Abstract
The Purdue-UAB Botanicals Research Center for Age Related Disease uses multidisciplinary and innovative technologies to investigate the bioavailability of bioactive polyphenolic constituents from botanicals and their relationship to human health. Many age-related diseases are associated with oxidative stress and tissue damage. One of the research goals of the Purdue-UAB Center is to investigate the bioavailability of bioactive natural compounds from a complex botanical mixture to the organ affected by the disease, determine the uptake and metabolism of these compounds, and relate these data to a protective mechanism. Equally important is to screen commercially available botanicals for their safety and efficacy. The central aims of the Center include the investigation of botanicals and their relationship to bone antiresorptive capacity, cognitive function, vascular effects, and cancer prevention.
Introduction
Due to the unprecedented use of botanical dietary supplements by the American public in recent years, following the 1994 Dietary Supplement Health and Education Act, the National Institutes of Health has mandated rigorous scientific study of plant-based dietary supplements. In 1999, the National Institutes of Health (NIH), through the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), established a Botanicals Centers program. In 2000, Purdue University and the University of Alabama-Birmingham (UAB), in collaboration with investigators at Rutgers University, Indiana University School of Medicine, and University of Illinois, received support from the NIH to establish a Center for Botanical Dietary Supplements Research (CBDSR) to study the effectiveness and mechanism of action of polyphenolic compounds purported to reduce the risk of cancer, osteoporosis, cardiovascular disease, cognitive function, and other age-related diseases. In 2005, the Purdue-UAB consortium was renewed as a CBDSR by NIH/NCCAM. The Purdue-UAB Botanicals Center is directed by Dr. Connie Weaver from Purdue University; Dr. Stephen Barnes from UAB is the Associate Director. The mission of the Purdue-UAB Center is to study the efficacy and mechanisms of action of those botanicals-based dietary supplements enriched in polyphenols, which have been shown to have antioxidant activities generally beneficial for human health. Polyphenols are compounds such as the soy isoflavones in soy and in kudzu root and the proanthocyanidins enriched in grape seed preparations. The Center uses a multidisciplinary team and innovative technologies to identify and evaluate bioactive ingredients from complex mixtures such as botanicals, and to evaluate their health benefits and mechanisms of action. Scientists with expertise in nutrition, plant physiology, horticulture, pharmacy, veterinary medicine, physics, chemistry, statistics, and medicine are part of our team. The Purdue-UAB Center’s central focus is age-related diseases, as many chronic diseases have an underlying etiology of oxidative damage and the polyphenolics in many botanicals have potent antioxidant properties. The incidences of bone loss, cognitive loss, hypertension, cardiovascular disease, type 2 diabetes, and stroke all increase with age, and dramatically in women after menopause; each of these components appears to be related to some common mechanisms that synergize, and thereby accelerate morbidity and mortality. Because the use of estrogen to offset these effects has been strongly questioned after the findings of the Women’s Health Initiative, it is clear that a better understanding of the basis for these postmenopausal symptoms and their alleviation is needed. Currently, there are three principal projects within the Purdue-UAB Botanicals Center: Project 1– Polyphenols and osteoporosis (Connie Weaver, Purdue); Project 2 – Cardio- and neuroprotective mechanisms of polyphenols (J. Michael Wyss, UAB); Project 3 – Singlet oxygen, polyphenols, and lens proteins (Stephen Barnes, UAB). The following is a brief overview of some of the ongoing research from each of these projects that is being conducted by the Center, specifically, screening botanical dietary supplements for their efficacy for the prevention of bone resorption in postmenopausal women, neuronal protection in animal models, anticancer potential in cell culture and animal models, and vascular effects and glucose tolerance in animal models.
Prevention of bone resorption by isoflavones
Starting plant materials of interest for this project are collected or cultivated in a greenhouse at Rutgers University or the University of Illinois. At the University of Illinois, 14C-enriched polyphenolics are produced using plant cell suspension and/or root culture technologies and grown in specially designed chambers (CitationYousef et al., 2004). The plant tissues are extracted and the crude extracts or fractions resulting from vacuum liquid chromatography are sent to Purdue University for the animal studies. The extracts are used in rat studies to determine the pharmacokinetics, tissue distribution, and metabolism of 14C-labeled or unlabeled bioactive compounds. These compounds are followed in serum or intestinal fluid using inserted membrane probes connected to a programmable automatic sampling station machine developed at Purdue Research Park (CitationJanle et al., 2005). The biological samples are analyzed for label by standard scintillation counting or for very low concentrations by accelerator mass spectrometry (AMS) at Purdue University (CitationLila et al., 2005) or for polyphenols and metabolites by mass spectrometry at UAB. AMS detects 14C with much greater efficiency than liquid scintillation counting, allowing for the measurement of even fentamole quantities to be characterized even for poorly absorbed compounds and across the blood–brain barrier.
Numerous commercially available botanical dietary supplements claim to be substitutes for estrogen therapy for preventing bone loss associated with menopause. Because the phytoestrogens in certain botanicals including soy, red clover, and kudzu are chemically similar to estrogen and bind to the estrogen receptor, much attention has been given to their ability to attenuate the accelerated bone resorption associated with estrogen deficiency accompanying menopause. However, a concern has been raised that these bioactive compounds may affect reproductive tissues similarly to estrogen (CitationReinwald & Weaver, 2006).
The barrier to evaluating effective interventions for preventing bone loss is that bone has a very slow turnover rate and it takes several years to properly monitor changes in bone mineral density, the classical approach and US Food and Drug Administration-approved method. Thus, it is very expensive to measure dose–response effects of a candidate therapy or compare multiple interventions. However, the Center has developed a novel method for rapidly and sensitively monitoring bone resorption by following 41Ca losses in urine from pre-labeled bone measured by AMS. Our study design screens multiple treatments per year in the same subjects using 50-d interventions administered in a randomized, crossover design. Recently, the report from the first intervention trial using 41Ca was published (CitationCheong et al., 2007). The results of this dose–response study of soy protein, containing 0–135.5 mg/day isoflavones, showed no effect on bone resorption in postmenopausal women. In contrast, estrogen therapy and a bisphosphate reduced bone resorption with sufficient power to see significant effects in four and six women, respectively.
Neuroprotective effects of proanthocyanidins
Botanical extracts such as grape seed extract (GSE) are enriched in proanthocyanidins (PAs; oligomeric polyphenols), and have been shown to have multiple health benefits due to their antioxidant, anti- inflammatory, and other activities. These activities have been attributed to the PAs; however, a systematic analysis of the molecular basis of these benefits has not been demonstrated. Since the brain is particularly vulnerable to age-related oxidative damage as well as inflammatory insults, we hypothesized that ingestion of GSE would affect specific proteins in the brains of animals in a manner consistent with neuroprotection.
Center investigators reported on the identification and quantitation of specific proteins in mammalian tissues modulated by GSE, and were first to demonstrate a link of ingesting any complex botanical ingredient with any disease (CitationKim et al., 2005). In this study, normal adult female rats were fed a defined (AIN-76A; Teklad Diets) diet supplemented with 5% GSE (Kikkoman Corporation, Chiba, Japan) for 6 weeks. The animals were sacrificed, and the brains were then removed and homogenized and the homogenates were subjected to proteomics analysis (2D electrophoresis and mass spectrometry). Image and statistical analysis of the 2D gel images determined that 13 brain proteins were altered in amount, charge, or both, qualitatively consistent with GSE being neuroprotective.
Tea catechins in cancer prevention
Epidemiological and animal data support a potential role for tea and tea catechins in the prevention of several chronic diseases including cancer, cardiovascular disorders, and obesity (CitationFeng, 2006). However, poor oral bioavailability of catechins is thought to minimize the potential efficacy of tea polyphenols by limiting concentrations at specific target tissues. Factors believed to contribute to poor bioavailability of catechins include digestive instability, poor transcellular efflux in intestinal cells, and rapid metabolism and excretion (CitationFeng, 2006). A further complication to understanding physiological catechin profiles is matrix effects associated with consumption of tea. Adjuncts that affect catechin stability in food systems are present in milk, juice, and antioxidants added to beverages. Currently at the Center, we are using a coupled in vitro digestion Caco-2 intestinal cell culture model to evaluate digestive stability and intestinal uptake of green tea catechins from various beverage blends (CitationFerruzzi et al., 2006).
The Center has also investigated the relationship between green tea catechins and cancer mediated through a cell surface protein, tNOX (Genbank Accession No. AF207881) (CitationChueh et al., 2002). tNOX appears to be a specific target for low-dose apoptosis of cancer cells by green tea catechins (CitationMorré et al., 2000), and its expression is restricted to cancer cells. Cancer specificity resides in its expression of a splice variant (CitationTang et al., 2007) that is uniquely drug inhibited and uniquely associated with human cancer (CitationChueh et al., 2002). When the tNOX of cancer cells is inhibited, the cells fail to enlarge after division, cease to divide, and, after a few days, undergo apoptosis. Polyphenolic constituents from plants that target tNOX were identified, along with combinations based on botanical sources that often duplicated the key ameliorating polyphenols and interacted synergistically. Botanical preparations that most effectively inhibited cancer cell growth were combinations of decaffeinated green tea concentrate and vanilloid-containing Capsicum L. (Solanaceae) preparations () (CitationMorré & Morré, 2003). A 25:1 ratio of green tea concentrate to Capsicum preparation killed cancer cells in culture 100-times greater, by weight, than green tea alone. Assuming that there are 2 g of green tea per cup in a usual green tea infusion, these findings suggest that a 350 mg capsule of the commercially available green tea extract–Capsicum preparations (Capsibiol-T® or Capsol-T®) is equivalent to drinking 16 cups of green tea based on the comparative responses of growth of 4T1 and HeLa cells in culture (CitationMorré & Morré, 2003). (–)-Epigallocatechin-3-gallate (EGCg), capsaicin, vanillylamine, and green tea mixtures alone or combined with vanilloids did not inhibit the growth of non- cancerous cell lines in culture nor did they inhibit the activity of the corresponding NOX protein constitutive to normal cells. This differential activity suggests cancer specificity and safety of the green tea–Capsicum mixtures. Further in vitro investigations demonstrated that the functional cell surface expression of tNOX was both necessary and sufficient for the cancer-specific cell growth inhibition attributed to EGCg (CitationChueh et al., 2004). In an in vivo experiment, transgenic mice over-expressing tNOX were generated, and these transgenic animals exhibited a level of unregulated cell enlargement and sensitivity to EGCg similar to the in vitro experiments in cancer cells (CitationYagiz et al., 2006).
Figure 1. Dose–response of HeLa cells at 72 h in cell culture to a 25:1 decaffeinated green tea concentrate–Capsibiol-T mixture prepared on an equivalent weight basis. Points are mean ± SD of 3–5 determinations. Growth responses to mixture dilutions were analyzed using a two-way analysis of variance (ANOVA) and considered to be significant at p < 0.05. One 350 mg capsule of Capsibiol-T is equivalent to drinking 16 cups of green tea. Modified from CitationTang et al. (2007).
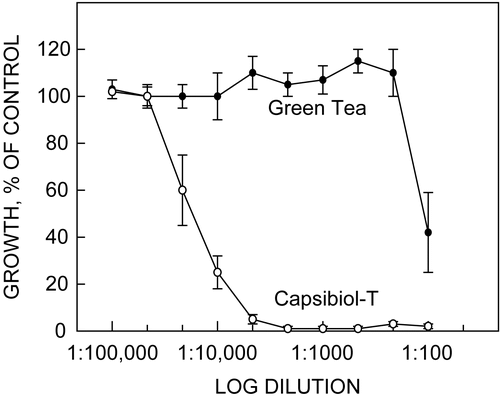
In some preliminary human studies conducted at the Center, 36% of 50 cancer patients receiving Capsol-T® had a significant prolongation of life or remained alive, 32% improved, and 32% had a normal course of disease (CitationMorré & Morré, 2006). Currently, a Phase II/III clinical study (toxicology and pharmacokinetics) of Capsol-T® with renal cancer and melanoma directed by Dr. Theodore Logan, MD at Indiana University School of Medicine, is under way, and a more extensive clinical study to evaluate efficacy is under development at the Goshen Cancer Center, Goshen, IN. A favorable interaction between a tea catechin–Capsicum preparation and radiation therapy was previously indicated from compassionate intervention studies with five cancer patients (CitationFernandez & Ganzon, 2003).
Interaction between polyphenols and antioxidants
Polyphenols in botanicals, including the bioflavonoids and stilbenoids, also play an important role in regulating oxidative stress in cardiovascular disease and other chronic diseases of aging (CitationBoersma et al., 2003; CitationD’Alessandro et al., 2003; CitationPrasain et al., 2003). These diseases are marked by the production of reactive nitrogen species (RNS; peroxynitrite, ONOO−; nitrogen dioxide) and reactive oxygen species (ROS; superoxide; hydrogen peroxide; hypobromous acid; hypochlorous acid, HOCl) by eosinophils, macrophages, and neutrophils. Although RNS and ROS modify DNA and proteins and disrupt gene expression and protein function in targeted cells, they also damage neighboring uninvolved normal cells. Because many of the protein modifications are on tyrosine (3-nitrotyrosine, 3-chlorotyrosine, 3-bromotyrosine), an aromatic group, the Center is investigating how the RNS and ROS react with dietary polyphenols. In vitro, both HOCl and ONOO− formed chloro and nitro derivatives with the soy isoflavones daidzein and genistein (CitationBoersma et al., 1999). When these experiments were extended to incubation with activated neutrophil-like HL-60 cells and freshly isolated human polymorphonuclear cells, greater than 90% of a bioflavonoid (10 μmol/L) in the medium was converted to chlorinated products within 30 min (CitationBoersma et al., 2003; CitationD’Alessandro et al., 2003; CitationPrasain et al., 2003). In addition, the isoflavones, as do other polyphenols, increase the lag time for Cu2+-induced oxidation of human low density lipoprotein (LDL) and reduce the rate of lipid peroxidation. However, their effect is weak. We hypothesized that polyphenols undergo a cycle. They react with lipid peroxide free radicals and are converted to free radicals themselves. These attack new lipid molecules and restart the process of lipid radical formation. Adding ascorbic acid in molar excess to the reaction markedly improves overall antioxidant activity to 2–3 times greater than the sum of the individual antioxidant species (CitationPatel et al., 2001). This is particularly relevant to the work being carried out on the prevention of lens cataract disease. Genistein administered in the diet enters the aqueous humor that flows in front of the lens (CitationBarnes et al., 2006). Because the aqueous humor contains 5–10 mmol/L ascorbic acid, the high ratio between ascorbic acid and genistein may create a strong antioxidant environment. It also suggests that the study of polyphenol effects in vivo has to consider the whole botanical and not the individual components (CitationBarnes & Prasain, 2005).
Beneficial effects of polyphenols in metabolic syndrome
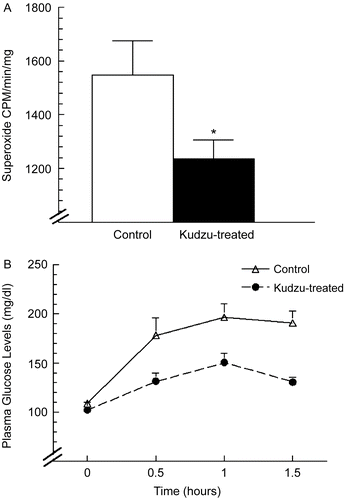
Polyphenols also appear to delay or partially prevent several aspects of the metabolic syndrome, but the mechanisms underlying these effects remain to be elucidated. Using middle-aged, ovariectomized spontaneously hypertensive rats (SHR), which are used as a model for menopause, hypertension, insulin resistance, and stroke, the effects of puerarin, a C-glucoside found in kudzu, was determined in the SHR model. Long-term feeding studies in 3-month-old female SHR given a casein-based AIN-76 diet containing either 0.3% kudzu root extract or no polyphenols for 3 months demonstrated that kudzu treatment prevented a blood pressure rise that occurred in control (non-kudzu-treated) rats. Heart rate was not affected by the treatment, but when the ganglionic blocker hexamethonium was administered, the decrease in arterial pressure was significantly greater in the control than in the treated rats, suggesting that sympathetic nervous system activity is required to maintain the higher arterial pressure in the control animals. Superoxide production was also significantly reduced in the kudzu-treated group as compared with control animals (). Kudzu root extract reduced resting blood glucose, insulin, serum cholesterol, and leptin. Glucose tolerance and glucose sensitivity were improved in the kudzu-supplemented groups by ~20%. Acute studies in rats and mice demonstrated that glucose tolerance was improved by ~50% when puerarin and glucose were administered simultaneously to otherwise naive SHR (CitationMeezan et al., 2005) (). Safety assessment trials demonstrated that kudzu root extract does not cause adverse side effects in rats even when administered at 1% of the diet for 2 months.
Figure 2. Effect of kudzu on superoxide anion formation and glucose tolerance in SHR rats. (A) In vitro measurement of superoxide anion formation in aortic ring segments. The assay was calibrated by monitoring the chemiluminescence signal of known amounts of superoxide generated by xanthine + xanthine oxidase (mean ± SEM; *p < 0.05 by t test; n = 5/group). (B) Dietary kudzu improved glucose tolerance in SHR. Glucose was given by gavage (2 g glucose/kg) at time 0. Note that the plasma glucose concentration was decreased by kudzu extract treatment at all times (mean ± SEM; p < 0.05 for main effect of treatment by one-way ANOVA; n = 5/group).
Conclusions
The Purdue-UAB Center is a multidisciplinary group using novel technologies to investigate age-related diseases. As many chronic diseases have an underlying etiology of oxidative damage, the investigation of the antioxidant effects of polyphenolic compounds found in many botanicals is scientifically rational. To this end, the Center uses a multidisciplinary approach, incorporating novel technologies to develop models for studying bioavailability, safety, and efficacy of bioactive compounds from complex mixtures. The Center has been instrumental in the development of novel methods for rapidly and sensitively monitoring bone resorption by following 41Ca losses in urine from pre-labeled bone as measured by accelerator mass spectrometry. Results from Center work have demonstrated that GSE has neuroprotective effects in rats, and have shown that green tea and combinations of green tea and Capsicum have effects on cancer treatment. This work has led to a Phase II/III clinical study (toxicology and pharmacokinetics) of Capsol-T® for renal cancer and melanoma. The Center work has further shown that polyphenols in botanicals play an important role in regulating oxidative stress in cardiovascular disease and other chronic diseases of aging, including the metabolic syndrome.
Thus, relating bioactive compounds in botanicals to the aging process and disease development through multiple mechanisms has presented significant challenges, but the outcomes have been very productive.
Acknowledgements
This article was presented at the Symposium: “Plants in the Service of Human Health: Continuing Search for Plant-based Therapies” – Society for Economic Botany 48th Annual Meeting in Chicago at Lake Forest, June 4, 2007.
Declaration of interest: This research was supported by Office of Dietary Supplements and NCCAM Grant P50 AT 00477. One of the authors (C.M.W.) is on the advisory boards of Pharmavite and Wyeth. She receives grant support from Wyeth, and reviews research proposals for the United Soybean Board. None of the other authors identified an affiliation.
The contents of this paper are the responsibility of the authors and do not necessarily represent the views of the funding agency.
References
- Barnes S, Prasain JK, Wang C-C, Moore DR II (2006): Applications of LC-MS in the study of the uptake, distribution, metabolism and excretion of bioactive polyphenols from dietary supplements. Life Sci 78: 2054–2059.
- Barnes S, Prasain JK (2005): Current progress in the use of traditional medicines and nutraceuticals. Curr Opin Plant Biol 8: 324–328.
- Boersma BJ, Patel RP, Kirk M, Darley-Usmar VM, Barnes S (1999): Chlorination and nitration of soy isoflavones. Arch Biochem Biophys 368: 265–275.
- Boersma BJ, D’Alessandro T, Benton MR (2003): Neutrophil myeloperoxidase chlorinates soy isoflavones and enhances their antioxidant properties. Free Radic Biol Med 35: 1417–1430.
- Cheong JMK, Martin BR, Jackson GS (2007): Soy isoflavones do not affect bone resorption in postmenopausal women: a dose response study using a novel approach with 41Ca. J Clin Endocrinol Metab 92: 577–585.
- Chueh P-J, Kim C, Cho N, Tang X, Morré DM, Morré DJ (2002): Molecular cloning and protein expression of a unique hydroquinone (NADH) oxidase (tNOX) of the cancer cell surface. Biochemistry 41: 3732–3741.
- Chueh P-J, Wu LY, Morré DM, Morré DJ (2004): tNOX is both necessary and sufficient as a cellular target for the anticancer action of capsaicin and the green tea catechin (–)-epigallocatechin-3-gallate. Biofactors 20: 235–249.
- D’Alessandro T, Prasain J, Botting NP (2003): Polyphenols, inflammatory response, and cancer prevention: chlorination of isoflavones by human neutrophils. J Nutr 133: 3773S–3777S.
- Feng WY (2006): Metabolism of green tea catechins: An overview. Curr Drug Metab 7: 755–809.
- Fernandez R, Ganzon DO (2003): Use of a green tea-capsicum supplement (Capsibiol-T) as adjuvant cancer treatment: Case study report. Philippine J Otolaryngol Head Neck Surg 18: 171–177.
- Ferruzzi MG, Lumpkin JL, Schwartz SJ, Failla ML (2006): Digestion, micellarization and intestinal cell uptake of β-carotene isomers. J Agric Food Chem 54: 2780–2785.
- Janle EM, Portocarrero C, Zhu Y, Zhou Q (2005): Effect of long-term oral administration of green tea extract on weight gain and glucose tolerance in Zucker diabetic (ZDF) rats. J Herb Pharmacother 5: 55–65.
- Kim H, Deshane J, Barnes S, Meleth S (2005): Proteomics analysis of the actions of grape seed extract in rat brain: Technological and biological implications for the study of the actions of psychoactive compounds. Life Sci 78: 2060–2065.
- Lila MA, Yousef GG, Jiang Y, Weaver CM (2005): Sorting out bioactivity in flavonoid mixtures. J Nutr 135: 1231–1235.
- Meezan E, Meezan EM, Jones K, Moore R, Barnes S, Prasain JK (2005): Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. J Agric Food Chem 53: 8760–8767.
- Morré DJ, Bridge A, Wu L-Y, Morré DM (2000): Epigallocatechin gallate inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Biochem Pharmacol 60: 937–946.
- Morré DJ, Morré DM (2003): Synergistic Capsicum-tea mixtures with anticancer activity. J Pharm Pharmacol 55: 987–994.
- Morré DM, Morré DJ (2006): Catechin-vanilloid synergies with potential clinical applications in cancer. Rejuvenation Res 9: 48–55.
- Patel RP, Boersma B, Crawford JH (2001): Antioxidant mechanisms of isoflavones in lipid systems: paradoxical effects of peroxyl radical scavenging. Free Radic Biol Med 31: 1570–1581.
- Prasain JK, Boersma BJ, Kirk M (2003): ESI-tandem mass spectrometric analysis of chlorinated and nitrated isoflavones. J Mass Spectrom 38: 764–771.
- Reinwald S, Weaver CM (2006): Soy isoflavones and bone health: A double-edged sword? J Nat Prod 69: 450–459.
- Tang X, Tian Z, Chueh P-J, Chen S, Morré DM, Morré DJ (2007): Alternative splicing at the basis for specific localization of tNOX, a unique hydroquinone (NADH) oxidase, to the cancer cell surface. Biochemistry 46: 12337–12346.
- Yagiz K, Morré DJ, Morré DM (2006): Transgenic mouse line over-expressing the cancer-specific tNOX protein has an enhanced growth and acquired drug-response phenotype. J Nutr Biochem 17: 750–759.
- Yousef GG, Seigler DS, Grusak MA (2004): Biosynthesis and characterization of 14C-enriched flavonoid fractions from plant cell suspension cultures. J Agric Food Chem 52: 1138–1145.