Abstract
Context: The fruit of Terminalia chebula Retz. (Combretaceae) has been used for several therapeutic purposes in Thai folk medicines. Currently, the ethanol extracts containing antioxidant compounds have shown the ability to promote collagen synthesis.
Objective: This purpose of this work was to study the effects of the ethanol extract from T. chebula fruit on the inhibition of cutaneous photodamage.
Materials and methods: The viability of human skin fibroblasts after incubation with T. chebula at concentration 0.5–50 μg/mL for 24, 48 and 72 h was assessed by using sodium 3′-[(phenyl-amino)-carbonyl]-3,4,tetrazolium-bis(4-methoxy-6-notro)benzene-sulphonic acid hydrate (XTT). The levels of type I procollagen and matrix metalloproteinases (MMP)-1 and MMP-13 produced by UVB-irradiated fibroblasts were determined by ELISA. Skin thickness and collagen content caused by long-term UVB irradiation in male ICR mice were determined from haematoxylin and eosin stained tissue sections and spectrophotometric measurement of hydroxyproline.
Results: The extract (0.5–50 μg/mL) had no effect on cell viability or morphology of the human fibroblasts. In vitro studies showed that the T. chebula extract reduced the UVB-induced MMP-1 and MMP-13 expression, whereas an increased production of type I procollagen was observed. In a UVB-irradiated animal model, male ICR mice with hair shaved were chronically exposed to UVB which lead to epidermal thickness and loss of hydroxyproline. However, these effects were fully prevented by the topical application of the T. chebula ethanol extract.
Discussion and conclusion: These data suggested that the T. chebula ethanol fruit extract is an efficacious pharmaceutical protectant of skin against photodamage.
Introduction
Ultraviolet (UV) irradiation accelerates ageing of the human skin, which is generally characterized by severe epidermal hyperplasia and skin elasticity reduction (Gilchrest Citation1996; Thurstan et al. Citation2012). Three types of UV radiation are classified according to their wavelengths: UVA (320–400 nm), UVB (280–320 nm) and UVC (<280 nm). Although UVB represents only a small part of the UV spectrum, it makes a major contribution to the degradation of dermal connective tissue (Viyoch et al. Citation2012). The most abundant structural protein in dermal connective tissue is type I collagen, which is primarily synthesized by fibroblast cells. Type I collagen confers strength and resilience on the skin. Repeated skin exposure to UVB can cause collagen breakdown resulting in an aged skin appearance such as wrinkle formation. The mechanism underlying UVB-induced collagen degradation is the generation of various reactive oxygen species (ROS) provoking the mitogen-activated protein (MAP) kinase pathway, which subsequently induces the expression and activation of matrix metalloproteinases (MMPs) in human skin fibroblasts (Moon et al. Citation2008; Chiang et al. Citation2012; Wen et al, Citation2012). These enzymes include collagenase-1 (MMP-1) and collagenase-3 (MMP-13), which are the primary mediators of connective tissue damage in skin following UV irradiation exposure (Ravanti et al. Citation1999; Brennan et al. Citation2003; Ahn et al. Citation2012). It has been proposed that inhibition of these enzymes might be a useful strategy for preventing UV-accelerated skin ageing by restoring the balance between collagen degradation and synthesis (Brennan et al. Citation2003; Ahn et al. Citation2012; Itsarasook et al. Citation2014; Park et al. Citation2014).
Nowadays, safe and effective ingredients extracted from natural sources have been frequently reported for their potential applications in preventing skin damage caused by UV exposure. This preventative action apparently associated with their antioxidant capacities (Katiyar et al. Citation2001; Kang et al. Citation2003; Lee et al. Citation2003). Compounds with antioxidant properties such as phenolics can prevent ageing by preventing ROS formation or by preventing ROS overproduced caused by external stimuli exposure. Eradicating ROS or depletion ROS levels impedes the activation of subsequence downstream signalling pathways corresponding to skin damage.
Terminalia chebula Retz. (Combretaceae) is a tall, deciduous tree grown throughout central and Southeast Asia. Its nut-like fruits are reputed to have many therapeutic uses including antibacterial, purgative, as an astringent, and as a blood purifier in Ayurvedic and Thai folk medicines (Gupta Citation2012). Recently, the appreciable free radical scavenging and antioxidant activities of T. chebula fruit extracts have been documented (Chulasiri et al. Citation2011; Weerapreeyakul et al. Citation2012). Certain activities of its extracts are the result of the presence of phenolic compounds such as gallic, ellagic, chebulagic and chebulinic acids (Juang et al. Citation2004; Gupta Citation2012). These compounds have anticancer, antimicrobial and anti-inflammatory properties (Surveswaran et al. Citation2007). The effect of hydroalcoholic extract of T. chebula fruit on the promotion of collagen synthesis in 3T3 fibroblasts has also been reported (Satardekar & Deodhar Citation2010). Notwithstanding this previously published research into the preventive effects of the ethanol fruit extract on UVB-irradiated skin cell and tissue, these beneficial effects have not been investigated.
Given the lack of past research into what can be considered an important and beneficial medicinal application, this study was undertaken to fully investigate the use of ethanol T. chebula fruit extract in the downregulation of MMP-1, MMP-13 and in the upregulation of type I procollagen in UVB-irradiated primary human dermal fibroblasts. The study was an examined of T. chebula ethanol fruit extract on epidermal skin thickness and collagen content in long-term UVB-irradiated ICR mice. The results confidently demonstrate the potential applications of T. chebula fruit extracts as an ingredient for preventing skin damage caused by UVB exposure.
Materials and methods
Extract preparation
The powder of T. chebula fruit was freshly prepared by CR Pure Light, Thailand. Its product specifications and the voucher specimen were kept in the Research & Development Division, S & J International Enterprises Public Co., Ltd., Bangkok, Thailand. The fruit powder was macerated with 70% v/v ethanol for 48 h, and the extract was dried under vacuum and then stored in a tight Amber glass bottle at 4 °C for further studies.
Assay for total phenolics
Phenolics in T. chebula were measured using the Folin-Ciocalteu method (Viyoch et al. Citation2012; Phetdee et al. Citation2014). Aliquots (40 μL) of standard gallic acid solutions (Sigma-Aldrich Co., St Louis, MO) at different concentrations or appropriately diluted extracts were added to 520 μL of sterile water. Then, 40 μL of Folin-Ciocalteu’s phenol reagent (Merck KGaA, Darmstadt, Germany) was added to the mixture and shaken. After 5 min, 400 μL of 7% Na2CO3 solution was added while mixing. After incubation for 30 min at room temperature, the absorbance of the incubated solution was measured at 760 nm (Spectra Count, Perkin Elmer, Waltham, MA). The amount of total phenolics in the solution was expressed as gallic acid equivalents from the calibration curve set up from the standards. This assay was performed in triplicate.
DPPH assay
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity was determined as previously described (Donsing et al. Citation2008; Itsarasook et al. Citation2014; Phetdee et al. Citation2014). Briefly, 1 mL of 0.2 mM DPPH solution (Sigma-Aldrich Chemic GmbH, Steinheim, Germany) was mixed with 0.5 mL of various concentrations of the samples of extracts previously dissolved in methanol. The mixture was kept at room temperature for 30 min, and then the absorbance at 515 nm was measured using a spectrophotometer. l-Ascorbic acid (vitamin C) was used as the positive control. The radical scavenging activity was calculated as a percentage of DPPH decolouration using the following equation:
where AS is the absorbance of DPPH with the tested sample and AB is the absorbance of DPPH without the tested sample. EC50, the equivalent concentration to give the 50% effect, was determined by log-probit analysis using 6–10 different concentrations of the tested samples. The study was run in triplicate.
Cytotoxicity of extract to human skin fibroblasts
Cell isolation and cultivation
Human skin fibroblasts were obtained from abdominal skin which remained after surgery (from a number of women aged 58–60, passage number not more than 8). The procedure was approved by the ethical committee of Naresuan University (Approval No. 55 01 02 0020). The dermal layer was washed with phosphate buffered saline (PBS) and antibiotic (penicillin/streptomycin). Then, the skin tissue samples were cut into 4–5 pieces (∼2 mm square) which were placed into 25 cm2 culture flasks and subsequently incubated in 5% CO2 at 37 °C. During this process the tissue adhered to the culture flask. A culture medium consisting of Dulbecco’s modified Eagle’s medium (DMEM, low glucose, Sigma-Aldrich Co., St Louis, MO) supplemented with 10% foetal bovine serum (Cultilab, Campinas, São Paulo, Brazil) and 1% of stock penicillin/streptomycin solution (100 U/mL penicillin and 100 μg/mL streptomycin, Gibco™, Invitrogen, New York), was added to each flask. After incubation for 3 weeks at 37 °C with 5% CO2, the fibroblast cells had migrated from the original explant site. After trypsinization, cells were seeded at 1 × 104 cells/cm2 in 75 cm2 flasks using the same medium. Passage numbers 2–8 were used in this study.
Cell viability test
Cell viability was assessed using sodium 3′-[(phenyl-amino)-carbonyl]-3,4,tetrazolium-bis(4-methoxy-6-notro)benzene-sulphonic acid hydrate (XTT, Boehringer Mannheim, Mannheim, Germany) assay (Inpanya et al. Citation2012; Phetdee et al. Citation2014). The cells were seeded into each well of 96 well plates (1 × 104 cells/cm2) and cultured with 250 μL supplemented DMEM at 37 °C with 5% CO2. After 24 h, cells cultured in serum free medium were treated with T. chebula extract (0.5–50 μg/mL). The highest concentration of the extract used in the study was at the limit of extract solubility in the final culture medium. Ethanol was used as a solubilizing enhancer and the final concentration of ethanol never exceeded 0.1% v/v (Lançon et al. Citation2007). After incubation for 24, 48 or 72 h, 200 μL of serum free medium and 50 μL of XTT solution were added. The cells were further incubated for 4 h. The absorbance was read on a microplate reader at 490 nm and the number of viable cells was calculated as the percentage of the optical density of the untreated group. The control group consisted of cells treated with 0.1% ethanol in DMEM.
Determination of preventive effects of the extract on UVB-induced alterations in fibroblasts
UVB irradiation
A fluorescent sun lamp (FL8BLB, Toshiba Co., Tokyo, Japan) with an emission spectrum between 275 and 305 nm was used as the source of UVB radiation, placed at 22 cm above the cell culture flasks. The UVB intensity used in this study was 128 J/cm2. The preliminary data indicate that this selected intensity is a sublethal dose, which inactivates the fibroblasts to undergo proliferation. These UVB-irradiated cells could perform normal cellular functions when they were re-cultured.
Human skin fibroblasts at 2.0 × 106 cells in a 25-cm2 flask were maintained overnight in culture media. The cells were pretreated with 50 μg/mL T. chebula extract for 24 h and were then washed and were covered by an ∼1 mm deep layer of PBS before exposing to UVB irradiation. Following the UVB irradiation, the PBS was replaced by a serum-free culture medium and the cells were incubated for further 4 or 24 h. At the end of the incubation period, the supernatant of the cultured fibroblasts was collected and stored at −80 °C until used for type I procollagen and MMP-1 and -13 analysis. Viability of irradiated cells was also determined by a XTT assay by the method described above. The number of viable cells was calculated as the percentage of the optical density of non UVB-irradiated group (control) compared to UVB-irradiated group.
Type I procollagen and matrix metalloprotease-1 and -13 immunoassay
ELISA kits were used to measure the levels of type I procollagen protein (Human Procollagen type I C-peptide (PIP) EIA Kit, Takara Bio Inc., Shiga, Japan) and MMP-1 and MMP-13 (RayBioTech Inc., Norcross, GA) in cell-free supernatants. The assay was performed in triplicate according to the manufacturer’s instructions.
Protection of skin ageing mice against UVB
Male mice (outbred ICR, 5 weeks old) were housed for 1 week in a temperature controlled room (25 ± 5 °C), relative humidity (55 ± 5 °C) and 12 h dark/light cycle, and given free access to a standard laboratory diet and water. All relevant protocols were approved by the Animal Ethics Committee of Naresuan University (Approval No. NU-AE550202). A total of 20 mice were randomized into five groups, four animals per group. One group of mice was a non-UVB-irradiated (control group). Two groups of mice were applied daily either 200 μL of 0.5% w/v extract solution or vehicle of extract solution. Another two groups were applied with 200 μL of 3% w/v vitamin C solution or deionized water. Therefore, 1 μg extract or 6 μg vitamin C was applied daily per mouse. Vitamin C was used as a positive control because sodium ascorbate at a concentration of 3 g/100 mL has been demonstrated to prevent UVB-induced skin damage (Kim et al. Citation2009). The dose of the extract was selected based on the results from a previous report indicating biological activity in animal models given this dose (Jayasree et al. Citation2013).
UVB irradiation
After anaesthetization, a 4 × 4 cm2 midline skin area behind the scapulae was treated with 8% sodium sulphide for 1 min and the hair was removed with wet cotton wool. The tested samples were applied topically with the ethanol T. chebula extract on the hairless region once a day for 12 weeks. Two hours after applying the extracts, each mouse was UVB irradiated. UVB irradiation was performed three times per week (Monday, Wednesday and Friday) as our previous studies (Viyoch et al. Citation2012). The initial dose of UVB was set at 18 J/cm2 (8 W, 8 min) at weeks 1–4, which was subsequently increased to 36 J/cm2 (8 W, 17 min) for weeks 4–7, 54 J/cm2 (8 W, 25 min) at weeks 7–10 and finally to 72 J/cm2 (8 W, 34 min) at weeks 10–12.
Measurement of epidermal thickness
After 12 weeks, all mice were pentobarbital (200 mg/kg) overdosed and skin samples (about 3 × 3 cm2) were then quickly removed. The samples were fixed in pre-labelled base moulds containing Tissue-Tek (Sakura Finetex USA Inc., Torrance, CA) and then frozen. The samples were sectioned at 8 μm thickness and mounted on a slide. The slides were stained with haematoxylin–eosin and the epidermal thickness measured using the AXIO program (Carl Zeiss Microscopy Ltd., Cambridge, UK). The epidermal thickness indicated the average value measured from three regions (left, right and centre).
Determination of hydroxyproline content
Hydroxyproline, a major component of collagen in skin, was measured as in previous studies (Faikrua et al. Citation2009; Inpanya et al. Citation2012). Briefly, 25 mg of each tissue sample was immersed in chloroform and methanol for 60 min. The tissue sample was then hydrolysed with 6 N hydrochloric acid at 110 °C for 18 h. After the sample was dried, PBS (pH 7.4) was subsequently added. The absorbance of the resultant solution was measured at 202 nm and the hydroxyproline content was calculated from a standard curve equation relating to hydroxyproline (Sigma-Aldrich, St Louis, MO). Each determination was performed in triplicate.
Statistical analysis
All values were expressed as means of each treatment group. The Student’s unpaired t-test was used to compare between the two groups. p < 0.05 was considered statistically significant.
Results
Total phenolic content and DPPH scavenging activity
The phenolic content of the ethanol T. chebula extract was equivalent to 443.2 ± 1.6 mg gallic acid in 1 g extract. Each tested sample showed a concentration-dependent reduction of DPPH activity and that the EC50 of the extract was 153.8 ± 0.2 μg/mL compared to 9.74 ± 0.1 μg/mL for the vitamin C, positive control.
The extract showed no cytotoxicity to human skin fibroblasts
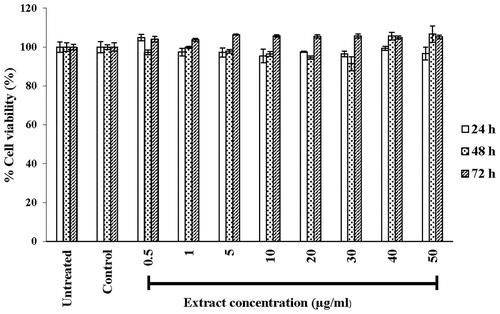
Irrespective of concentration (0.5–50 μg/mL) and incubation time (24 or 72 h), the treatment of the human skin fibroblast with the T. chebula extract had no effect on the viability of the human fibroblasts (). In addition, during these treatments, no changes in cell morphology were observed. The fibroblasts still retained the typical spindle-shape even at the highest concentration tested (50 μg/mL, ).
Figure 1. Effect of the extract on viability of primary human skin fibroblasts. Cells were treated with 0.1% ethanol (control group) or the extract at concentrations in range of 0.5 to 50 μg/mL for 24, 48 and 72 h. Results are expressed as percentage of cell viability as compared to untreated cells for which the optical density was adjusted to 100%. Each bar represents mean ± SD of triplicate study.
T. chebula extract enhanced type I procollagen synthesis but inhibited MMP-1 and MMP-13 productions in UVB-irradiated fibroblasts
The level of type I procollagen in UVB-irradiated cells and non-UVB-irradiated cells is shown in . At 24 h after UVB irradiation, the procollagen level of the UVB-irradiated group decreased as compared to that of the non-irradiated (control) group. The fibroblast pretreated with the extract (pretreated group) before exposure to UVB irradiation exhibited markedly increased type I procollagen secretion compared to the UVB irradiation only (p < 0.05) or control group.
Figure 3. Effects of the extract (50 μg/mL) on type I procollagen (A), MMP-1 (B) and MMP-13 (C) productions by UVB-irradiated human skin fibroblasts at 4 and 24 h after irradiation. Each bar represents mean ± SD of triplicate study. *p <0.05 and **p <0.01, when compared between two groups (Student’s t-test).
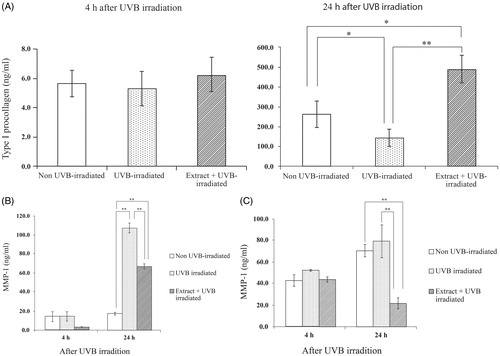
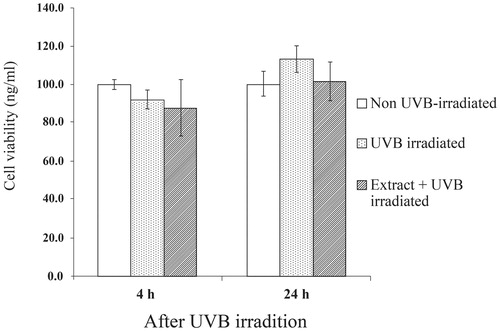
The fibroblasts produced substantial amounts of MMP-1 after UVB irradiation for 24 h () but UVB-induced MMP-1 expression was impeded in cells pretreated with the T. chebula extracts. For MMP-13, we found no difference in MMP-13 levels between UVB-irradiated fibroblasts for 24 h and the non-irradiated fibroblasts (). However, the reduction of the MMP-13 level was detected in the UVB-irradiated cells pretreated with the extract, particularly at 24 h after irradiation. To rule out the possibility that the reduction of MMP-1 and MMP-13 may have been caused by the cytotoxicity of the UV irradiation and the extracts, the viability of the cells after irradiation at 4 and 24 h was assessed and the result is depicted in . With or without the extract, no difference in viability was observed between UVB-irradiated groups and non-irradiated groups. Thus, the depletion of MMP-1 and MMP-13 was not due to the low number of cells, but was indeed caused by the inhibitory effects of the T. chebula extracts.
Extract prevented the epidermal thickening and collagen damage after UVB irradiation in mice
None of the animals showed any skin blistering on the body. As expected, epidermal thicknesses of 30–35% were observed on the skins of the mice irradiated with UVB and also of the mice pretreated with water or vehicles ( and ). In sharp contrast, the UVB radiation did not cause such skin thickening in the animal skins pretreated with the T. chebula extract or vitamin C.
Figure 5. Epidermal thickness of mice skin tissues at 12 weeks after UVB irradiation. Skin was daily applied deionized water, vehicle for extract solution, 3% w/v vitamin C solution or 5% w/v extract solution at 2 h after UVB irradiation. Each bar represents mean ± SD of triplicate study. *p <0.05, when compared between two groups (Student’s t-test).
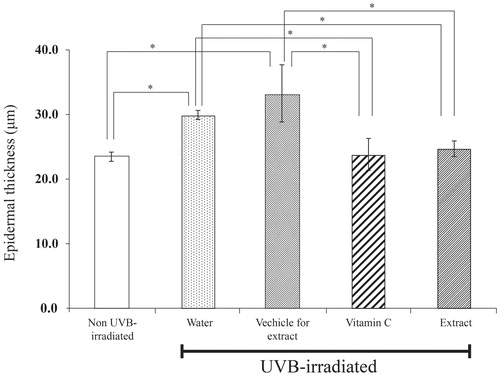
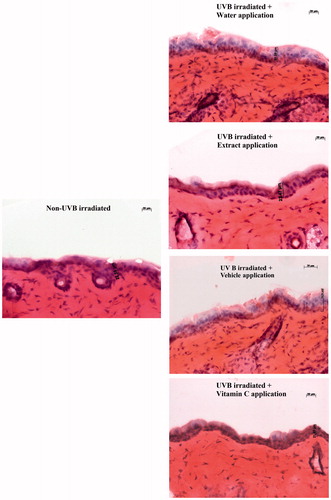
Figure 6. Light microscope (magnification 20×) of 12 week-UVB irradiated mice skin tissues stained with haematoxylin–eosin to show the effects of extract and vitamin C on epidermal thickness.
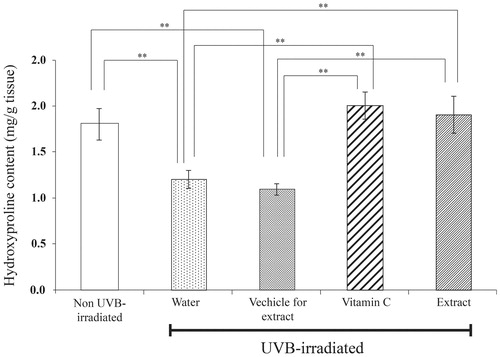
Hydroxyproline content was lower in both UVB-irradiated groups pretreated with water and vehicle than that of the non-UVB irradiated group. This indicates that the collagen degradation and/or synthesis was apparently changed when the skin was expose to UVB. In contrast, the hydroxyproline content was not altered in the UVB-irradiated mice pretreated on the skin with the extract or vitamin C ().
Figure 7. Hydroxyproline content in dermal skin tissues of mice after UVB irradiation for 12 weeks. Skin was daily applied deionized water, vehicle for extract solution, 3% w/v vitamin C solution or 5% w/v extract solution at 2 h after UVB irradiation for 12 weeks. Each bar represents mean ± SD of triplicate study. **p <0.01, when compared between two group (Student’s t-test).
Discussion
Free radicals generated from UVB exposure play a pivotal role in skin damage and/or accelerated ageing. A topical application of compounds with antioxidation would be useful to minimize such harmful effects. We expected that T. chebula fruit extract containing poly compounds could improve activities of skin fibroblasts, which are normally changed in biological activities after UVB exposure.
Free radical scavenging and antioxidant activities of phenolic compounds containing in T. chebula fruits have not previously been reported (Aqil et al. Citation2006; Chulasiri et al. Citation2011; Chang & Lin Citation2012). In the present study, the total phenolic content of the ethanol fruit extract was 443.2 ± 1.6 mg gallic/g extract, which was lower than reported previously (867.2 ± 18.1, 95% ethanol extract) (Chang & Lin Citation2012). This difference may be due to the differences in the extraction process, the percent of alcohol used and/or the season. For the DPPH scavenging activity, we found that the extract exhibited a lower activity than that of vitamin C, a well-known skin antiageing compound. However, this lower activity, at least in part, might be due to the slow reaction of the phenolics containing in the extract to DPPH compared to non-phenolic antioxidants such as vitamin C. This non-phenolic vitamin C has rapid reaction to the DPPH radical (Sánchez et al. Citation1998).
We found that the T. chebula extract at all the concentrations used (0.5–50 μg/mL) showed it to be non-toxic to human skin fibroblasts and thus the extract at the concentration of 50 μg/mL was used for subsequent experiments. Changes in biological activities of skin cells would markedly influence the skin physiology and function. During UVB exposure of fibroblasts, several biological pathways including MAP kinase are provoked, resulting in up-regulation of MMPs. Such up-regulation causes the down-regulation of procollagen expression. In this study, we found that UVB irradiation on fibroblasts suppressed type I procollagen production and, by contrast, enhanced MMP-1 expression. For MMP-13, after 24 h the UVB-irradiated cells showed no differences in the MMP-13 level compared to that of the non-irradiated fibroblasts. These findings are in line with previous finding indicating that irradiation does not evoke MMP-13 production in human skin (Fisher et al. Citation2001).
MMP-mediated collagen damage is a major contributor to the phenotype of photodamaged human skin (Fisher et al. Citation2000; Quan et al. Citation2004; Moon et al. Citation2008). UVB irradiation induces the secretion of various MMPs and simultaneously transiently suppresses type I collagen gene expression. This can cause the impairment of the structural integrity of the dermis (Brenneisen et al. Citation2002; Varani et al. Citation2002). Therefore, an alleviated UV-induced MMPs expression leads to photodamage caused by collagen loss (Rakic et al. Citation2000; Moon et al. Citation2008). Among other MMPs, MMP-1 induction has the greatest impact on collagen breakdown. In the current study, pre-treatment with T. chebula extract reduced both MMP-1 and -13 secretions. The decrease of these MMPs was not due to any cytotoxic effect of the extracts because cell viability of cells treated with the extracts was comparable to non-treated cells. Moreover, the extract could enhance the level of extracellular type I procollagen. This could imply that the antioxidant effect of the extract inhibits UVB-induced ROS formation, which attenuates a provocation of the MAP kinase pathway. Another possibility is that the extract might improve the activities of the transcription factor corresponding to procollagen expressions. These hypotheses are based on the results from a previous study showing that phenolic compounds such as gallic acid can enhance transcription factors determining antioxidant repose element (Yeh & Yen Citation2006). The mechanisms underlying the enhancement of type I collagen production and inhibition of MMPs need to be further clarified.
Photodamage of the skin manifests as skin thickness, reduction in skin elasticity and formation of wrinkles. These signs are fundamentally associated with reductions in collagen type I, the principal component of the dermal layer of the skin. Chronic UVB irradiation induced epidermal thickness and collagen loss (as hydroxyproline) was seen in our present study. These results closely match those of others studies which showed a reduction of collagen synthesis in mouse skin with repeated UVB exposure (Brenneisen et al. Citation2002; Cho et al. Citation2008). It seems likely that the protective effect of the T. chebula fruit extract on such skin photodamage caused by chronic UVB exposure may be mediated through the free radical scavenging activity and the preservation of MMPs and procollagen expressions in dermal fibroblast. The results provide a mechanism by which the ethanol extract of T. chebula fruits can prevent photodamage.
Conclusions
This study demonstrated the effectiveness and efficacy of the ethanol fruit extract of T. chebula on the reduction of MMP-1 and MMP-13 and the preservation of cutaneous collagen. Thus, the ethanol fruit extract of T. chebula can be used as a protectant against skin photodamage.
Funding information
We would like to thank Thailand Research Fund (TRF) and S & J International Enterprises Public Company Limited for financial support and the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education on facility supports. We would like to thank Dr. C. Norman Scholfield for useful discussions.
Acknowledgements
Many thanks to Mr. Roy Morien of the Naresuan University Language Centre for his editing assistance and advice on English expression in this document.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahn BN, Kim JA, Himaya SWA, Bak SS, Kong CS, Kim SK. 2012. Chitooligosaccharides attenuate UVB-induced damages in human dermal fibroblasts. Naunyn-Schmiedeberg’s Arch Pharmacol. 385:95–102.
- Aqil F, Ahmad I, Mehmood Z. 2006. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol. 30:177–183.
- Brennan M, Bhatti H, Nerusu KC, Bhaqavathula N, Kang S, Fisher GJ, Varani J, Voorhees JJ. 2003. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 78:43–48.
- Brenneisen P, Sies H, Scharffetter-Kochanek K. 2002. Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signaling to initial events. Ann NY Acad Sci. 973:31–43.
- Chang CL, Lin CS. 2012. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid Based Complement Alternat Med. 2012:1–7.
- Chiang HM, Chen HC, Lin TJ, Shin IC, Wen KC. 2012. Michelia alba extract attenuates UVB-induced expression of matrix metalloproteinases via MAP kinase pathway in human dermal fibroblasts. Food Chem Toxicol. 50:4260–4269.
- Cho S, Kim HH, Lee MJ, Lee S, Park CS, Nam SJ, Han JJ, Kim JW, Chung JH. 2008. Phosphatidylserine prevents UV-induced decrease of type I procollagen and increase of MMP-1 in dermal fibroblasts and human skin in vivo. J Lipid Res. 49:1235–1245.
- Chulasiri M, Wanaswas P, Sriaum D, Nakamat S, Wongkrajang Y, Kongsaktrakoon B, Phornchirasilp S, Songchitsomboon S, Leelarungrayub D. 2011. Utilizing hydroglycolic extract from myrobalan fruits to counteract reactive oxygen species. Int J Cosmet Sci. 33:371–376.
- Donsing P, Limpeanchob N, Viyoch J. 2008. Evaluation of the effect of Thai breadfruit’s heartwood extract on melanogenesis – inhibitory and antioxidation activities. J Cosmet Sci. 59:41–58.
- Faikrua A, Jeenapongsa R, Sila-asna M, Viyoch J. 2009. Properties of β-glycerol phosphate/collagen/chitosan blend scaffolds for application in skin tissue engineering. ScienceAsia. 35:247–254.
- Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, Kang S, Voorhees JJ. 2000. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 106:663–670.
- Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, Kang S, Voorhees JJ. 2001. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol. 117:219–226.
- Gilchrest BA. 1996. A review of skin ageing and its medical therapy. Br J Dermatol. 135:867–875.
- Gupta PC. 2012. Biological and pharmacological properties of Terminalia chebula Retz. (haritaki) – an overview. Int J Pharm Pharm Sci. 4:62–68.
- Inpanya P, Faikrua A, Ounaroon A, Sittichokechaiwut A, Viyoch J. 2012. Effects of the blended fibroin/aloe gel film on wound healing in streptozotocin-induced diabetic rats. Biomed Mater. 7:035008.
- Itsarasook K, Ingkaninan K, Viyoch J. 2014. Artocarpin-enriched extract reverses collagen metabolism in UV-exposed fibroblasts. Biologia. 69:943–951.
- Jayasree T, Nagesh CH, Prakash M, Shankar J, Chandra Sekhar A. 2013. Evaluation of surface anesthetic activity of alcoholic extract of fruit of Terminalia chebula on the cornea of albino rabbits. Bull Pharm Med Sci. 1:16–19.
- Juang LJ, Sheu SJ, Lin TC. 2004. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J Sep Sci. 27:718–724.
- Kang DG, Yun CK, Lee HS. 2003. Screening and comparison of antioxidant activity of solvent extracts of herbal medicines used in Korea. J Ethnopharmacol. 87:231–236.
- Katiyar SK, Afaq F, Perez A, Mukhtar H. 2001. Green tea polyphenols (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation induced oxidative stress. Carcinogenesis. 22:287–294.
- Kim GY, Sumiyoshi M, Sakanaka M, Kimura Y. 2009. Effects of ginseng saponins isolated from red ginseng on ultraviolet B-induced skin aging in hairless mice. Eur J Pharmacol. 602:148–156.
- Lançon A, Hanet N, Jannin B, Delmas D, Heydel JM, Lizard G, Chagnon MC, Artur Y, Latruffe N. 2007. Resveratrol in human hepatoma HepG2 cells: Metabolism and inducibility of detoxifying enzymes. Drug Metab Dispos. 35:699–703.
- Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. 2003. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 73:167–179.
- Moon HJ, Lee SR, Shim SN, Jeong SH, Stonik VA, Rasskazov VA, Zvyagintseva T, Lee YH. 2008. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol Pharm Bull. 31:284–289.
- Park JE, Pyun HB, Woo SW, Jeong JH, Hwang JK. 2014. The protective effect of Kaempferia parviflora extract on UVB-induced skin photoaging in hairless mice. Photodermatol Photoimmunol Photomed. 37:237–245.
- Phetdee K, Rakchai R, Rattanamanee K, Teaktong T, Viyoch J. 2014. Preventive effects of tamarind seed coat extract on UVA-induced alterations in human skin fibroblasts. J Cosmet Sci, 65:11–24.
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. 2004. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 165:741–751.
- Rakic L, Lapière CM, Nusgens BV. 2000. Comparative caustic and biological activity of trichloroacetic and glycolic acids on keratinocytes and fibroblasts in vitro. Skin Pharmacol Appl Skin Physiol. 13:52–59.
- Ravanti L, Heino J, López OC, Kähäri VM. 1999. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 274:2446–2455.
- Sánchez MC, Larrauri JA, Saura CF. 1998. A procedure to measure the antiradical efficiency of polyphenols. J Sci Food Agric. 76:270–276.
- Satardekar KV, Deodhar MA. 2010. Anti-ageing ability of Terminalia species with special reference to hyaluronidase, elastase inhibition and collagen synthesis in vitro. Int J Pharmacog Phytochem Res. 2:30–34.
- Surveswaran S, Cai YZ, Corke H, Sun M. 2007. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 102:938–953.
- Thurstan SA, Gibbs NK, Langton AK, Griffiths CE, Watson RE, Sherratt MJ. 2012. Chemical consequences of cutaneousphotoageing. Chem Cent J. 6:34–40.
- Varani J, Perone P, Fligiel SE, Fisher GJ, Voorhees JJ. 2002. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 119:122–129.
- Viyoch J, Mahingsa K, Ingkaninan K. 2012. Effects of Thai Musa species on prevention of UVB-induced skin damage in mice. Food Chem Toxicol. 50:4292–4301.
- Wen KC, Fan PC, Tsai SY, Shih IC, Chiang HM. 2012. Ixora parviflora protects against UVB-induced photoaging by inhibiting the expression of MMPs, MAP kinases, and COX-2 and by promoting type I procollagen synthesis. Evid Based Complement Alternat Med. 2012;417346.
- Weerapreeyakul N, Seebundit K, Pra-yong P. 2012. Antioxidative and tyrosinase inhibitory activities of indigenous plants. KKU Sci J. 40:572–583.
- Yeh CT, Yen GC. 2006. Involvement of p38 MAPK and Nrf2 in phenolic acid-induced P-form phenol sulfotransferase expression in human hepatoma HepG2 cells. Carcinogenesis. 27:1008–1017.