Abstract
Context: Curcumin, an active principal of Curcuma longa Linn. (Zingiberaceae), has potent antioxidant and anti-inflammatory properties.
Objectives: This study investigated the effects of curcumin on hyperlipidemia and hepatic steatosis in high-fructose-fed Wistar rats.
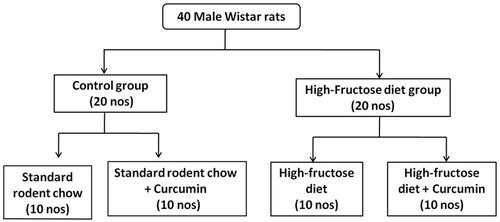
Materials and methods: Forty male Wistar rats were divided into four groups with 10 rats in each. Two groups were fed with standard rodent diet and the other two with 60% high-fructose diet for 10 weeks. Curcumin (200 mg/kg body weight) was administered along with the diets simultaneously to each of the aforementioned diet groups. After 10 weeks of experiment, blood samples were collected from tail vein. Liver, adipose and epididymal tissues were collected after sacrifice of the animals and stored for further analyses.
Results: Administration of curcumin reduced body weight (280.6 ± 7.4 g), liver weight (2.5 ± 0.2 g/100 g BW), adipose weight (1.4 ± 0.3 g/100 g BW), plasma levels of TAG (86.1 ± 13.5 mg/dL), VLDL-C (17.2 ± 2.7 mg/dL), lipid ratios and increased HDL-C (28.4 ± 4.5 mg/dL) in fructose-fed rats. Curcumin supplementation significantly lowered TAG content and decreased the protein expression of LXR-α (43%) and SREBP1c (59%) in the liver. Furthermore, curcumin suppressed the expression of lipogenic enzymes, ACLY (95%), ACC (50%) and FAS (77%) in rats fed with high-fructose diet. No significant change was found in the expression of PPAR-α.
Discussion and conclusion: Curcumin prevented the high-fructose induced hyperlipidemia and hepatic steatosis.
Keywords:
Introduction
Consumption of fructose-rich diet has risen considerably in recent years, which has led to major public health problems. High-fructose corn syrup (HFCS) is the major source of caloric sweeteners found in beverages, jams, jellies, baked foods and dairy products (Yudkin Citation1967). Several studies suggest that increased fructose ingestion may be responsible for the present epidemic of obesity and the increased incidence of metabolic syndrome (Huang et al. Citation1997; Bantle et al. Citation2000; Bray et al. Citation2004). Metabolic syndrome is defined as a cluster of metabolic disorders, including hypertension, insulin resistance, dyslipidemia, diabetes and cardiovascular disease (Simmons et al. Citation2010).
There is growing evidence that intake of fructose-rich diets is associated with dyslipidemia, insulin resistance, cardiovascular disease and hypertriglyceridemia both in humans and rodents (Tappy & Lê Citation2010). Hypertriglyceridemia is a major risk factor for cardiovascular diseases (Brunzell Citation2007). This may be due to the stimulation of hepatic de novo lipogenesis (DNL) which enhances free fatty acid generation leading to hypertriglyceridemia. The excess triglycerides can be stored as lipid droplets within hepatocytes or secreted in circulation as very-low density lipoprotein (VLDL) (Mayes Citation1993). Excessive hepatic TAG (triacylglycerol) accumulation is the hallmark of non-alcoholic fatty liver disease (NAFLD). NAFLD is considered as one of the most common causes of liver injury and often coexists with diabetes and obesity (Marchesini et al. Citation2001). NAFLD refers to a wide spectrum of liver damage, which ranges from simple steatosis or intracellular triglyceride accumulation to inflammation (steatohepatitis), fibrosis and cirrhosis (Neuschwander-Tetri & Caldwell Citation2003). If this benign form of simple hepatic steatosis is uncontrolled, it may progress to cirrhosis with subsequent liver failure and increases the risk for hepatocellular carcinoma. Management of dyslipidemia in early stages may halt the progression of hepatic steatosis. Although a wide range of lipid lowering agents are available, the metabolic complications associated with dyslipidemia still persist. Recent evidence indicates that dietary phytochemicals with hypolipidemic properties may be the prime choice in the prevention of dyslipidemia (Sikder et al. Citation2014).
Curcumin, a major active component of turmeric [Curcuma longa Linn, (Zingiberaceae)], is used as a spice and food-colouring agent in several food preparations. In vitro and in vivo studies have reported that curcumin exhibits potent antioxidant, anti-inflammatory and anticancer activities (Bengmark Citation2006). Experimental and clinical studies have proved that protection of non-alcoholic steatohepatitis (NASH) by curcumin could be attributed to its beneficial effects on inflammation, hyperlipidemia and leptin resistance (Shapiro & Bruck Citation2005; Jang et al. Citation2008; Tang et al. Citation2009). A study reported that curcumin improves hypertriglyceridemia and insulin sensitivity through the suppression of protein tyrosine phosphatase 1B (Li et al. Citation2010). Studies in animal models have proved that curcumin effectively prevented high-fat diet induced obesity (Um et al. Citation2013).
Although the hypolipidemic properties of curcumin have been reported in certain studies, the molecular mechanism underlying its protective effects in fructose induced lipid derangements and obesity remain unclear. Hence, the present study was designed to investigate the molecular effects of curcumin on fructose-induced obesity, hyperlipidemia and fat accumulation in male Wistar rats.
Materials and methods
Materials
Curcumin was procured from Sigma-Aldrich, St. Louis, MO. All reagents and chemicals used for Western blotting were of molecular reagent-grade and were obtained from Sigma Aldrich (St. Louis, MO), Merck (Mumbai, India) and SRL (Mumbai, India). Horseradish peroxidase-conjugate goat anti-rabbit IgG and goat anti-mouse IgG were procured from Santa Cruz Biotechnology (Santa Cruz, CA) and β-actin from Biolegend (San Diego, CA). The primary antibodies LXR, PPAR α and SREBP1c, FAS were obtained from Abcam (Cambridge, MA). ATP citrate lyase and acetyl CoA carboxylase were sourced from Cell Signaling Technology (Danvers, MA). The enhanced chemiluminescence substrate (ECL) was procured from Pierce, West Pico Super Signal (Thermo Fisher Scientific, Marietta, OH).
Animals
Five-month-old male Wistar rats of body weight ranging 250–300 g were used. They were separately housed in cages and maintained under standard laboratory conditions (temperature 22 ± 2 °C, 12 h dark/light cycle). All animals had free access to diet and water. All experimental procedures were approved by the Institute Animal Ethics Committee (Protocol no. 5 DEM/JSRC/10). Rats were randomly divided into four experimental groups with 10 rats in each group (). The fructose-rich diet was prepared by mixing 60% wt/wt fructose with standard rodent diet (Hwang et al. Citation1987).
Groups: Diet and treatment protocol.
Control: rats were fed with standard rodent chow throughout the experimental period of 10 weeks.
Control + curcumin: rats were fed with standard rodent chow and curcumin throughout the experimental period of 10 weeks.
Fructose group: rats were fed with 60% fructose diet throughout the experimental period of 10 weeks.
Fructose + curcumin: rats were fed with 60% fructose diet and curcumin throughout the experimental period of 10 weeks.
Curcumin (200 mg/kg body weight) was dissolved in 0.1% carboxy methyl cellulose (Na et al. Citation2011) and given orally. Body weight-baseline and final was noted. Blood samples were collected at the end of 10 weeks of experimental period from all the animals of different groups. The plasma separated from the blood was used for all the investigations. At the end of the experiment, animals were sacrificed under anaesthesia; the tissues such as liver, muscle and adipose tissue were collected and immediately frozen in liquid nitrogen. The frozen tissues were stored at −80 °C for proteomics and histological analysis.
Measurement of plasma biochemical parameters
The plasma total protein, albumin, AST, ALT and lipid profile were estimated using reagent kits adapted for fully Automated Clinical Chemistry analyzer (Olympus AU 400 Siemens, Tokyo, Japan). The indices of atherogenic lipid risk factors were assessed by calculating the lipid ratios: non-HDL-cholesterol, non-HDL-cholesterol/HDL-cholesterol, total cholesterol/HDL-cholesterol and triacylglycerol/HDL-cholesterol (Dobiásová & Frohlich Citation2000). Atherogenic index of plasma (AIP) [log10 (TG/HDL-c)] was calculated as described previously (Dobiásová Citation2006).
Estimation of liver triacylglycerol
The tissues were homogenized in chloroform and methanol (2:1, v/v) mixture (1 g tissue in 20 mL of solvent mixture). About 0.2 mL (4 mL for 20 mL) of distilled water or 0.9% NaCl solution was added to the homogenate, mixed and centrifuged at 2000 rpm for 15 min to separate into two phases. The lower chloroform phase containing lipid was collected and evaporated. Triacylglycerol (TAG) content was estimated using commercial enzyme kits (Genuine Biosystems, Chennai, India). TAG was expressed as milligrams TAG per gram of tissue (Folch et al. Citation1957).
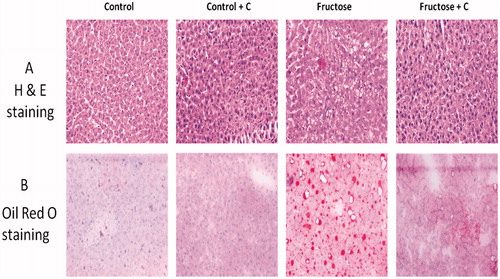
Liver histology and oil red O staining
Formalin-fixed liver tissue was embedded in paraffin, cut into 4 μm sections and stained with haematoxylin and eosin (H & E). Oil Red O (Sigma Aldrich, St. Louis, MO) staining of neutral lipids was done on frozen sections. Images were captured using phase contrast microscopy (Olympus BX43, Tokyo, Japan) at a magnification of 20×.
Western blot analysis
Liver homogenates were prepared in lysis buffer (50 mM Tris, pH 8.0, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM sodium chloride, 0.1% sodium dodecyl sulphate (SDS), 1 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM phenylmethylsulphonyl fluoride, 20 mM dithiothreitol, 1 mM aprotinin and 0.5% okadaic acid) as described previously (Laemmli Citation1970). The homogenate was then centrifuged at 12,000 rpm for 20 min at 4 °C. The protein concentrations were measured in the supernatant (Lowry et al. Citation1951). An equal amount of proteins was subjected to SDS–polyacrylamide gel electrophoresis (Mini Protean II System, Bio-Rad, Hercules, CA) according to their molecular weight and electro transferred to nitrocellulose membranes (Sigma, St. Louis, MO). The membranes were blocked with 5% BSA or 5% non-fat milk powder. The membranes were incubated with primary antibody specific to LXR, ATP Citrate Lyase, PPAR alpha, SREBP, FAS and ACC at 4 °C overnight. The membranes were then washed three-times with 0.05% Tween-20 in Tris-Buffered Saline (TBS) and incubated with secondary antibody [anti-mouse/anti-rabbit] (1:5000) conjugated with horseradish peroxidase reagent 1 h at room temperature. Membranes were stripped of all bound antibodies and then reprobed with antibody specific to β actin. Band intensities were visualized by enhanced chemiluminescence method using ECL kit (Pierce, Thermoscientific Inc., Marietta, OH). Images were captured with GS-800 densitometer and quantified using quantity 1 software (Biorad Laboratories Inc., Hercules, CA). The experiments were repeated three-times.
Statistical analysis
All values were expressed as mean ± SD. The difference between the groups was analysed by one-way analysis of variance (ANOVA) followed by the Tukey post hoc test. All the statistical analysis was done using Statistical Package for the Social Sciences (SPSS version 19.0, SPSS Inc., Chicago, IL) software. A p value less than 0.05 was considered as statistically significant.
Results
Effect of curcumin on body weight, food intake and organ weights in high-fructose-fed rats
shows the effect of curcumin on food intake, body weight and relative organ weights. High-fructose feeding significantly increased the body weight, liver and adipose mass when compared with the control group. No significant difference was found in the food intake and epididymal fat mass between the control group and the fructose group. Curcumin significantly reduced body weight, liver and adipose mass in high-fructose-fed rats.
Table 1. Effect of curcumin on relative weight of organs in high-fructose-fed rats.
Effect of curcumin on lipid profile in high-fructose-fed rats
shows the effect of curcumin on lipid profile in high-fructose-fed rats. High-fructose feeding significantly elevated the plasma levels of triacylglycerol (TAG), very low-density lipoprotein cholesterol (VLDL-c), atherogenic index of plasma (AIP), non HDL-c, non HDL-c/HDL-c, TC/HDL-c, TAG/HDL-c ratios and decreased high-density lipoprotein cholesterol HDL-c. The hypertriglyceridemia, elevated lipid indices and liver TAG levels in fructose-fed rats were reduced with curcumin treatment.
Table 2. Effect of curcumin on lipid profile and lipid ratios in high-fructose-fed rats.
Effect of curcumin on leptin, liver and kidney functions in high-fructose-fed rats
The hepatic enzyme alanine transaminase (ALT) was significantly elevated upon fructose feeding (). There was no change in the total protein, albumin and renal function parameters such as urea, creatinine in fructose-fed rats. Curcumin treatment significantly decreased ALT level. Plasma leptin levels were significantly elevated upon fructose feeding and treatment with curcumin decreased leptin levels in fructose-fed rats.
Table 3. Effect of curcumin on plasma leptin, liver and renal function in high-fructose-fed rats.
Effect of curcumin on liver fat accumulation in high-fructose-fed rats
The TAG levels were elevated in the liver of high-fructose-fed rats (). Similarly, H & E and oil O red staining () showed fat accumulation in the liver (hepatic steatosis) of high-fructose-fed rats. The hepatic TAG level and hepatic steatosis were significantly reduced upon curcumin treatment.
Figure 2. Effects of curcumin on liver fat accumulation in high-fructose-fed rats by (A) haematoxylin and eosin staining, (B) oil O red staining. Rats (n = 4) were fed with high-fructose diet and high-fructose diet + curcumin. Images were captured using phase contrast microscope Olympus BX43, Tokyo, Japan under 20× resolution. Fructose-fed rats showed marked fat accumulation. Fructose diet + curcumin prevented fat accumulation.
Protein expression of lipogenic transcription factors and enzymes
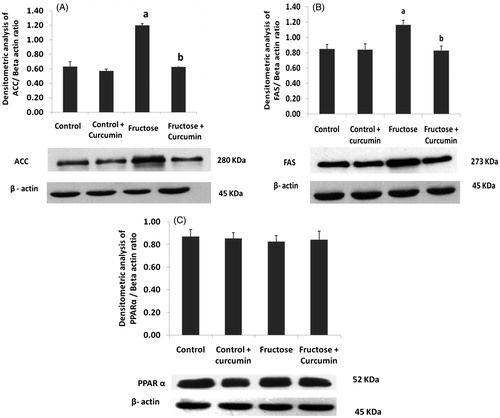
shows the immunoblot analysis of hepatic key proteins involved in lipid metabolism. ATP citrate lyase (ACLY) () was up-regulated during high-fructose feeding. The lipogenic transcription factor sterol regulatory element-binding protein 1c (SREBP 1c) () and liver X receptor (LXR-α) () were also up-regulated upon fructose feeding. These two nuclear transcription factors regulate the fatty acid synthesis. The two other key lipogenic enzymes: fatty acid synthase (FAS) () and acetyl CoA carboxylase (ACC) () were also up-regulated upon high-fructose feeding. Supplementation with curcumin significantly down-regulated the protein expression of ACLY, LXR-α, SREBP-1c, ACC and FAS about 95, 43, 59, 50 and 77%, respectively, in fructose-fed rats. There was no significant difference in PPAR α expression () among the experimental groups.
Figure 3. Immunoblot and densitometric analysis of the effects of curcumin on hepatic expression of (A) ACLY, (B) SREBP1c and (C) LXR-α (n = 4). One-way ANOVA followed by Tukey as post hoc test was used for analyzing differences between the groups. Data represent the mean ± SD. p < 0.05. ACLY, ATP citrate lyase; mSREBP1c, mature Sterol regulatory element binding protein; LXRα, liver X receptor. aIn comparison with control. bIn comparison with the fructose group.
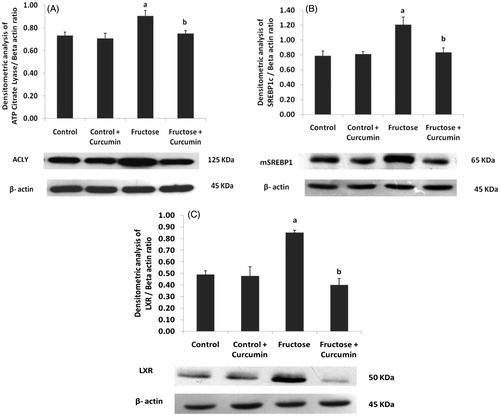
Figure 4. Immunoblot and densitometric analysis of the effects of curcumin on hepatic expression of (A) ACC, (B) FAS and (C) PPAR α (n = 4). One-way ANOVA followed by Tukey as post hoc test was used for analysing differences between the groups. Data represent the mean ± SD. p < 0.05. aIn comparison with control. bIn comparison with the fructose group. ACC: acetyl CoA carboxylase; FAS: fatty acid synthase; PPAR-α: peroxisome proliferator activated receptor.
Discussion
The present study investigated the beneficial effects of curcumin on high-fructose-diet induced metabolic complications in rats. Curcumin significantly reduced fructose induced increment in the body weight and relative organ weights (liver and adipose tissue weight). These rats did not show significant change in the food intake but showed elevated levels of plasma leptin. Leptin is an adipokine secreted by the adipose tissue that regulates food intake and body weight by stimulating anorexigenic pathways in hypothalamus (Ahima et al. Citation2000). Increase in adiposity triggers leptin secretion leading to stimulation of lipolysis and decreased lipogenesis in peripheral tissue thereby aids in the maintenance of adequate body-fat reserve. However, chronic consumption of high-fructose diet may lead to increased adiposity, which causes elevated circulating leptin levels resulting in leptin resistance. Leptin resistance is associated with induction of diet-induced obesity (Shapiro et al. Citation2008). Curcumin treatment decreased leptin resistance by decreasing plasma leptin levels, which is associated with reduced adipose mass.
High-fructose feeding caused increased plasma triacylglycerol (TAG) and VLDL-C, lipid indices and decreased HDL-C. These results were consistent with the previous study (Anitha Nandhini et al. Citation2002). Curcumin administration along with high-fructose diet improved HDL-C and decreased TAG, lipid indices and VLDL-C. Increased lipid level causes deposition of TAG in the liver and adipose tissue thereby leads to tissue damage. Consistent with this, plasma ALT level was significantly elevated with fructose feeding which is indicative of liver damage. Curcumin notably reversed the hepatic steatosis and lowered hepatic TAG levels. Curcumin supplementation along with high-fructose diet decreased the ALT levels thereby prevented the fructose induced hepatotoxicity. These findings indicated the hypolipidemic properties of curcumin. To illustrate the molecular mechanisms by which curcumin exhibits their hypolipidemic effect, we investigated the expression of key proteins involved in the lipid homeostasis.
Excess fructose when ingested enters glycolysis and generates pyruvate, which leads to increased production of acetyl CoA. The acetyl-CoA thus formed serves as the major substrate for lipid biosynthesis. The excess acetyl CoA exits the mitochondria and enters the cytosol in the form of citrate (Park et al. Citation1992). The available citrate is acted upon by the citrate cleavage enzyme, ATP citrate lyase (ACLY) which breaks down citrate into acetyl CoA and oxaloacetate. The resultant acetyl CoA serves as the major substrate for de novo lipogenesis which leads to overproduction of fatty acids (Chypre et al. Citation2012). In the current study, fructose feeding caused up-regulation of hepatic ACLY and led to enhancement of lipogenesis. Treatment with curcumin significantly reduced hepatic expression of ACLY. The inhibitory effect of curcumin on ACLY led to decreased TAG synthesis, which was reflected as decreased plasma TAG level in this study.
The expression of ACLY in the liver is regulated, at least in part (Shimano et al. Citation1999) at the transcriptional level by sterol responsive element binding protein 1c (SREBP-1c). Factors stimulating SREBP1c activation and its downstream actions on lipogenesis remain unclear. To confirm the activation of SREBP1c and ACLY, we investigated the other key proteins involved in lipogenesis. LXR-α is a nuclear transcriptional factor which regulates cholesterol metabolism via its target genes (Repa et al. Citation2000; Zhao & Dahlman-Wright Citation2010). In addition to its role in cholesterol metabolism, over-expression of LXR stimulates de novo FA synthesis leading to hypertriglyceridemia and hepatic steatosis via induction of SREBP1c. Enhanced fat deposition and VLDL secretion in liver were observed in mice upon treatment with synthetic LXR-α agonist (Chen et al. Citation2004). LXR activation stimulates SREBP1c which further up-regulates the expression of key lipogenic enzymes, ACC, FAS and SCD (Grefhorst et al. Citation2002). In addition, it has been noted that the maximal stimulation of the lipogenesis requires activation of both LXR and SREBP1c (Joseph et al. Citation2002). Therefore, inhibition of LXR-SREBP axis prevents the development of hypertriglyceridemia and hepatic steatosis.
In the present study, high-fructose fed rats showed increased expression of LXR, SREBP1c, ACC and FAS in the liver. It has been shown that excess fructose consumption activates the lipogenic enzymes (ACL, ACC, FAS and SCD) through induction of SREBP-1c leading to enhanced lipogenesis (Miyazaki et al. Citation2004). The observed hypertriglyceridemia and elevated lipid fractions upon high-fructose feeding are due to the stimulation of these lipogenic enzymes. Treatment with curcumin decreased the hepatic expression of lipogenic transcription factors LXR-α and SREBP1c. Furthermore, curcumin treatment along with high-fructose diet down-regulated the hepatic expression of ACC and FAS. Curcumin competitively binds to malonyl/acetyl transferase domain and covalently modifies FAS thereby decreasing TAG synthesis (Zhao et al. Citation2011). Another study reported that curcumin inhibits hepatic lipogenesis through down-regulation of SREBP1c expression (Shao et al. Citation2012). The reduction in the level of SREBP-1c and LXR-α might have turned off the lipogenic genes, thereby suppressing the lipogenesis. Curcumin exhibits potent antioxidant property. This anti-oxidant property might have contributed to the suppression of SREBP-1c, LXR-α and its target lipogenic enzymes FAS and ACC. The decrease in these lipogenic proteins with curcumin treatment could explain the reduction of TAG in plasma and tissue.
Peroxisome proliferator-activated receptor α (PPAR-α) is a nuclear hormone receptor that regulates the expression of fatty acid oxidation enzymes (Aoyama et al. Citation1998; Leone et al. Citation1999). There was no significant difference in the hepatic expression of PPAR-α. These results are in agreement with similar studies (Yokozawa et al. Citation2008; Kim et al. Citation2010).
The decrease in these lipogenic proteins with curcumin could explain the reduction in plasma and tissue TAG and seems to be involved in the control of weight of liver and body. Hence, it appears that the protective effect of curcumin on hepatic steatosis may be partly explained by its inhibitory effect of curcumin on SREBP1c and LXR thereby suppressing its target lipogenic genes, resulting in decreased TAG synthesis and liver fat accumulation.
In the present study, diet induced animal models were used to investigate the effects of curcumin on obesity and related metabolic complications. Although our results demonstrated the beneficial role of curcumin, the use of genetically knocked out obese and transgenic mice models can provide more focused information for better understanding of the efficacy of curcumin on obesity-associated metabolic derangements. Comparison with standard therapy might have provided more insights into the effectiveness of curcumin.
In conclusion, we found that high-fructose feeding caused hyperlipidemia, hepatic steatosis and lipid derangements through up-regulation of ACLY, FAS, ACC, LXR and SREBP1c. Curcumin treatment improved high-fructose induced fatty liver, lipid derangements and obesity through modulation of lipid metabolism in the liver as evidenced by decreased expression of lipogenic enzymes and transcription factors. Therefore, it is suggested that the use of curcumin may be beneficial as an adjuvant in the prevention and management of diet-induced obesity and its associated complications
Funding information
The authors are grateful to the Indian Council of Medical Research (ICMR), New Delhi, India for providing financial support in the form of Senior Research Fellowship to Mrs. Maithilikarpagselvi. N. The work was also supported by an intramural grant from Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 21:263–307.
- Anitha Nandhini AT, Balakrishnan SD, Anuradha CV. 2002. Taurine improves lipid profile in rats fed a high fructose-diet. Nutr Res. 22:343–354.
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. 1998. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem. 273:5678–5684.
- Bantle JP, Raatz SK, Thomas W, Georgopoulos A. 2000. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 72:1128–1134.
- Bengmark S. 2006. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enteral Nutr. 30:45–51.
- Bray GA, Nielsen SJ, Popkin BM. 2004. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 79:537–543.
- Brunzell JD. 2007. Clinical practice. Hypertriglyceridemia. N Engl J Med. 357:1009–1017.
- Chen G, Liang G, Ou J, Goldstein JL, Brown MS. 2004. Central role for liver X receptor in insulin-mediated activation of SREBP-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci USA. 101:11245–11250.
- Chypre M, Zaidi N, Smans K. 2012. ATP-citrate lyase: a mini-review. Biochem Biophys Res Commun. 422:1–4.
- Dobiásová M. 2006. AIP – atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr˘ Ní Lékar˘ Ství. 52:64–71.
- Dobiásová M, Frohlich J. 2000. The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy. Vnitr˘ Ní Lékar˘ Ství. 46:152–156.
- Folch J, Lees M, Sloane Stanley GH. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Grefhorst A, Elzinga BM, Voshol PJ, Plösch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ et al. 2002. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 277:34182–34190.
- Huang YJ, Fang VS, Juan CC, Chou YC, Kwok CF, Ho LT. 1997. Amelioration of insulin resistance and hypertension in a fructose-fed rat model with fish oil supplementation. Metab Clin Exp. 46:1252–1258.
- Hwang IS, Ho H, Hoffman BB, Reaven GM. 1987. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 10:512–516.
- Jang E-M, Choi M-S, Jung UJ, Kim MJ, Kim HJ, Jeon SM, Shin SK, Seong CN, Lee MK. 2008. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metab Clin Exp. 57:1576–1583.
- Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TF, Tontonoz P. 2002. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 277:11019–11025.
- Kim HY, Okubo T, Juneja LR, Yokozawa T. 2010. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br J Nutr. 103:502–512.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685.
- Leone TC, Weinheimer CJ, Kelly DP. 1999. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 96:7473–7478.
- Li J-M, Li Y-C, Kong L-D, Hu Q-H. 2010. Curcumin inhibits hepatic protein-tyrosine phosphatase 1B and prevents hypertriglyceridemia and hepatic steatosis in fructose-fed rats. Hepatology (Baltim MD). 51:1555–1566.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. 2001. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 50:1844–1850.
- Mayes PA. 1993. Intermediary metabolism of fructose. Am J Clin Nutr. 58:754S–765S.
- Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. 2004. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and independent mechanisms. J Biol Chem. 279:25164–25171.
- Na L-X, Zhang Y-L, Li Y, Liu LY, Li R, Kong T, Sun CH. 2011. Curcumin improves insulin resistance in skeletal muscle of rats. Nutr Metab Cardiovasc Dis. 21:526–533.
- Neuschwander-Tetri BA, Caldwell SH. 2003. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 37:1202–1219.
- Park OJ, Cesar D, Faix D, Wu K, Shackleton CH, Hellerstein MK. 1992. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J. 282:753–757.
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXR-alpha and LXR-beta. Genes Dev. 14:2819–2830.
- Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, Lu H, Fantus IG, Jin T. 2012. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 7:e28784.
- Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. 2008. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 295:1370–1375.
- Shapiro H, Bruck R. 2005. Therapeutic potential of curcumin in non-alcoholic steatohepatitis. Nutr Res Rev. 18:212–221.
- Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, et al. 1999. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 274:35832–35839.
- Sikder K, Das N, Kesh SB, Dey S. 2014. Quercetin and beta-sitosterol prevent high fat diet induced dyslipidemia and hepatotoxicity in Swiss albino mice. Indian J Exp Biol. 52:60–66.
- Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich Mirchov I, Ben-Nakhi A, et al. 2010. The metabolic syndrome: useful concept or clinical tool? Report of a WHO Expert Consultation. Diabetologia. 53:600–605.
- Tang Y, Zheng S, Chen A. 2009. Curcumin eliminatesleptin's effects on hepatic stellate cell activation via interrupting leptin signaling. Endocrinology. 150:3011–3020.
- Tappy L, Lê K-A. 2010. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 90:23–46.
- Um MY, Hwang KH, Ahn J, Ha TY. 2013. Curcumin attenuates diet-induced hepatic steatosis by activating AMP-activated protein kinase. Basic Clin Pharmacol Toxicol. 113:152–157.
- Yokozawa T, Kim HJ, Cho EJ. 2008. Gravinol ameliorates high-fructose-induced metabolic syndrome through regulation of lipid metabolism and pro-inflammatory state in rats. J Agric Food Chem. 56:5026–5032.
- Yudkin J. 1967. Evolutionary and historical changes in dietary carbohydrates. Am J Clin Nutr. 20:108–115.
- Zhao C, Dahlman-Wright K. 2010. Liver X receptor in cholesterol metabolism. J Endocrinol. 204:233–240.
- Zhao J, Sun X-B, Ye F, Tian W-X. 2011. Suppression of fatty acid synthase, differentiation and lipid accumulation in adipocytes by curcumin. Mol Cell Biochem. 351:19–28.