Abstract
Context: Antiglycative potential of Psidium guajava L. (Myrtaceae) leaves has been established. However, the molecular basis of its antiglycative potential remains unknown.
Objective: The ethyl acetate fraction of P. guajava leaves (PGEt) was evaluated to determine the cardioprotective effect and its mechanism of action compared to quercetin.
Materials and methods: After the induction of diabetes by streptozotocin (55 mg/kg body weight), PGEt and quercetin (50 mg/kg body weight) was administered for 60 days. Rats were grouped as follows: Group C: Control, Group D: Diabetic, Group D + E: Diabetic rats treated with PGEt, Group D + Q: Diabetic rats treated with quercetin. The antiglycative potential was evaluated by assaying glycosylated haemoglobin, serum fructosamine and advanced glycation end product levels. The differential receptor for advanced glycation end products and nuclear factor kappa B (NFκB) protein levels was determined by western blot and the transcript level changes of connective tissue growth factor (CTGF), brain natriuretic peptide (BNP) and TGF-β1 in heart tissue were assessed by RT-PCR analysis.
Results: Glycated haemoglobin and serum fructosamine levels were found to be enhanced in diabetic rats when compared with control. Administration of PGEt significantly reduced the glycated haemoglobin and fructosamine levels to a larger extent than quercetin treated diabetic rats. PGEt reduced the translocation of NFκB from cytosol to nucleus when compared with diabetic rats. Expression of TGF-β1, CTGF and BNP was downregulated in PGEt treated groups compared with diabetic controls.
Discussion and conclusion: Administration of PGEt ameliorated diabetes associated changes in the myocardium to a greater extent than quercetin.
Keywords:
Introduction
Diabetes mellitus is associated with a number of secondary complications. Among them, cardiovascular disease is one of the major causes of morbidity and mortality associated with diabetes. There is increasing evidence that advanced glycation end products (AGEs) play a central role in cardiac complications associated with diabetes (Baynes & Thorpe Citation2000).
In patients with diabetes, hyperglycemia increases oxidative stress and accelerates the accumulation of AGEs. Glycation products are capable of generating reactive oxygen species either themselves or by interacting with receptor for advanced glycation end products (RAGE) (Ramasamy et al. Citation2005). Binding of AGEs to RAGE activates endothelial cells, resulting in higher levels of endothelial adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1), and activation of transcription factor, nuclear factor kappa B (NFκB) (Kunt et al. Citation1999). NFκB and VCAM-1 promote atherosclerosis by enhancing adhesivity of monocytes and vascular permeability (Libby et al. Citation2002). Retardation of AGE formation in experimental diabetic animals can prevent diabetic complications (Ahmed & Ahmed Citation2006). Transforming growth factor β1 (TGF-β1) is highly expressed in heart and kidney of experimental diabetes in association with extracellular matrix accumulation (Yagi et al. Citation1997). TGF-β1 also induces the expression of collagen and fibronectin from cardiac fibroblasts and myocytes (Noh et al. Citation2006). Connective tissue growth factor (CTGF) is critical for the development of cardiovascular complications associated with diabetes mellitus (Candido et al. Citation2002). The increased expression of brain natriuretic peptide (BNP) has also been suggested as a molecular marker for cardiomyocyte hypertrophy (Nishikimi et al. Citation2006).
Our previous in vitro and in vivo studies proved significant antioxidant, antiglycative and hyperglycemic potential of PGEt (Soman et al. Citation2010, Citation2013). In vitro studies have shown that most of the antiglycative as well as antioxidant potential could be accounted to the high polyphenol content present in the ethyl acetate fraction of Psidium guajava L. (Myrtaceae) leaves (Soman et al. Citation2010). Quercetin was reported as one of the most abundant and active compounds present in P. guajava leaves (Gutiérrez et al. Citation2008). Thus, the current study was designed to evaluate the antiglycative potential of PGEt in comparison with quercetin, a well-known standard flavonoid. The mechanism of action of PGEt on diabetic cardiovascular complications was also attempted.
Materials and methods
Plant material
Budding leaves of P. guajava were collected from nearby areas of Thiruvananthapuram in December 2008 and the plant was identified and authenticated by Dr. G. Valsala Devi (Curator, Department of Botany, University of Kerala) and a voucher herbarium specimen (No. KUBH 5796) was deposited in the herbarium of Department of Botany, University of Kerala, India. The freshly collected leaves were shade dried and powdered.
Preparation of P. guajava extracts
The powdered plant material (500 g) was extracted three times with 80% methanol (MeOH), at room temperature overnight. The MeOH extracts were combined and concentrated under reduced pressure to yield the total extract (71 g). It was further extracted with petroleum ether to remove low polar substances. The lower layer obtained after petroleum ether fractionation was subjected to extraction with ethyl acetate. The ethyl acetate fraction (6.5 g), which showed maximum in vitro antioxidant and antiglycative potential, was further used for the in vivo studies. The ethyl acetate fraction of the plant was denoted as PGEt and kept at −20 °C until used.
Induction of experimental diabetes
Female Sprague Dawley rats (200–250 g) were used for the study. Animals were kept in polypropylene cages with a 12-h light/dark cycle. Rats were fed with rat feed (Lipton India Ltd., Mumbai, India). Food and water was given ad libitum. All procedures were approved by the animal ethical committee of Kerala University. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ) (55 mg/kg body weight) dissolved in citrate buffer (pH 4.6). After 3 days of STZ injection, blood from tail vein was collected to determine the fasting blood glucose level. Only rats with fasting blood glucose level above 250 mg/dL were considered diabetic and were included for the experiments. All studies were carried out after 1 week of STZ injection.
Experimental design
The rats were divided into four groups of six rats each and were treated as follows:
Group C: Control rats with saline,
Group D: Diabetic rats with saline,
Group D + Q: Diabetic rats treated with quercetin (50 mg/kg body weight/day) and
Group D + E: Diabetic rats treated with PGEt (50 mg/kg body weight/day).
The dose of PGEt was selected based on our preliminary dose-dependent study (Soman et al. Citation2010). PGEt and quercetin were administered in normal saline solution by intragastric administration for 60 days. At the end of the experimental period, the rats were fasted overnight and sacrificed. Blood and tissues were removed to ice-cold containers for various estimations.
Analytical methods
Blood glucose was estimated using a standard glucometer (one touch horizon, Johnson & Johnson, Mumbai, India). Glycated haemoglobin (HbA1c) was estimated by Micromat 2 haemoglobin Acc test using micromat II instrument catalogue no. 280-00016XI. Serum fructosamine was determined by kit purchased from Spinreact, Spain (Spinreact, GIRONA, Spain).
Reverse transcription – PCR
Total RNA was extracted using ribopure TM kit (Ambion, Austin, TX) according to the manufacturer’s protocol. Total RNA was determined by the OD 260 nm method. Total RNA (4 μg) was reverse transcribed with the first-strand cDNA synthesis kit (Eppendorf, Hamburg, Germany) using oligo dT according to the manufacturer’s instructions. PCR reaction was carried out using Eppendorf Thermocycler. Primer sequences, annealing temperature and the product length of each assayed genes are given in .
Table 1. Primer sequences used for RT-PCR analysis.
Western blot analysis
Cardiac tissue (100 mg) of rats from each group was used for the preparation of the cytoplasmic and nuclear extracts by standard protocols as described by Andrews and Faller (Citation1991) and used for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and western blotting, respectively. Protein concentrations of the extracts were determined by the method of Lowry et al. (Citation1951). The cytosolic and nuclear extracts were equalized for protein concentration before loading in 10% SDS–PAGE (Laemmli Citation1970). It was then transblotted to nitrocellulose membranes (Bio-Rad, Hercules, CA) in a Biometra (Biometra GmbH, Göttingen, Germany) apparatus using standard protocol described by Burnette (Citation1981). Detection of NFκB in the cytosolic and nuclear fraction was done by incubating with an NFκB p65-specific antibody at 25 °C. Mouse monoclonal anti-NFκB (p65) and horseradish peroxidase-conjugated anti-IgG antibody used as a secondary antibody were purchased from Sigma Chemicals (Bangalore, India). The concentration of NFκB was then determined using a Gel-doc.
Histopathological analysis of heart
The heart tissues were rapidly dissected out and fixed by immersion at room temperature in 10% formalin solution. For the histological examinations, paraffin embedded tissue sections of heart (5 μm) were stained with haematoxylin–eosin. The tissue samples were then examined and photographed under a light microscope for observation of structural abnormality.
Statistical analysis
The results were analysed using a statistical program SPSS/PC+, Version 10.0 (SPSS Inc., Chicago, IL). One-way ANOVA was employed for comparison among the five groups. Duncan’s post hoc multiple comparison tests of significant differences among groups were determined. p < 0.05 was considered to be significant. All the results were expressed as mean ± SD for six animals in each group.
Results
shows the fasting blood glucose level and serum insulin level in control and experimental groups of rats. Blood glucose level was significantly higher in diabetic control than the treated groups. The reduction was more prominent in PGEt treated group compared to quercetin treated group. Serum insulin level was less in the diabetic group. However, administration of PGEt restored the level considerably in comparison to the quercetin treated group.
Table 2. Blood glucose and serum insulin.
depicts the levels of glycated haemoglobin and fructosamine, which are indicators of early glycation end products. Level of glycated haemoglobin was enhanced in the diabetic group. There was significant reduction in HbA1C levels in both the treated groups with a considerable effect in PGEt treated group. Serum fructosamine level was higher in the diabetic group and both quercetin and PGEt decreased its level.
Table 3. Glycated haemoglobin and serum fructosamine.
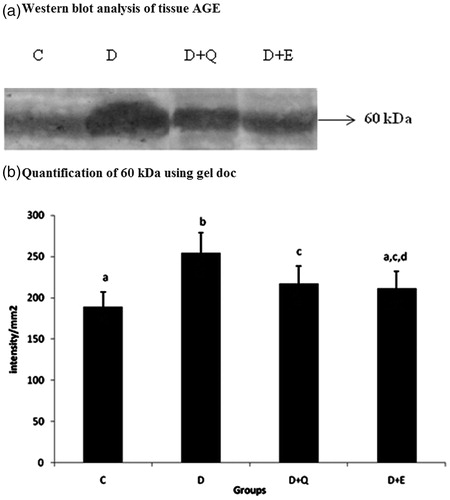
In order to confirm the effect of PGEt on AGE in heart tissue, the protein level of AGE was analysed by western blotting (). Total AGE level was increased markedly in the diabetic group. However, PGEt treated group showed decreased AGE expression. Though quercetin treatment also produced a reduction in AGE level, it was comparatively less when compared to PGEt treated group.
Figure 1. Equal concentrations of protein aliquots from heart cell lysates were separated using SDS–PAGE and blotted on to nitrocellulose membranes and later probed with anti-AGE antibody. (a) Location of bands ∼60 kDa in the heart extracts of experimental groups. (b) Intensity of bands ∼60 kDa was quantified using Biorad gel doc and plotted. The results presented are average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
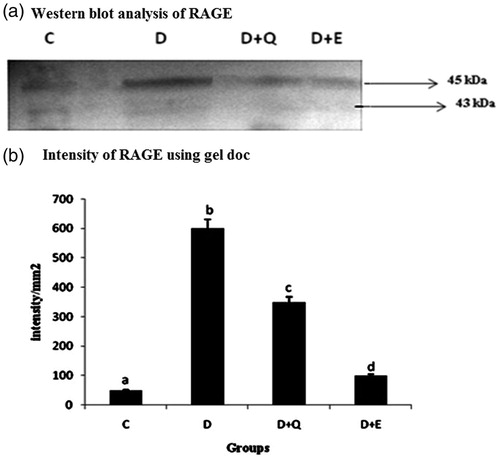
The effect of PGEt on the expression of RAGE was studied (). The relative mRNA expression of RAGE was high in diabetic myocardium. It was significantly reduced on the treatment with both PGEt and quercetin. RAGE expression at the protein level also showed a similar pattern with that of its mRNA expression ().
Figure 2. The relative amount of RAGE mRNA was estimated by semi-quantitative RT-PCR. The PCR products were quantified by densitometry and standardized to their respective GAPDH controls. The mean intensity was measured and expressed as INT/mm2. Results are expressed as average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
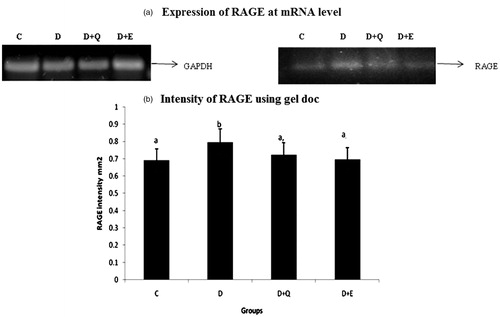
Figure 3. Equal concentrations of protein aliquots from heart cell lysates were separated using SDS–PAGE and blotted on to nitrocellulose membranes and later probed with anti RAGE antibody. (a) Location of bands ∼45 kDa in the heart extracts of experimental groups. (b) Intensity of bands ∼45 kDa was quantified using Biorad gel doc and plotted. The results presented are average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
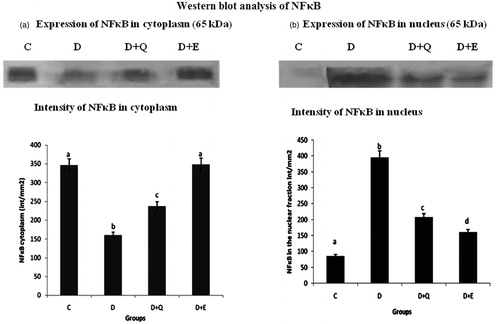
NFκB is a ubiquitous inducible transcription factor that activates expression of groups of genes, including those that promote cardiac hypertrophy. In order to study the effect of PGEt and quercetin on the activation and subsequent nuclear translocation of NFκB, western blot analysis was carried out using anti-NFκB antibody. Expression of NFκB was analysed in both the cytosolic and nuclear fraction of the heart tissue. Pattern of expression of NFκB in the cytoplasmic and nuclear fraction shows the activation and translocation of NFκB in the diabetic group (). Treatment with PGEt and quercetin inhibited the translocation of NFκB and this effect was relatively higher in PGEt treated group.
Figure 4. Equal concentrations of protein aliquots (Lowry’s method) from heart cell lysates were separated using SDS–PAGE and blotted on to nitrocellulose membranes and later probed with anti-NFκB p65 antibody. Location of bands ∼65 kDa in the nuclear and cytoplasmic fraction of heart extracts of experimental groups. (b) Intensity of bands ∼65 kDa was quantified using Biorad gel doc and plotted. The results presented are average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
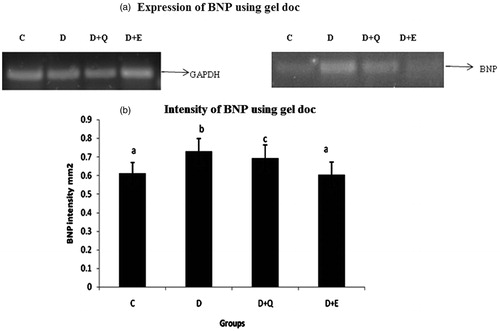
The mRNA expression of TGF-β1, CTGF and BNP were evaluated by RT-PCR. The expression of TGF-β1, CTGF and BNP was increased markedly in the myocardial tissue of diabetic rats when compared to control rats. Treatment with PGEt resulted in the decreased mRNA expression of TGF-β1 (), CTGF () and BNP (). Though quercetin showed a decreased tendency in the mRNA expression of CTGF and BNP, the reduction was not significant for TGF-β1.
Figure 5. The relative amount of TGF-β1 mRNA was estimated by semi-quantitative RT-PCR. The PCR products were quantified by densitometry and standardized to their respective GAPDH controls. The mean intensity was measured and expressed as INT/mm2. Results are expressed as average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
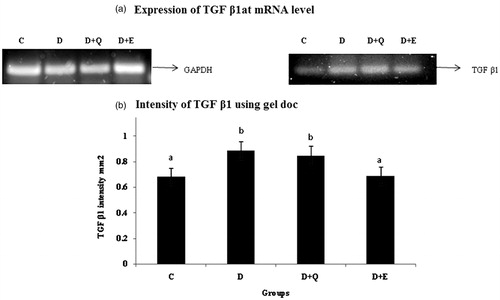
Figure 6. The relative amount of CTGF mRNA was estimated by semi-quantitative RT-PCR. The PCR products were quantified by densitometry and standardized to their respective GAPDH controls. The mean intensity was measured and expressed as INT/mm2. Results are expressed as average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
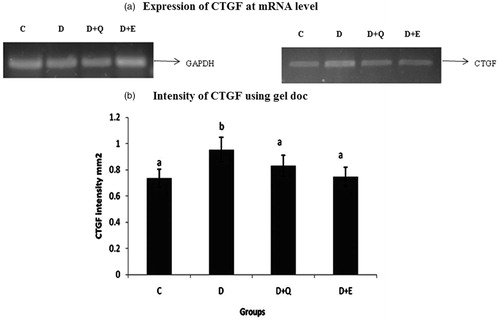
Figure 7. The relative amount of BNP mRNA was estimated by semi-quantitative RT-PCR. The PCR products were quantified by densitometry and standardized to their respective GAPDH controls. The mean intensity was measured and expressed as INT/mm2. Results are expressed as average of quadruplicate experiments ± SD statistically significant p ≤ 0.05.
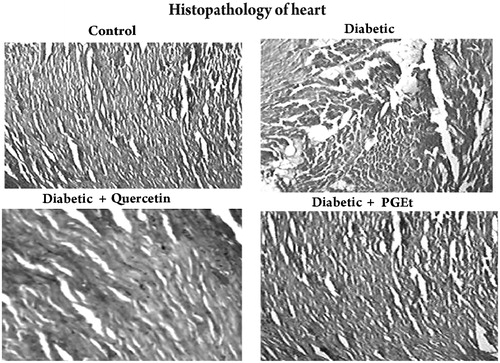
Histopathological analysis of the myocardium also supports the cardioprotective effect of PGEt (). The control rat showed normal myocardial fibres with normal cell structure whereas the heart of diabetic rats showed severe necrosis, inflammatory infiltration, and oedema with degeneration. Treatment with PGEt and quercetin improved the myocardial degeneration. Mild inflammatory infiltration was observed in quercetin treated groups when compared to PGEt treated rats.
Figure 8. Light microscopic appearance of the heart sections stained with haematoxylin and eosin (magnification 40×). Group – Control: Heart of control rat showing normal myocardial fibres with normal cell structure. Group – Diabetic: Heart of diabetic rats showing severe necrosis, inflammatory infiltration, oedema with degeneration. Group – Diabetic + Quercetin: Quercetin treatment improved the myocardial degeneration with mild inflammatory infiltration when compared to the heart of diabetic rats. Group – Diabetic + Extract: Administration of PGEt also improved the myocardial degeneration when compared to diabetic control.
Discussion
The pathological features of diabetes and its complications caused by hyperglycemia and AGEs in myocardium had been studied extensively (Inamoto et al. Citation2006). Our previous in vitro studies proved both the antioxidant and antiglycative potential of ethyl acetate fraction of P. guajava leaves (Soman et al. Citation2010). In the current study, the mechanism of PGEt in diabetes associated changes in myocardium was studied and compared to quercetin, a known standard reference flavonoid. The dose of PGEt for the present study (50 mg/kg body weight) was selected based on the results obtained from the preliminary dose-dependent study conducted by us (Soman et al. Citation2010). Quercetin was also administered at the same dose as there are reports that quercetin constitutes a major known active component in P. guajava leaves (Gutiérrez et al. Citation2008).
Our results showed that administration of PGEt and quercetin reduced blood glucose levels and enhanced insulin level in STZ induced diabetic rats. Current data support the finding of Gopal and Indira (Citation2010) who reported that glycosylated haemoglobin and fructosamine were significantly increased in diabetic control rats and the increase is directly proportional to fasting blood glucose.
The contribution of AGEs in diabetes mellitus has received considerable attention (Jakus & Rietbrock Citation2004), and free radicals have been reported to contribute in the formation of AGEs (Halliwell Citation2001). AGE deposition and expression of the receptor for AGEs were observed in the myocardium of diabetic rats (Sun et al. Citation1998). Serum levels of AGEs are found to be elevated in type 2 diabetic patients with coronary heart disease, and are associated with left ventricular diastolic function in patients with type 1 diabetes (Berg et al. Citation1999). Present results also support the increased AGE deposition in the myocardium and serum of diabetic rats. Reports also suggest the importance of antioxidants and radical scavengers in hindering these processes (Nakagawa et al. 2002). Wu and Yen (Citation2005) demonstrated the inhibitory effect of naturally occurring flavonoids on the formation of AGEs. Current data also support the finding that increased level of AGEs in serum and heart tissues was reversed on the treatment with PGEt and quercetin.
AGE cross-links are permanent and irreversible complexes, which bind to the target proteins and accumulate in diabetic conditions and results in cardiovascular complications (Singh et al. Citation2001). AGEs are thought to act through receptor-dependent and -independent mechanisms to promote myocardial damage; fibrosis and inflammation with accelerated diabetic cardiomyopathy (Goldin et al. Citation2006). Interaction of AGEs with its receptor RAGE activates various signalling pathways, leading to the activation of profibrosis and proinflammatory genes responsible for endothelial cell dysfunction in diabetic rats (Li et al. Citation2004). At the cellular level, AGE/RAGE interaction induces the activation and nuclear translocation of NFκB, and is implicated in the initiation of atherosclerotic lesions and other vascular disorders (Collins Citation1993). Activation of NFκB is well established in the development of cardiac hypertrophy (Li et al. Citation2004). The activation and translocation of NFκB would transcribe various pathological mediators like cytokines and chemokines (Newton et al. Citation1999).
To understand the role of PGEt on receptor-dependent pathway, we performed RT-PCR analysis of certain genes in the myocardial tissue of diabetic rats. Our results suggest that AGEs triggered the activation of NFκB by interacting with RAGE. Increased expression of RAGE protein in the myocardium of diabetic animals implies a role for this receptor in AGE induced myocardial structural alterations. This was in accordance with the finding of Candido et al. (Citation2003). The intensities of NFκB in the cytoplasmic fraction of the heart showed that the band intensities were higher in the control group compared to the diabetic group. PGEt and quercetin treated groups also possess comparable band intensities with that of control group. However, there is no detectable band in the nuclear fraction of the control group. PGEt and quercetin treated group showed lighter bands when compared to the diabetic control. This substantiates the fact that PGEt and quercetin reduced the nuclear translocation of NFκB. NFκB mediates its action through activation of cytokines like TGF-β1. Studies also highlight the increased expression of profibrotic cytokines and unusual distribution of extracellular matrix components are associated with enhanced expression of AGE and RAGE levels in diabetes (Candido et al. Citation2003). TGF-β1 is highly expressed in the heart and kidney in experimental diabetes in association with extracellular matrix accumulation (Sharma & Ziyadeh Citation1994) and there is increasing evidence that CTGF also plays an important role in the development of diabetic renal and cardiovascular complications (Candido et al. Citation2002). The profibrotic growth factor, TGF-β1 induces extracellular matrix accumulation and fibrosis in diabetes (Zhou et al. Citation2004). Our results also support the increased expression of TGF-β1 in cardiovascular tissue of diabetic rats. TGF-β1 is also known to induce the expression of CTGF in cardiac myocytes (Chen et al. Citation2000). Earlier studies have reported CTGF as a mediator of myocardial remodelling and fibrosis (Koitabashi et al. Citation2007).
BNP is an established marker of cardiac dysfunction (Nishikimi et al. Citation2006). BNP is released from ventricular myocardium in response to increased wall stress and reveals both systolic and diastolic cardiac dysfunction (Nishikimi et al. Citation2006). In diabetic rats, the development of cardiac dysfunction is also associated with activation of cardiac BNP gene expression. The myocardial expression of CTGF and BNP plays a crucial role in the development of myocardial fibrosis in an experimental animal model of diastolic heart failure (Koitabashi et al. Citation2007). Our results demonstrate that AGE–RAGE interaction increased the expression of NFκB mediated upregulation of TGF-β1 and CTGF, which subsequently promoted myocardial hypertrophy and fibrosis as evidenced by increased expression of BNP. Thus, in the present study, the reduction in BNP and CTGF expression strongly suggests improved cardiac function upon treatment with PGEt and quercetin.
As discussed above, it was found that both PGEt and quercetin showed beneficial effects on the diabetic myocardium. The data also suggest that PGEt is mediating its effect on diabetic heart by reducing the translocation of NFκB into the nucleus and downregulating TGF-β1 and thereby protecting the heart. The relatively decreased expression of TGF-β1 in the myocardium of quercetin treated animals shows that it may be acting through some other mechanisms to mediate its action on diabetic heart. Though both PGEt and quercetin showed cardioprotective effects, PGEt was more potent than quercetin. This may be due to the fact that antiglycation and cardioprotective effect of PGEt is not solely dependent upon its reported active compound quercetin. It could be due to the synergistic effects of quercetin with other active compounds present in P. guajava.
Conclusion
In short, PGEt ameliorated alterations caused by diabetes in the myocardium. The mechanism may be by reducing the concentration of AGEs in heart and thereby downregulating the expression of RAGE and further lowering AGE/RAGE induced NFκB signalling to a greater extent when compared to quercetin. PGEt significantly reduced the nuclear translocation of NFκB and also reduced the expression of CTGF and BNP, which are involved in the myocardial fibrosis. Though both PGEt and quercetin showed beneficial effects in the myocardium of diabetic rats, the effect was more significant in PGEt treated diabetic group, which may be due to the synergistic effects of quercetin with other active components present in PGEt.
Disclosure statement
The authors declare no conflict of interest.
Funding
This work was supported by Kerala State Council for Science, Technology and Environment (KSCSTE), Pattom, Thiruvananthapuram [(T) 176/SRS/2006/CSTE].
References
- Ahmed MS, Ahmed N. 2006. Antiglycation properties of aged garlic extract: possible role in prevention of diabetic complications. J Nutr. 136:796–799.
- Andrews NC, Faller DV. 1991. A rapid micropreparation technique for extraction of DNA binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19:2499.
- Baynes JW, Thorpe SR. 2000. Glycoxidation and lipoxidation in atherogenesis. Free Radic Biol Med. 28:1708–1716.
- Berg TJ, Snorgaard O, Faber J, Torjesen PA, Hildebrandt P, Mehlsen J, Hanssen KF. 1999. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 22:1186–1190.
- Burnette WN. 1981. Western blotting: electrophoretic transfer of proteins from sodium dodecyl sulfate polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radio iodinated protein A. Anal Biochem. 112:195–203.
- Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, et al. 2003. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 92:785–792.
- Candido R, Jandeleit-Dahm KA, Cao Z, Nesteroff SP, Burns WC, Twigg SM, Dilley RJ, Cooper ME, Allen TJ. 2002. Prevention of accelerated atherosclerosis by angiotensin-converting enzyme inhibition in diabetic apolipoprotein E-deficient mice. Circulation. 106:246–253.
- Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. 2000. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 32:1805–1819.
- Collins T. 1993. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 68:499–508.
- Goldin A, Beckman JA, Schmidt AM, Creager MA. 2006. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 114:597–605.
- Gopal VR, Indira M. 2010. Investigations on the correlation of advanced glycated end products (AGE) associated fluorescence with blood glucose and oxidative stress in ethanol-administered diabetic rats. Exp Toxicol Pathol. 62:157–162.
- Gutiérrez RM, Mitchell S, Solis RV. 2008. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 117:1–27.
- Halliwell B. 2001. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 18:685–716.
- Inamoto S, Hayashi T, Tazawa N, Mori T, Yamashita C, Nakano D, Matsumura Y, Okuda N, Sohmiya K, Sakai A, et al. 2006. Angiotensin-II receptor blocker exerts cardioprotection in diabetic rats exposed to hypoxia. Circ J. 70:787–792.
- Jakus V, Rietbrock N. 2004. Advanced glycation end products and the progress of diabetic vascular complications. Physiol Res. 53:131–142.
- Koitabashi N, Arai M, Kogure S. 2007. Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension. 49:1120–1127.
- Kunt T, Forst T, Früh B, Flohr T, Schneider S, Harzer O, Pfützner A, Engelbach M, Löbig M, Beyer J. 1999. Binding of monocytes from normo lipidemic hyperglycemic patients with type 1 diabetes to endothelial cells is increased in vitro. Exp Clin Endocrinol Diabetes. 107:252–256.
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685.
- Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. 2004. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 164:1389–1397.
- Libby P, Ridker PM, Maseri A. 2002. Inflammation and atherosclerosis. Circulation. 105:1135–1143.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein determination with Folin reagent. J Biol Chem. 195:133–140.
- Nakagawa T, Yokozawa T, Terasawa K, Shu S, Juneja LR. 2002. Protective activity of green tea against free radical-and glucose-mediated protein damage. J Agr Food Chem. 50:2418–2422.
- Newton TR, Patel NM, Bhat-Nakshatri P, Stauss CR, Goulet RJ Jr, Nakshatri H. 1999. Negative regulation of transactivation function but not DNA binding of NF-kappaB and AP-1 by IkappaBbeta1 in breast cancer cells. J Biol Chem. 274:18827–18835.
- Nishikimi T, Maeda N, Matsuoka H. 2006. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 69:318–328.
- Noh HJ, Kim HC, Lee SS, Kang YN, Chae YM, Park KK. 2006. The inhibitory effect of siRNAs on the high glucose-induced overexpression of TGF-beta1 in mesangial cells. J Korean Med Sci. 21:430–435.
- Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. 2005. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neuro degeneration, and inflammation. Glycobiology. 15:16–28.
- Sharma K, Ziyadeh FN. 1994. Renal hypertrophy is associated with upregulation of TGF-β1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol. 267:1094–1101.
- Singh R, Barden A, Mori T, Beilin L. 2001. Advanced glycation end-products: a review. Diabetologia. 44:129–146.
- Soman S, Rajamanickam C, Rauf AA, Indira M. 2013. Beneficial effects of Psidium guajava leaf extract on diabetic myocardium. Exp Toxicol Pathol. 65:91–95.
- Soman S, Rauf AA, Indira M, Rajamanickam C. 2010. Antioxidant and antiglycative potential of ethyl acetate fraction of Psidium guajava leaf extract in streptozotocin-induced diabetic rats. Plant Foods Hum Nutr. 65:386–391.
- Sun M, Yokoyama M, Ishiwata T, Asano G. 1998. Deposition of advanced glycation end products (AGE) and expression of the receptor for AGE in cardiovascular tissue of the diabetic rat. Int J Exp Pathol. 79:207–222.
- Wu CH, Yen GC. 2005. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem. 53:3167–3173.
- Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H. 1997. Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima Fatty rats. Hypertension. 29:728–735.
- Zhou G, Li C, Cai L. 2004. Advanced glycation end-products induce connective tissue growth factor-mediated renal fibrosis predominantly through transforming growth factor beta-independent pathway. Am J Pathol. 165:2033–2043.
