuged and the separated serum was assessed for liver functions and estimation of lipid peroxidation. After blood samples were taken, the animals were sacrificed and dissected. The livers were removed, washed with water, dried gently with filter paper, and preserved in 10% formalin to be ready for histopathological and histochemical examination.
Biochemical analysis
The activities of serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) were measured according to the colorimetric method described by CitationBegmeyer et al. (1986). The estimation of serum alkaline phosphatase (ALP) and total bilirubin were carried according to the colorimetric methods of CitationTietz et al. (1983) and CitationTolmen and Rej (1999). Lipid peroxidation (LPO) was estimated by determination of malondialdehyde (MDA) according to the modification of the CitationYagi method (1992). The percentage protection by test extracts against hepatotoxin-induced changes was calculated by considering the difference in parameter levels between rats treated with hepatotoxin and control. A 100% protection indicates that there is complete inhibition of the paracetamol-induced increase in the level of the biochemical parameters, while a 0% protection indicates that there is no reduction in the paracetamol-induced elevation in the level of the biochemical parameters.
Histopathological examination of the liver
The fixed liver samples were dehydrated by passing successively in different grades of ethyl alcohol (50%, 80% and 95%, and finally in absolute alcohol), cleared in xylene and embedded in paraffin. Sections of 6 μm thickness were prepared and stained with hematoxylin and eosin stain for microscopic observations (CitationDrury & Wallington, 1980).
Histochemical examination of the liver
The demonstration of the liver total proteins was applied using mercury bromophenol blue method (CitationMazia et al., 1953).
Carrageenan-induced hind rat paw edema
Twenty-four male rats were divided into four groups of six animals each; Group I, control group, received vehicle. Group II, positive control, received voltarin (0.7 mg/100 g BW per ora (p.o.)). Group III received 70% methanol extract (500 mg/100 g BW p.o.). Group IV received chloroform extract (500 mg/100 g BW p.o.). After 30 min, 0.1 mL of 1% w/v carrageenan solution was injected subcutaneously into the plantar tissue of the right hind paw, while the left hind paw was injected with 0.1 mL saline solution. After 3 h, all the animals were sacrificed, both the right and left hind paws were cut and their volume was measured by the use of mercury plethysmograph (CitationRouleau et al., 1997). The percentage edema and percentage reduction were calculated according to the following equations:
where Vi = the volume of the left hind paw, and Vf = the volume of the right hind paw.
SRB assay
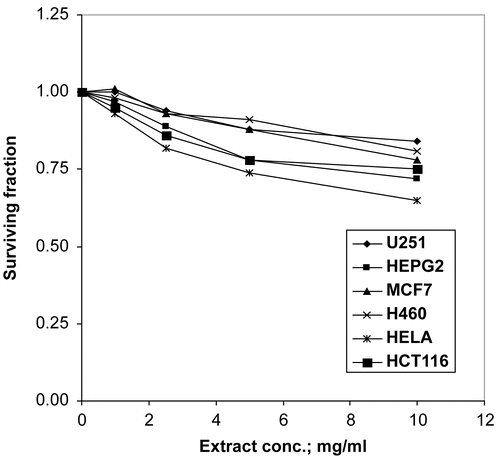
The 70% methanol extract was tested for cytotoxic activity against six human tumor cell lines, brain tumor (U251), liver carcinoma (HEPG2), breast carcinoma (MCF7), lung carcinoma (H460), cervix carcinoma (HELA) and colon carcinoma (HCT116), supplied by the National Cancer Institute, Egypt. Cells were plated in 96-multiwell plates (104 cells/well) for 24 h before treatment with the extract to allow attachment of cells to the wall of the plate. Different concentrations of the extract under test (0, 1, 2.5, 5 and 10 μg/mL) were added to the cell monolayer; triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the extracts for 48 h at 37°C and in an atmosphere of 5% CO2. After 48 h, cells were fixed, washed, and stained with Sulforhodamine B stain (SRB). Excess stain was washed with acetic acid and attached stain was recovered with Tris EDTA buffer. Color intensity was measured in an ELISA reader. At the end IC50 (concentration which reduces survival to 50%) and IC10 (concentration which reduces survival to 10%) were calculated (CitationSkehan et al., 1990).
Statistical analysis
The statistical analysis of all the biochemical analyses was carried out using SPSS 11.0 for Windows. The values were represented as mean ± standard error (SE). Paired t-test (CitationSnedecor & Cochran, 1971) was used for reporting the P value and significance with respect to the control group and ANOVA for the comparison between more than two groups. Results with P < 0.05 were considered as statistically significant.
Results
Chemical characterization of isolated compounds
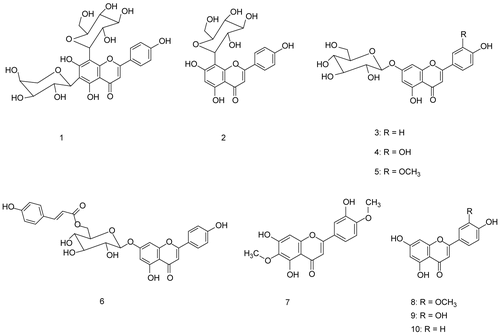
Ten flavonoid compounds were isolated and identified as one diglycoside flavone, apigenin 6-C-α-arabinoside-8-C-β-glucoside (1); four monoglycoside flavones, apigenin 8-C-β-glucoside (2), apigenin 7-O-β-glucoside (3), luteolin 7-O-β-glucoside (4), and luteolin 7-O-β-glucoside-3’-methyl ether (5); one acylated monoglycoside flavone, apigenin 7-O- (6’’-O-p-coumaroyl)-β-glucoside (6); two methylated flavones, 6-methoxyluteolin-4’-methyl ether (7) and luteolin 3’-methyl ether (8); and two flavone aglycons, luteolin (9) and apigenin (10) (CitationMabry et al., 1970; CitationMarkham, 1982; CitationHarborne & Mabry, 1982; CitationHarborne, 1994). These compounds were isolated for the first time from Leonotis leonurus plant and even from the genus Leonotis.
The structures of the isolated compounds were established by means of a UV-visible spectrophotometer (Shimadzu model UV-240 and 2401 PC), MS (Finnigan MAT SSQ 7000, 70 ev) and NMR spectrometer (Jeol ECA 500), NRC, Egypt as follows.
Apigenin 6-C-α-arabinoside-8-C-β-glucoside (1):
yellow powder, PC Rf 18 (BAW) and 40 (15% HOAc). UV λmax (MeOH): 273, 332; (NaOMe): 282, 332 sh, 399; (AlCl3): 264, 280, 305, 348, 387; (AlCl3/HCl): 262, 280, 305, 346, 385; (NaOAc): 282, 337; (NaOAc/H3BO3): 279, 348. 1H-NMR (DMSO-d6, 500 MHz): δ 8.02 (2H, d, J = 8 Hz, H-2’,6’), 6.91 (2H, d, J = 8 Hz; H-3’,5’), 6.78 (1H, s, H-3), 4.78 (d, J = 9.7 Hz, H-1’’’), 4.68 (d, J = 9.2 Hz, H-1’’). 13C-NMR (DMSO-d6, 125 MHz) : δ 182.6(C-4), 164.4(C-2), 161.7(C-7, 4’), 158.8(C-5), 155.6(C-9), 129.5(C-2’,6’), 122(C-1’), 116.3(C-3’,5’), 108.6(C-6), 105.6(C-8), 103.8(C-10), 103(C-3), 82.4(C-5’’’), 79.4(C-3’’’), 74.7(C-1’’), 74.4(C-3’’), 73.8(C-1’’’), 71.5(C-2’’’), 71(C-5’’), 70.6(C-4’’’), 70(C-2’’), 69(C-4’’), 61.7(C-6’’’). HMBC spectrum showed correlations between the anomeric proton of arabinose (H-1’’) at δH 4.68 with C-5, C-6 and C-7 at δC 158.8, 108.6 and 161.7 respectively confirming that the arabinose moiety is attached to C-6 of apigenin. While the anomeric proton of glucose (H-1’’’) at δH 4.78 showed correlations with C-7, C-8 and C-9 at δC 161.7, 105.6 and 155.6 respectively confirming that the attachment of the glucose moiety to the aglycone is at C-8. The other correlations are in a good agreement with the proposed structure.
Apigenin 8-C-β-glucoside; vitexin (2):
yellow powder, PC Rf 38 (BAW) and 20 (15% HOAc). UV λmax (MeOH): 269, 331; (NaOMe): 279, 327 sh, 392; (AlCl3): 276, 303, 346, 382; (AlCl3/HCl): 277, 303, 343, 381; (NaOAc): 279, 389; (NaOAc/H3BO3): 270, 319, 345. 1H-NMR (DMSO-d6, 500 MHz): δ 7.96(2H, d, J = 8.4 Hz; H-2’, 6’), 6.87(2H, d, J = 8.4 Hz; H-3’, 5’), 6.59(1H, s, H-3), 5.97(1H, s, H-6), 4.71(1H, d, J = 9.2 Hz, H-1’’).
Apigenin 7-O-β-glucoside (3):
yellowish white powder, PC Rf 50 (BAW) and 19 (15% HOAc). UV λmax (MeOH): 268, 330; (NaOMe): 271, 388; (AlCl3): 276, 299, 346, 382; (AlCl3/HCl): 277, 298, 342, 382; (NaOAc): 267, 337; (NaOAc/H3BO3): 267, 334. 1H-NMR (DMSO-d6, 500 MHz): δ 7.95(2H, d, J = 8.4 Hz; H-2’, H-6’), 6.93(2H, d, J = 8.4 Hz; H-3’, H-5’), 6.8(2H, d; H-3, H-8), 6.44(1H, d, J = 1.5 Hz; H-6), 5.07(1H, d, J = 6.9 Hz; H-1’’). 13C-NMR (DMSO-d6, 125 MHz) : δ 182.4(C-4), 164.8(C-2), 163.4(C-7), 162.3(C-5), 161.6(C-4’), 157.4(C-9), 129.1(C-2’,6’), 121.2(C-1’), 116.6(C-3’,5’), 105.8(C-10), 103.4(C-3), 100.4(C-1’’), 100(C-6), 95.3(C-8), 77.7(C-5’’), 76.9(C-3’’), 73.6(C-2’’), 70.1(C-4’’), 61.1(C-6’’).
Luteolin 7-O-β-glucoside (4):
yellowish brown powder, PC Rf 47 (BAW) and 9 (15% HOAc). UV λmax (MeOH): 255, 348; (NaOMe): 262, 402; (AlCl3): 272, 297sh, 328sh, 405; (AlCl3/HCl): 274, 295sh, 358sh, 388; (NaOAc): 258, 406; (NaOAc/H3BO3): 258, 371. 1H-NMR (DMSO-d6, 500 MHz): δ 7.4(2H, m; H-2’,6’), 6.9(1H, d, J = 8.3 Hz; H-5’), 6.78 (1H, d, J = 1.3 Hz; H-8), 6.74(1H, s; H-3), 6.44(1H, d, J = 1.3 Hz; H-6), 5.08(1H, d, J = 7.6 Hz; H-1’’). 13C-NMR (DMSO-d6, 67.5 MHz): δ 181.9(C-4), 164.4(C-2), 162.9(C-7), 161.1(C-5), 156.9(C-9), 150(C-4’), 145.8(C-3’), 121.2(C-1’), 119.1(C-6’), 115.9(C-5’), 113.5(C-2’), 105.3(C-10), 103.1(C-3), 99.8(C-1’’), 99.5(C-6), 94.6(C-8), 77.1(C-3’’), 76.3(C-5’’), 73(C-2’’), 69.5(C-4’’), 60.5(C-6’).
Luteolin 7-O-β-glucoside-3’-methyl ether (5):
yellow powder, PC Rf 54 (BAW) and 10 (15% HOAc). UV λmax (MeOH): 253, 268, 345; (NaOMe): 262, 406; (AlCl3): 263, 274, 294, 357, 386; (AlCl3/HCl): 262, 275, 294, 354, 384; (NaOAc): 254, 407; (NaOAc/H3BO3): 269, 346. 1H-NMR (DMSO-d6, 500 MHz): δ 7.53(1H, d, J = 8.4 Hz; H-6’), 7.44(1H, d, J = 1.6 Hz; H-2’), 6.8(3H, m; H-3, H-8, H-5’), 6.4(1H, d, J = 1.5 Hz; H-6), 5.04(1H, d, J = 7.6 Hz; H-1’’), 3.84(3H, s; -OCH3). 13C-NMR (DMSO-d6, 125 MHz): δ 182(C-4), 165.3(C-2), 163.2(C-7), 161.7(C-5), 157.3(C-9), 149.5(C-3’,4’), 121.9(C-1’,6’), 116(C-5’), 110(C-2’), 105.7(C-10), 102.1(C-3), 100.5(C-1’’), 99.8(C-6), 95.2(C-8), 77.7(C-3’’), 77(C-5’’), 73.6(C-2’’), 70.1(C-4’’), 61.1(C-6’’), 56.2(-OCH3).
Apigenin 7-O-(6’’-O-p-coumaroyl)-β-glucoside (6):
yellowish green powder, PC Rf 65 (BAW) and 9 (15% HOAc). UV λmax (MeOH): 269, 317; (NaOMe): 269, 375; (AlCl3): 278, 300, 324, 382; (AlCl3/HCl): 279, 300, 324, 382; (NaOAc): 269, 317, 383; (NaOAc/H3BO3): 268, 319. 1H-NMR (DMSO-d6, 500 MHz): δ 7.94(2H, d, J = 9.2 Hz; H-2’,6’), 7.49(1H, d, J = 16 Hz; H-7’’’), 7.36(2H, d, J = 8.4 Hz; H-2’’’,6’’’), 6.91(2H, d, J = 9.2 Hz; H-3’,5’), 6.82(1H, s, H-3), 6.8 (1H, d, J = 2.3 Hz; H-8), 6.65(2H, d, J = 8.4 Hz; H-3’’’,5’’’), 6.46(1H, d, J = 2.3 Hz; H-6), 6.3(1H, d, J = 16 Hz; H-8’’’), 5.16(1H, d, J = 6.9 Hz; H-1’’). 13C-NMR (DMSO-d6, 125 MHz): δ 182.4 (C-4), 166.9(C-9’’’), 164.8(C-2), 163.2(C-7), 161.9(C-5), 161.6(C-4’),160.3(C-4’’’), 157.4(C-9), 145.4(C-7’’’), 130.6(C-2’’’,6’’’), 129(C-2’,6’), 125.4(C-1’’’), 121.4(C-1’), 116.5(C-3’,5’), 116.1(C-3’’’, 5’’’), 114.2(C-8’’’), 105.8(C-10), 103.5(C-3), 99.9 (C-6, 1’’), 95.2(C-8), 76.7(C-3’’), 74.3(C-5’’), 73.4(C-2’’), 70.4(C-4’’), 63.9(C-6’’).
6-Methoxyluteolin-4’-methyl ether (7):
yellowish brown powder, PC Rf 41(BAW) and 27(50% HOAc). EI-MS: m/z 330 (100%). UV λmax (MeOH): 284, 339; (NaOMe): 269, 313, 370; (AlCl3): 258, 372; (AlCl3/HCl): 257, 366; (NaOAc): 286, 334; (NaOAc/H3BO3): 287, 334. 1H-NMR (DMSO-d6, 500 MHz): δ 7.52(1H, dd, J = 2.3, 8.6 Hz; H-6’), 7.45(1H, d, J = 2.3 Hz; H-2’), 7.06(1H, d, J = 8.6 Hz; H-5’), 6.86(1H, s; H-3), 6.74(1H, s; H-8), 3.82(3H, s;-OCH3), 3.89(3H, s; -OCH3). 13C-NMR (DMSO-d6, 125 MHz): δ 182.6 (C-4), 164(C-2), 154.9(C-7), 151.6(C-4’), 150.1(C-9), 147.3(C-5), 146.6(C-3’), 130.4(C-6), 123.6(C-1’), 119.1(C-6’), 113.4(C-2’), 112.5(C-5’), 105.5(C-10), 103.6(C-3), 91.6(C-8), 56.8 (-OCH3), 56.2(-OCH3).
Luteolin 3’-methyl ether; chrysoeriol (8):
yellow powder, PC Rf 76 (BAW) and 37 (50% HOAc). EI-MS: m/z 300 (100%). UV λmax (MeOH): 264, 295 sh., 341; (NaOMe): 266, 328 sh, 400; (AlCl3): 260, 275, 298, 355, 384; (AlCl3/HCl): 260, 276, 297, 352, 385; (NaOAc): 270, 314, 394; (NaOAc/H3BO3): 269, 343. 1H-NMR (DMSO-d6, 500 MHz): δ 7.52(1H, d, J = 8.4 Hz; H-6’), 7.5 (1H, d, J = 1.5 Hz; H-2’), 6.9(1H, d, J = 8.4 Hz; H-5’), 6.77(1H, s, H-3), 6.31 (1H, d, J = 1.5 Hz; H-8), 6.01 (1H, d, J = 1.5 Hz; H-6), 3.85 (3H, s; -OCH3).
Luteolin (9):
yellow powder, PC Rf 69 (BAW) and 27 (50% HOAc). EI-MS: m/z 286 (100%). UV λmax (MeOH): 254, 264, 346; (NaOMe): 260, 310sh, 395; (AlCl3): 272, 300, 327, 418; (AlCl3/HCl): 276, 296, 358, 385; (NaOAc): 270, 390, (NaOAc/H3BO3): 260, 370. 1H-NMR (DMSO-d6, 500 MHz): δ 7.29(1H, d, J = 8.4 Hz; H-6’), 7.2(1H, d, J = 2 Hz; H-2’), 6.6(1H, d, J = 8.4 Hz; H-5’), 6.36(1H, s; H-3), 6.18(1H, d, J = 2 Hz; H-8), 5.93(1H, d, J = 2 Hz; H-6).
Apigenin (10):
yellowish white powder, PC Rf 81(BAW) and 37(50% HOAc). EI-MS: m/z 270 (100%). UV λmax (MeOH): 268, 337; (NaOMe): 274, 322 sh, 392; (AlCl3): 276, 301, 346, 382; (AlCl3/HCl): 276, 300, 344, 380; (NaOAc): 274, 387; (NaOAc/H3BO3): 268, 344. 1H-NMR (DMSO-d6, 500 MHz): δ 7.88(2H, d, J = 8 Hz; H-2’,6’), 6.89(2H, d, J = 8 Hz; H-3’,5’), 6.67(1H, s; H-3), δ 6.33(1H, d, J = 2 Hz; H-8), 6.05(1H, d, J = 2 Hz; H-6).
Acute toxicity
Both 70% methanol and chloroform extracts of flowering aerial parts of Leonotis leonurus were found to be practically non-toxic when administered orally to rats and its LD50 value was found to be higher than 5000 mg/100 g BW. This data enabled us to select the dose to be administered to rats for assessing its hepatoprotective and anti-inflammatory activities. The doses used in paracetamol-induced hepatotoxicity and carrageenan-induced hind rat paw edema methods were 200 mg/100 g BW and 500 mg/100 g BW, respectively.
Paracetamol-induced hepatotoxicity
The results of paracetamol-induced hepatotoxicity are presented in . In rats treated with paracetamol alone, there was a significant elevation in AST, ALT, ALP, bilirubin and MDA levels as compared with the control rats. Pretreatment with the flowering aerial parts of Leonotis leonurus extracts (70% methanol or chloroform) exhibited a significant (P < 0.05) protection against the elevation of AST, ALT, ALP, bilirubin and MDA levels as compared with the paracetamol-treated rats. On the other hand, the results indicated that the total 70% methanol extract showed higher protection effect against paracetamol-induced elevations in the levels of serum parameters and MDA than that of the chloroform extract. The protection percentages of pretreatment with 70% methanol were 68.72% (AST), 83.06% (ALT), 89.68% (ALP), 76.92% (bilirubin) and 85.41% (MDA). While that of pretreatment with chloroform extract were 65.63% (AST), 74.14% (ALT), 60.54% (ALP), 69.23% (bilirubin) and 64.58% (MDA).
Table 1. Effect of 70% methanol and chloroform extracts of L. leonurus on serum biochemical parameters and lipid peroxidation in paracetamol-induced hepatic damage in rats.
Histopathological changes in paracetamol- intoxicated liver
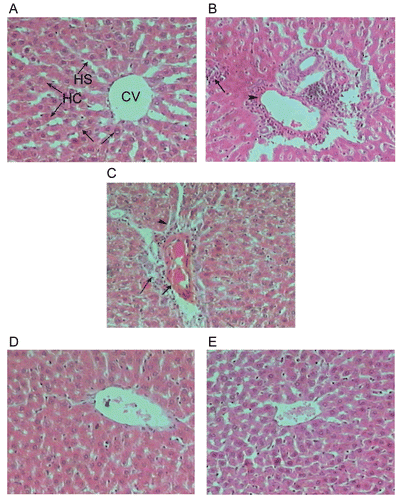
The hepatic lobules are the structural units of the liver; each is formed of cords of hepatocytes with blood sinusoids in between. The sinusoids run radially, converging at the center of the hepatic lobule to form the central or centrolobular vein ().
Examination of liver sections of rats given an oral dose equivalent to 3 g/kg BW of paracetamol demonstrated mild lymphocytic infiltration in the portal and periportal areas associated with dilated and congested veins (). In some animals, the hepatocytes presented small vacuoles of fatty change mainly in the inner and outer regions of the lobules. The cytoplasm and the nuclei of these hepatocytes exhibited pale stainability. The hepatocytes around the dilated congested vessels appeared variable in shape and size ().
Examination of liver sections of rats treated with a daily dose of 200 mg/100 g rats of the methanolic or chloroform extract for seven days and received a single oral dose of paracetamol showed that architecture appear more or less near the control (, E).
Figure 1. A photomicrograph of section of liver of (A) control rat showing the architecture of a hepatic lobule, (B) rat liver given an oral dose of paracetamol equivalent to 3 g/kg BW showing focal necrosis associated with inflammatory infiltration. Notice the venous congestion in the portal area, (C) rats given an oral dose of paracetamol equivalent to 3 g/kg BW showing a portal tract with dilated and congested vein. Notice the periportal necrosis of the hepatocytes that surround the portal area, and the inflammatory infiltration, (D) rat daily given an oral dose equivalent to 200 mg/100 g BW of total 70% methanol extract of L. leonurus for seven successive days and treated with a dose of paracetamol equivalent to 3 g/kg BW on day 8 showing the liver architecture that appears more or less as control, and (E) rats daily given an oral dose equivalent to 200 mg/100 g BW of chloroform extract of L. leonurus for seven successive days and treated with a dose of paracetamol equivalent to 3 g/kg BW on day 8 showing the liver architecture that appears more or less as control (H & E stain-X 300).
Histochemical changes in paracetamol-intoxicated liver
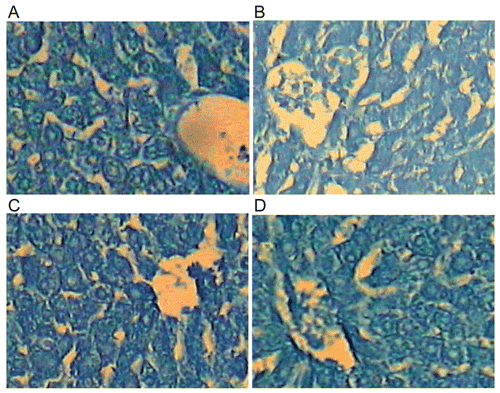
Examination of sections of the liver of the control rats displayed the proteinic inclusions in the hepatocytes as grayish blue irregular particles of various sizes against weakly to moderately stained ground cytoplasm. The nuclear chromatin and the nucleoli are densely stained indicating their rich content of proteinic constituents (). In rats administrated with a single oral dose of paracetamol equivalent to 3 g/kg BW, the proteinic inclusions showed marked diminution in many liver cells and the stainability was mostly diffused ().
Daily treatment of the rats 200 mg/100 g BW of the methanolic or chloroform extract for seven days and received a single oral dose of paracetamol revealed relative diffusion and diminution of the stainability of the proteinic content in both the cytoplasm and nucleus of the hepatocytes of the treated animals (, D).
The above results showed that paracetamol induced a decrease in the proteinic content of the liver of treated animals. The protein inclusions became more or less as control in the rats treated with 200 mg/100 g BW of the methanolic or chloroform extract for seven days and received a single oral dose of paracetamol.
Figure 2. A photomicrograph of section of liver of (A) control rat showing the proteinic contents. Notice the irregular particles of various sizes that are equally distributed in the cytoplasm of the liver cells. The nucleoli are intensely stained while the ground cytoplasm and nucleoplasm display faint stainability, (B) rat liver received a single oral dose of paracetamol equivalent to 3 g/kg BW showing the stainability of the proteinic inclusions of the hepatocytes. The stainability is relatively diffused in both the cytoplasm and nucleus, (C) rat daily received an oral dose equivalent to 200 mg/100 g BW of total 70% methanol extract of L. leonurus for seven successive days and received a single oral dose of paracetamol (3 g/kg BW) on day 8 showing the proteinic inclusions in many liver cells. Notice that the stainability is mostly diffused in cytoplasm and nuclei, and (D) rat daily received an oral dose equivalent to 200 mg/100 g of chloroform extract of L. leonurus for seven days and received a single oral dose of paracetamol (3 g/kg BW) on day 8 showing the proteinic inclusions. Notice that in some cells the proteinic particles are relatively few in number. No change was observed in most hepatocytes (Bromophenol blue reaction-X 600).
Anti-inflammatory results
The results of the anti-edematous effect of oral administration of 70% methanol or chloroform extracts of flowering aerial parts of Leonotis leonurus are shown in . The 70% methanol and chloroform extracts (500 mg/100 g BW) caused a significant reduction (20% and 41%, respectively) in the paw edema. Voltarin (0.7 mg/100 g BW), utilized as a reference anti- inflammatory, produced significant inhibition by 26%.
Table 2. Effects of total 70% methanol and chloroform extracts on the carrageenan-induced hind paw edema in rats.
Discussion
Flavonoids are diphenyl propanoids that occur everywhere in plant foods and form important constituents of the human diet. The 70% methanol extract of the flowering aerial parts of Leonotis leonurus was shown by preliminary 2D-PC screening to contain a complicated flavonoid mixture ().
Ten known flavonoid compounds were isolated and purified by standard methods. All compounds appeared as dark purple spots on PC under UV light, changing to yellow when exposed to ammonia vapor, except compound (7) where no change in color was observed. Chemical investigations: complete acid hydrolysis for O-glycosides and ferric chloride degradation for C-glycosides were carried out, and followed by paper co-chromatography with authentic samples to identify the hydrolytic flavonoid glycoside products whether aglycons and sugar moieties. The structures of the isolated flavonoids were determined from UV, MS and NMR spectral data.
Both 70% methanol and chloroform extracts of L. leonurus were found to be safe for further biological studies, as no lethality was observed even at 5000 mg/100 g BW p.o. in rats. This is the first study to show the hepatoprotective activity of L. leonurus on rats using paracetamol-induced hepatotoxicity method. The hepatoprotective activity of 70% methanol and chloroform extracts was evaluated by measuring their protective effects on liver functions and structure as well as lipid peroxidation. Protection against paracetamol induced toxicity has been used as a test for a potential hepatoprotective agent by several investigators (CitationDwivedi et al., 1991; CitationVisen et al., 1993; CitationSingh & Handa, 1995). Paracetamol is a common antipyretic agent which is safe in therapeutic doses but can produce fatal hepatic necrosis in man, rats and mice with toxic doses (CitationPrescott et al., 1971; CitationMitchell et al., 1973). Paracetamol is mainly metabolized in liver to excretable glucuronide and sulfate conjugates (CitationJollow et al., 1974; CitationWong et al., 1981). However, hepatotoxicity of paracetamol has been attributed to the formation of toxic metabolites when a part of paracetamol is activated by hepatic cytochrome P-450 (CitationSavides & Oehme, 1983) to a highly reactive metabolite; N-acetyl-p-benzoquinone imine (CitationVermeulen et al., 1992). This reactive metabolite binds to macromolecules and cellular proteins and also causes peroxidation of the biological membrane leading to the formation of lipid radicals and a variety of degraded products (the majority of which is malondialdehyde) and eventually liver injury (CitationUpasani et al., 2001). Due to liver injury, the transport function of the hepatocytes gets disturbed, resulting in the leakage of plasma membrane (CitationZimmerman & Seeff, 1970), thereby causing an increased enzyme level in the serum. Also bilirubin concentration is increased in the serum as a result of liver dysfunction (CitationHall, 2001). Leakage of large quantities of enzymes into the blood stream is often associated with massive necrosis of the liver (CitationRees & Spector, 1961).
The present study confirms the better hepatoprotective effect of the 70% methanol and chloroform extracts of L. leonurus against experimentally induced hepatic damage by paracetamol. The biochemical results of the present work were in accordance with histopathological results. The hepatoprotective effect of the extract was due to flavonoid constituents (CitationRebrin & Sohal, 2004; CitationZheng et al., 2005). Such flavonoids, as apigenin-7-O-glucoside, luteolin-7-O-glucoside, and quercitin prevented the glutathione depletion and lipid peroxidation induced by an acute intoxication with carbon tetrachloride (CCl4), ethanol, acetominophen and bromobenzene in the liver and in the rats with biliary obstruction (CitationFerrandiz et al., 1994; CitationPeres et al., 2000; CitationQiusheng et al., 2004).
The results obtained here also showed that L. leonurus had anti-inflammatory activity, where the 70% methanol extract was almost as effective as voltarin while the chloroform extract showed higher activity. This activity may be due to the action of the flavonoid compounds (CitationModnicki et al., 2007). Anti-inflammatory activity of the aqueous leaf extract of L. leonurus was reported in vivo using fresh egg albumin-induced paw edema method (CitationOjewole, 2005) and in vitro using cyclooxygenase (COX-1) anti-inflammatory inhibition assay (CitationJäger et al., 1996; CitationStafford et al., 2005).
The 70% methanol extract showed no cytotoxic activity against the human tumor cell lines tested at the chosen extract concentrations.
In conclusion, the present study reveals that the 70% methanol and chloroform extracts of L. leonurus possess promising protective activity against paracetamol-induced hepatic damage and anti-inflammatory activity in rats. These activities may be attributed to the presence of flavonoids.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdallah MF, Saleh NM, Gabr S, Abu-Eyta AM El-Said H (1983): Flavone glycosides of Salvia triloba. Phytochemistry 22: 2057–2060.
- Begmeyer HU, Horder M, Rej R (1986): IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate and alanine aminotransferases. J Clin Chem Clin Biochem 24: 481–495, 497–510.
- Drury RAB, Wallington EA (1980): Carleton’s Histological Technique, 5th edn. Oxford, Oxford University Press, pp.195–196.
- Dwivedi Y, Rastogi R, Garg NK, Dhawan BN (1991): Prevention of paracetamol-induced hepatic damage in rats by picroliv, the standard active fraction from Picrorhiza Kurroa. Phytother Res 5: 115–119.
- Feinbrun-Dothan N (1978): Flora Palaestina Jerusalem. The Israel Academy of Sciences and Humanities, p. 481.
- Ferrandiz ML, Bustos G, Paya M, Gunasegaran R, Alcaraz MS (1994): Hispidulin protection against hepatotoxicity induced by bromobenzene in mice. Life Sci 55: 145–150
- Gerstner J (1941): A preliminary check list of Zulu names of plants with short notes. Bantu Studies 15: 277–301, 369–383.
- Hall RL (2001): Principles of clinical pathology for toxicology studies, in: Hayes AW, ed., Principles and Methods of Toxicology, 4th edn. Philadelphia, Taylor and Francis, p. 1001.
- Harborne JB, Mabry TJ (1982): The Flavonoids - Advances in Research. London, Chapman and Hall, p. 19.
- Harborne JB (1994): The Flavonoids - Advances in Research Since 1986. London, Chapman and Hall, p. 441.
- Jafri MA, Subhani MJ, Javed K, Singh S (1999): Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats. J Ethnopharmacol 66: 355–361.
- Jäger AK, Hutchings A, van Staden J (1996): Screening of Zulu medicinal plants for prostaglandin-synthesis inhibitors. J Ethnopharmacol 52: 95–100.
- Jollow DJ, Thorgeirsson SS, Potter WZ, Hashimoto M, Mitchell JR (1974): Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and non-toxic doses of acetaminophen. Pharmacology 12: 251–271.
- Litchfield JT, Wilcoxon FWJ (1949): A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96: 99–113.
- Mabry TJ, Markham KR, Thomas MB (1970): The Systematic Identification of Flavonoids. New York, Springer.
- Markham KR (1982): Techniques of Flavonoid Identification. London, Academic Press.
- Mazia D, Brewer PA, Afert M (1953): The cytochemical staining and measurement of protein with the mercuric bromophenol blue. Biol Bull 104: 57–67.
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BN (1973): Acetaminophan induced hepatic necrosis. 1. Role of drug metabolism. J Pharmacol Exp Ther 187: 185–194.
- Modnicki D, Tokar M, Klimek B (2007): Flavonoids and phenolic acids of Nepeta cataria L. var. citriodora (Becker) Balb. (Lamiaceae). Acta Pol Pharm 64: 247–252.
- Ojewole JA (2005): Antinociceptive, anti-inflammatory and antidiabetic effects of Leonotis leonurus (L.) R. BR. (Lamiaceae) leaf aqueous extract in mice and rats. Methods Find Exp Clin Pharmacol 27: 257–264.
- Peres W, Tunon MJ, Collado PS, Herrmann S, Marroni N, Gonzalez-Gallego J (2000): The flavonoid quercetin ameliorates liver damage in rats with biliary obstruction. J Hepatol 33: 742–750.
- Prescott LF, Wright N, Roscoe P, Brown SS (1971): Plasma paracetamol half life and hepatic necrosis in patients with paracetamol overdose. Lancet 1: 519–522.
- Qiusheng Z, Xiling S, Xubo Meng, S, Changhai W (2004): Protective effects of luteolin-7-glucoside against liver injury caused by carbon tetrachloride in rats. Pharmazie 59: 286–289.
- Rebrin I, Sohal RS (2004): Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp Gerontol 39: 1513–1519.
- Rees KR, Spector WG (1961): Reversible nature of liver cell damage due to carbon tetrachloride as demonstrated by the use of Phenergan. Nature 189: 821–829.
- Rouleau A, Garbarg M, Ligneau X, Mantion C, Lavie P, Advenier C, Lecomte JM, Krause M, Stark H, Schunack W, Schwartz, JC (1997): Bioavailability, antinociceptive and antiinflammatory properties of BP 2-94, a histamine H3 receptor agonist prodrug. J Pharmacol Exp Ther 281: 1085–1094.
- Sanford HS (1954): Method for obtaining venous blood from the orbital sinus of the rat or mouse. Science 119: 100.
- Savides MC, Oehme FW (1983): Acetaminophen and its toxicity. J Appl Toxicol 3: 95–111.
- Singh A, Handa SS (1995): Hepatoprotective activity of Apium graveolens and Hygrophila auriculata against paracetamol and thioacetamide intoxication in rats. J Ethnopharmacol 49: 119–126.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990): New colourimetric cytotoxicity assay for anticancer drug Screening. J Nat Cancer Inst 82: 1107–1112.
- Snedecor WG, Cochran GW (1971): Statistical Methods. Ames, Iowa State University Press.
- Stafford GI, Jäger AK, Van Staden J (2005): Effect of storage on the chemical composition and biological activity of several popular South African medicinal plants. J Ethnopharmacol 97: 107–115.
- Tietz NW, Rinker D, Shaw LM (1983): IFCC methods for the measurement of catalytic concentration of enzymes. Part 5. IFCC method for alkaline phosphatase. J Clin Chem Clin Biochem 21: 731–748.
- Tolmen KG, Rej R (1999): Liver function, in: Burtis CA & Ashwood ER, ed., Tietz Book of Clinical Chemistry, Philadelphia, Saunders Company, p.1136.
- Ulubelen A, Brieskorn CH (1977): Chemical study of the herba of Salvia amplexicaulis. Planta Med 31: 80–82.
- Upasani CD, Khera A, Balaraman R (2001): Effect of lead and vitamin E, C or spiruline on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol 39: 70–74.
- Van Wyk BE, Van Oudtshoorn B, Gericke N (2000): Medecinal Plants of South Africa, 2nd edn. Pretoria, Briza, p.166.
- Vermeulen NPE, Bessems JGM, Van de Streat R (1992): Molecular aspects of paracetamol induced hepatotoxicity and its mechanism based prevention. Drug Metab Rev 24: 367–407.
- Visen PKS, Shukla B, Patnaik GK, Dhawan BN (1993): Andrographalide protects rat hepatocytes against paracetamol induced damage. J Ethnopharmacol 40: 131–136.
- Watt JM, Breyer-Brandwijk BN (1962): Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd edn. Edinburgh and London, Churchill Livingstone, p.514.
- Wollenweber E (1974): Flavones and flavonols in exudates of Salvia glutinosa. Phytochemistry 13: 753.
- Wong LT, Whitehouse LW, Solemonraj G, Paul CJ (1981): Pathways of disposition of acetaminophen conjugate in the mouse. Tox Lett 9: 145–151.
- Yagi K (1992): Assay for serum lipid peroxide level and its clinical significance, in: Yagi K, ed., Lipid Peroxides in Biology and Medicine, New York, Academic Press.
- Zimmerman HJ, Seeff LB (1970): Enzymes in hepatic disease, in: Goodly EI, ed., Diagnostic Enzymology, Philadelphia, Lea & Febiger, p.1.
- Zheng QS, Sun X, Xu B, Li G, Song M (2005): Mechanisms of apigenin-7-glucoside as a hepatoprotective agent. Biomed Environ Sci 18: 65–70.