Abstract
Context: Cyclocarya paliurus (Batal) Iljinskaja (Juglandaceae) is an edible and medicinal plant; the leaves are used in Chinese folkloric medicine to treat dyslipidaemia and diabetes.
Objective: This study evaluates the antihyperlipidaemic potential of the triterpenic acid-enriched fraction (TAE) from C. paliurus and the underlying mechanism.
Materials and methods: The hyperlipidaemic rats were induced by high fat diet for 6 weeks. After oral administration of TAE (200 and 400 mg/kg), the neutral fraction (150 and 300 mg/kg) and statin (4 mg/kg) to the hyperlipidaemic rats for 4 weeks, lipid profile and apolipoprotein (apoB48) level in plasma, and the expression levels of apoB48, microsomal triglyceride transfer protein (MTP), phosphorylation of mitogen-activated protein kinase (MAPK) and tumour necrosis factor α (TNF-α) in intestine were examined. The main constituents in the TAE were identified by HPLC-MS.
Results: TAE administration (400 mg/kg) decreased the levels of atherogenic lipids in serum and liver (p < 0.05) and increased serum high-density lipoprotein cholesterol by 19.7%. Furthermore, TAE treatment (200 and 400 mg/kg) decreased plasma apoB48 level by 15.3 and 19.5%, downregulated intestinal apoB48 and MTP expression levels (p < 0.05), and inhibited TNF-α expression by 36.2 and 56.2% and the phosphorylation level of MAPK by 8.8 and 13.2%, respectively. HPLC analysis revealed the presence of pentacyclic- and tetracyclic-triterpene acids in TAE.
Conclusion and discussion: These findings suggested that TAE possessed antihyperlipidaemic activity partially involved in the inhibitory effect on apoB48 overproduction, which may provide evidence about its potential role in ameliorating dyslipidaemia.
Introduction
Cardiovascular disease is a leading cause of death and it is clinically associated with dyslipidaemia characterized by elevated levels of plasma triglyceride (TG), total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C), as well as a low concentration of the high-density lipoprotein cholesterol (HDL-C) (Natarajan et al. Citation2010). Furthermore, the excessive intake of dietary lipids can lead to hyperlipidaemia with an elevated incidence of atherosclerotic disease (Zilversmit Citation1995).
Apolipoprotein B48 (apoB48) is the constitutive protein of chylomicron, which is involved in dietary lipid absorption (Ros Citation2000). The overproduction of intestinal apoB48 might contribute to the elevation of atherogenic triglyceride-rich lipoprotein (TRL) particles in the circulation (Cohn et al. Citation1993) and an elevated risk of coronary artery disease (Masuda et al. Citation2011; Mori et al. Citation2013). Microsomal triglyceride transfer protein (MTP) catalyzes the transfer of the lipids to apoB48 in endoplasmic reticulum lumen to assemble chylomicrons. In addition, MTP binds to nascent apoB48 to prevent dislocation from the endoplasmic reticulum and avoid proteosomal degradation (Hussain et al. Citation2003; Pal et al. Citation2005).
Epidemiological studies revealed that elevated level of lipoproteins containing apoB could trigger inflammation and initiate atherosclerosis in human subjects and experimental animals (Zilversmit Citation1995; Glass & Witztum Citation2001; Mori et al. Citation2013). Tumour necrosis factor (TNF-α), a major proinflammatory cytokine, also plays a critical role in the lipid metabolism, especially lipid absorption (Chen et al. Citation2009). TNF-α has been demonstrated to stimulate p38 mitogen-activated protein kinase (MAPK) pathway activation and intestinal production of apoB48-containing lipoprotein (Qin et al. Citation2010). Moreover, SB203580, the pharmacological p38 inhibitor, could block TNF-α-induced apoB48 oversecretion in vivo and ex vivo (Qin et al. Citation2007).
Cyclocarya paliurus (Batal) Iljinskaja (Juglandaceae), known as the ‘sweet tea tree’, is an edible and medicinal plant growing on the highlands in Southern China (Shu et al. Citation1995; Xie & Li Citation2001). The leaves of the plant are often consumed as a beverage to prevent and treat hyperlipidaemia and diabetes mellitus (Xie & Li Citation2001). The plant extract has shown therapeutic effects in the treatment of dyslipidaemia, diabetes and hypertension (Ren Citation1994), as well as the inhibition of lipid peroxidation (Dong et al. Citation2007).
Polysaccharides, flavonoids and triterpenoids have been isolated from C. paliurus (Xie & Xie Citation2008). However, the active antihyperlipidaemic ingredients remain unknown. Previous work revealed that the water extract could reduce postprandial triglyceride level in hyperlipidaemic mice (Kurihara et al. Citation2003), and polysaccharides might be the active components (Huang et al. Citation2011). However, our previous findings demonstrated that the triterpenoids might be the antihyperlipidaemic constituents (Wang et al. Citation2013a), including cyclocaric acid B, arjunolic acid, corosolic acid, oleanolic acid and ursolic acid which have been isolated from the leaves of C. paliurus (Cui & Li Citation2012). Oleanolic acid and ursolic acid, naturally occurring in a large variety of plants, have been shown to increase the HDL-C level and decrease the atherogenic lipid level (Somova et al. Citation2003). Corosolic acid is reported to inhibit cholesterol acyltransferase activity (Han et al. Citation2014). Interestingly, we found that the total extract of C. paliurus leaves abundant in triterpernoids decreased the serum level of total apoB48 and the expression of TNF-α in hyperlipidaemic mice (Jiang et al. Citation2014; Ma et al. Citation2015).
Therefore, the present study was designed to evaluate the antihyperlipidaemic activity of triterpenic acid-enriched fraction of C. paliurus leaves, and probe into its potential mechanism by inhibiting apoB48 secretion in high fat diet (HFD)-induced hyperlipidaemic rats.
Materials and methods
Plant material
Fresh leaves of Cyclocarya paliurus (Batal) Iljinskaja were collected in October 2010 in the campus of the Nanjing Forestry University, China (GPS coordinates: 118.822414, 32.085054), and was authenticated by Prof. Minjian Qin of the China Pharmaceutical University. Voucher specimen (No. L20100033) was deposited in Department of Natural Medicinal Chemistry of the university.
Drugs and reagents
Simvastatin tablet from Yichang Changjiang Pharmaceutical Enterprise (Yidu, Hubei, China) was used as a reference antihyperlipidaemic drug. Assay kits for TG, TC, LDL-C, HDL-C, apoB48, superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), and glutathione peroxidase (GSH-Px) were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). Commercial diagnostic kit was obtained from the Nanjing Senbeijia Biological technology Co. (Nanjing, Jiangsu, China) for the determination of serum apoB48. Anti-apoB, anti-MTP, anti-MAPK, anti-phospho-MAPK, horseradish peroxidase-conjugated anti-rabbit and anti-goat antibodies were obtained from the Cell Signaling Technology, Inc. (Beverly, MA). Arjunolic acid, 2α, 3α, 23-trihydroxyursa-12, 20(30)-dien-28-oic acid, cyclocaric acid B, pterocaryoside B, 2α, 3α, 23-trihydroxyurs-12-en-28-oic acid, hederagenin, 3β, 23-dihydroxy-12-ene-28-ursolic acid and oleanolic acid were isolated from our laboratories with >98% purity. All the other chemicals were of analytical grade.
Preparation and analysis of the plant extracts
The dried and ground leaves (2.5 kg) of C. paliurus were extracted with 80% ethanol (3 × 20 L, each for 2 h). The combined extracts were filtered and concentrated under reduced pressure to obtain a semi-solid residue, which was suspended in water, defatted with petroleum ether and partitioned with chloroform to afford a chloroform fraction (171.9 g). The chloroform fraction was dissolved in chloroform and partitioned with 3% NaOH aqueous solution to obtain an organic phase labelled as the neutral fraction (NeE, 56.2 g). The aqueous phase was neutralized with 5% HCl and re-extracted three times with chloroform to provide the fraction enriched in triterpenic acids (TAE, 84.7 g) (Paraschos et al. Citation2007).
The triterpenic acid-enriched fraction was dissolved in HPLC grade methanol to prepare sample solution (5 mg/mL) for analysis. HPLC analysis was performed on an Agilent 1260 Infinity HPLC system equipped with a UV detector. Chromatographic separation was conducted on an Alltima C18 column (4.6 × 250 mm, 5 μm, from Alltech, Milan, Italy). The solvent system composed of solvent A (acetonitrile) and solvent B (0.01% formic acid in H2O) in the following gradient: 0–15 min, 45% A; 15–25 min, 45% A to 52% A; 25–30, 52% A; 30–40 min, 52% to 55% A; 40–50 min, 55% A; 50–80 min, 55% A to 100% A. Operating conditions were as follows: detection wavelength, 205 nm; flow rate, 1.0 mL/min; column temperature, 30 °C; injection volume, 5 μL.
The presence of triterpenoids in TAE was confirmed by a Bruker AmaZon SL LC-MS (Sparta, NJ). The MS conditions were as follows: capillary, 4500 V; end plate offset, −500 V; dry heater temperature, 180 °C; dry gas flow rate, 8.0 L·min−1; nebulizer pressure, 17.0 psi. The data acquisition was accomplished by Bruker Daltonics for MS2/MS2 fragmented ions of the components.
Animals
SD male rats (180–220 g) were purchased from the Experimental Animal Center of Nantong University (Nantong, Jiangsu, China) [Certificate No. SCXK (SU 12014-000)] and acclimatized for 7 days in the animal room. They were kept in controlled ambient temperature (24 ± 2 °C) and humidity (60 ± 10%) under a 12 h light-dark cycle; water was available ad libitum. The study protocol was approved by the Animal Ethics Committee of the Jiangsu Province Hospital of Integration of Chinese and Western Medicine. The animal protocol was approved by the Animal Care Ethics Committee of Southeast University (Dingjiaqiao Campus, Nanjing, Jiangsu, China) [Approval No. 20,140,809].
Induction of hyperlipidaemia
After the adaptation period, rats were fed with high-fat diet, consisting of 2% cholesterol, 10% lard, 10% egg yolk, 0.5% bile sodium, 77.5% standard diet (w/w) (Pang et al. Citation2002). After six weeks, the hyperlipidaemic rats showing serum cholesterol level >140 mg/dL were used as described below (Hirunpanich et al. Citation2006).
Experimental procedures
Animals were divided into 7 groups of 8 rats each as follows ():
Figure 1. Experimental procedure. High-fat-diet induced hyperlipidaemic rats were treated with Cyclocarya paliurus extracts for 4 weeks, following with the determination of lipid metabolism, antioxidant activity, serum apoB48 content and intestinal apoB48, MTP, MAPK and TNF-α expression levels. Groups: normal control (NC), hyperlipidaemic control (HC), hyperlipidaemic rats treated with neutral fraction (NL and NH) at 0.15 and 0.3 g/kg doses and triterpenic acids-enriched fraction (TL and TH) at 0.2 and 0.4 g/kg doses, respectively, and simvastatin tablets (ST).
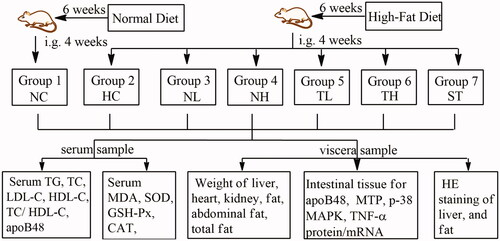
Group 1: normal control (NC), administered with 0.5% sodium carboxyl methyl cellulose (CMC-Na, 10 mL/kg/day).
Group 2: hyperlipidaemic control (HC), administered with 0.5% CMC-Na (10 mL/kg/day).
Group 3 and 4: hyperlipidaemic rats treated with the triterpenic acids-enriched fraction (TL and TH) at 200 and 400 mg/kg doses, respectively.
Group 5 and 6: hyperlipidaemic rats treated with neutral fraction (NL and NH) at 200 and 400 mg/kg doses, respectively.
Group 7: hyperlipidaemic rats treated with simvastatin (ST, positive control, 4 mg/kg) (Yi et al. Citation2001; Ma et al. Citation2015; Wang et al. Citation2013a).
The test samples were dissolved or suspended in 0.5% CMC-Na. Each group was administered by oral gavage once a day for 4 weeks. Behavioural activity, fur condition, water and food intake of the rats were observed daily. Body weight and food intake were recorded at weekly intervals until the end of the experiment.
Blood sampling and biochemical analysis
After 4-week treatment, blood samples were drawn after overnight fast from the orbital venous plexus and centrifuged at 3000 rpm for 15 min, and then the serum was stored at −80 °C until analysis.
Serum lipids level indices of TG, TC, HDL-C, LDL-C and apoB48 were measured using commercial assay kits according to the manufacturer’s directions by an automatic biochemical analyzer (Roche Modular DP, Basel, Switzerland). MDA, GSH-Px, CAT and SOD were determined following the standard protocols.
Analyses of relative organs
At the end of the experiment, overnight-fasted animals were intraperitoneally anesthetized with 10% chloral hydrate (0.3 mL/100 g), and then bloods were collected from the abdominal aorta. The heart, liver, kidney and adipose tissues (including the epididymal, perirenal and abdominal adipose tissues) were removed immediately and weighed after rinsed with physiological saline. They were stored at −80 °C until use.
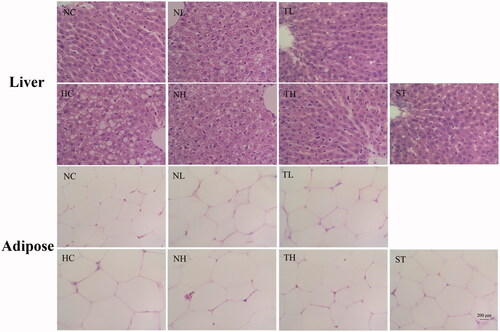
Histological evaluation of liver and fat samples by haematoxylin-eosin (HE) staining
The liver and adipose tissues were collected, immediately fixed in 10% neutral formalin for 48 h, dehydrated though the standard procedure, and embedded in low-melting point paraffin wax. Sample sections (5 μm thick) were cut and stained with haematoxylin-eosin solution.
Western blotting analysis
Intestinal tissue (100 mg) was lysed with cold lysis buffer (0.5 mL, Beyotime, Jiangsu, China) and centrifuged (13,000g for 15 min at 4 °C). The supernatants were separated for the measurement of protein concentration using a Bicinchoninic Acid Protein Assay Kit (St. Louis, MO). Proteins (50 μg) were subjected to 5% SDS-PAGE and immunoblotting was performed using Anti-apoB, anti-MTP, anti-MAPK, anti-phospho-MAPK. Then the blots were incubated with secondary horseradish peroxidase-conjugated anti-rabbit antibody. The band intensity was quantized using the IPP 6.0 software (Qin et al. Citation2009).
Real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis
Total RNA samples from small intestines were isolated using Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Wilmington, DE) with a S1000™Thermal cycler (Bio-Rad, Hercules, CA). Real-time PCR was performed in 25 μL with a Stratagene Mx3000P QPCR System (Agilent Technologies, La Jolla, CA) using the Maxima SYBR Green/ROX qPCR Master Mix (2x) (Thermo Scientific, Wilmington, DE). The program had a UDG pretreatment of 50 °C and an initial incubation of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. The sense and antisense primers sequence (5eque) for analysis are shown as follows:
apoB: TGTCTGAAGCCATCTGTAA and TCTGAAGAAGCGACTGTT;
MTP: AATAATGAGCGGCTATACAAG and TTCCTCCACAGTAACACAA;
TNF-α: GCCCTACGGGTCATTGAGAG and GAGAGACGACAGACGCAGAC;
β-actin: GTGGGTATGGGTCAGAAG and AAAGTGTGGTGCCAAATC, respectively.
The gene expression from each sample was analyzed in triplicates. mRNA abundance was calculated according to the 2-ΔΔCt method and normalized to β-actin mRNA (Jiang et al. Citation2014). Levels of the NC group were arbitrarily assigned a value of 1.
Statistical analysis
Data are expressed by the means ± standard error. The data were evaluated using the SPSS software package, Version 19.0 (Chicago, IL). Statistical significance was performed using one-way analysis of variance (ANOVA) followed by Turkey post hoc test. p values <0.05 were considered significant.
Results
Phytochemical analysis
TAE typical chromatogram was shown in by HPLC analysis. A comparison with corresponding reference standards () revealed the presence of arjunolic acid, 2α, 3α, 23-trihydroxyursa-12, 20(30)-dien-28-oic acid, cyclocaric acid B, pterocaryoside B, 2α, 3α, 23-trihydroxyurs-12-en-28-oic acid, 3β, 23-dihydroxy-12-ene-28-ursolic acid, hederagenin and oleanolic acid (), which was confirmed by LC-MS analysis (). These results indicated pentacyclic triterpenic acids were the major components of the TAE fraction. The relative content of the individual constituent was presented in .
Figure 2. The structures of the compounds (A) and high performance liquid chromatography profiles of standard mixture (B1) as well as the triterpenic acid-enriched fraction (B2) of Cyclocarya paliurus using UV detection. External standards: (1) arjunolic acid; (2) 2α, 3α, 23-trihydroxyursa-12, 20(30)-dien-28-oic acid; (3) cyclocaric acid B; (4) pterocaryoside B; (5) 2α, 3α, 23-trihydroxyurs-12-en-28-oic acid; (6) 3β, 23-dihydroxy-12-ene-28-ursolic acid; (7) hederagenin; (8) oleanolic acid.
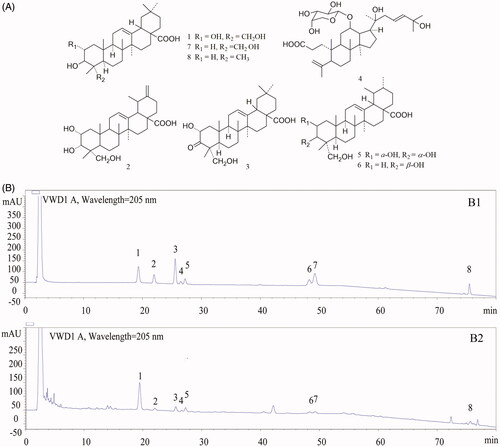
Table 1. Identification and determination of the compounds in the triterpenic acid-enriched fraction of Cyclocarya paliurus by HPLC.
Body weight, food intake and organ weight
The food consumption of all animals was monitored during the experiment. Rats in the HC group consumed a little less amount of food than that in the NC group presumably because of the unpleasant taste of the high-fat diet (). However, there was no obvious difference in food intake among the groups fed with HFD.
Table 2. Food intake of hyperlipidaemic rats (g, daily average, n = 8).
No significant difference was detected in the weight of the heart, liver and kidney in the experimental groups (). The body weight in the HC rats was significantly less than that in the NC rats, whereas the weight of adipose tissue in the HC group was remarkably higher than that in the NC group (p < 0.05). Interestingly, high-dosage of TAE treatment (TH group) led to a significant reduction in body weight, as well as in the weight of adipose tissues including abdominal fat and epididymal fat, compared to the HC group (p < 0.05). However, treatment with the neutral fraction has no observable effect on the weight of both body and the adipose tissue compared with the HC group.
Table 3 Body weight, weight of organs and adipose tissues (n = 8, mean ± S.E.).
Lipid profile in hyperlipidaemic rats
Effects of the plant extracts on serum lipid profiles in the experimental rats are shown in . It is clear that high-fat diet significantly led to the increased levels of TC, TG and LDL-C, as well as the decreased level of HDL-C compared to the NC group (p < 0.01). After TAE treatment, the levels of TC, TG and LDL-C were significantly reduced (p < 0.05), while the level of HDL-C was elevated compared to the HC group (TH, 19.7%, p < 0.05, respectively). These results suggested TAE administration led to a notable reduction of serum atherogenic index (TC/HDL-C) in treated rats compared with the HC group (TH, p < 0.01). However, little change was observed in the NL and NH groups. Additionally, the increased levels of hepatic TC and TG induced by the HFD were notably alleviated in the TH group (p < 0.01) (), whereas no similar effect was observed in the NL and NH groups.
Figure 3. Effects of different extracts on hepatic TG and TC levels in hyperlipidaemic rats (mmol/L, n = 8, mean ± S.E.). *p < 0.05, **p < 0.01 compared with NC; #p < 0.05, ##p < 0.01 compared with HC.
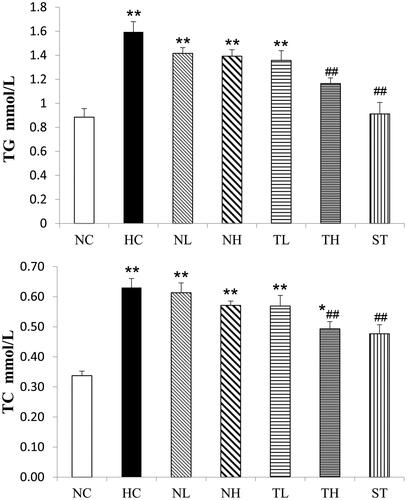
Table 4. Effects of different extracts on lipids and lipoproteins in hyperlipidaemic rats (n = 8, mean ± S.E.).
MDA and antioxidant enzyme activities in hyperlipidaemic rats
Compared to the NC group, a significant increase in serum MDA concentration and decrease in GSH-Px, SOD and CAT activities were observed in the HC group (, p < 0.01). However, the changes of serum MDA level and SOD and CAT activities were clearly improved by treatment with high-dose of TAE (p < 0.05), not by the NeE treatment.
Table 5. Effects of different extracts on serum MDA level, SOD, GSH-Px and CAT activities in hyperlipidaemic rats (n = 8, mean ± S.E.).
Histopathological analysis
Hepatohistological analysis indicated the presence of steatosis and cytoplasmic vacuoles in the livers of hyperlipidaemic rats (). Nevertheless, treatment with TAE alleviated the liver lesions in rats. When compared to the NC group, an enlargement of the adipocytes in the adipose tissues from the HC group was observed by light microscopy, while the rats in the TAE and ST treatment groups exhibited a reduction in size of the adipocytes ().
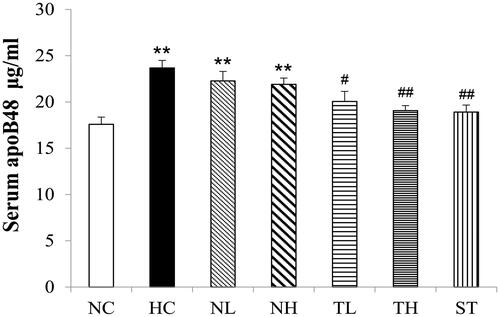
Serum apoB48 in hyperlipidaemic rats
Data from indicated that HFD feeding led to an elevation of serum apoB48 level compared to the normal control (p < 0.01). However, TAE treatment (200 and 400 mg/kg) decreased the serum apoB48 levels by 15.3 and 19.4%, respectively. High-dose treatment of TAE (400 mg/kg) brought the concentrations back to a level comparable the NC group (p < 0.01). No significant effects were observed in rats treated with NeE.
Intestinal apoB48, MTP protein and mRNA levels in hyperlipidaemic rats
Intestinal apoB48, MTP protein and mRNA levels were presented in . HFD feeding resulted in the remarkable elevation of intestinal apoB48 protein and mRNA level to 1.68-fold and 1.56-fold that of the NC group, respectively (p < 0.01). Both TAE and simvastatin treatment reduced the increased protein and mRNA level of apoB48 (p < 0.05), but NeE group did not show similar effect.
Figure 6. Effects of different extracts on intestinal protein (A) and mRNA (B) levels of apoB48 and MTP protein. Results are expressed as mean ± S.E. of three independent experiments. *p < 0.05, **p < 0.01 compared with NC; #p < 0.05, ##p < 0.01 compared with HC.
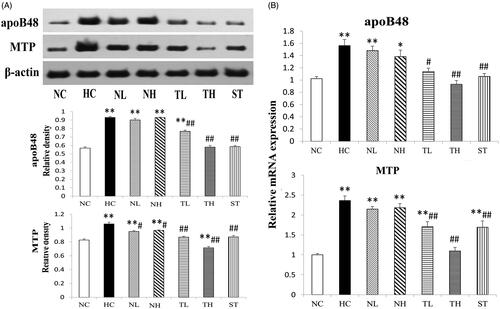
The protein and mRNA expression levels of MTP in HFD-fed rats were dramatically up-regulated by 1.28-fold and 2.36-fold compared to the NC group (p < 0.01), while down-regulated levels were obtained in rats with TAE (21.7% and 48.4% decrease at 200 and 400 mg/kg, respectively) and ST administration (p < 0.01). NeE treatment led to a significant regulation on MTP protein (p < 0.05) and a weak effect on the RNA expression level (not significantly).
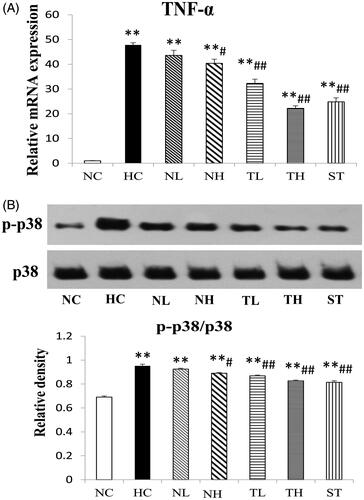
TNF-α and MAPK expressions in hyperlipidaemic rats
To demonstrate the effect of TAE on intestinal TNF-α/p38 MAPK pathway, the mRNA of TNF-α and the phosphorylation level of p38 MAPK in intestinal tissue were determined by RT-PCR and western blot experiment, respectively (). As expected, the mRNA level of TNF-α in the HC group was up-regulated about 47-fold compared to NC group, while significantly down-regulated by TAE treatment by 36.2 and 56.2% (p < 0.01), respectively (). The phosphorylation level of p38 MARK were markedly increased about 1.38-fold in the HC group (, p < 0.01), but subsequently decreased after treatment with the plant extracts (p < 0.05), especially TAE (TL and TH, 8.8 and 13.2% decrease, respectively, p < 0.01). These data indicated C. paliurus down-regulated the TNF-α/MAPK pathway in the hyperlipidaemic rodents, which was consistent with the previous study (Ma et al. Citation2015).
Figure 7. Effects of different extracts on p38 MAPK phosphorylation (A) and TNF-α mRNA levels (B) in hyperlipidaemic rats. P-p38 was determined by western blot. Results are expressed as mean ± S.E. of three independent experiments. *p < 0.05, **p < 0.01 compared with NC; #p < 0.05, ##p < 0.01 compared with HC.
Discussion
Evidence was available to demonstrate the beneficial effects of C. paliurus extracts on hyperlipidaemia (Ren Citation1994; Chen et al. Citation2002; Kurihara et al. Citation2003). However, the active compounds remain unclear. In the present study, a triterpenic acid-enriched fraction of C. paliurus leaves exerted the antihyperlipidaemic effect on a HFD-induced hyperlipidaemia rats. The results indicated that TAE could decrease the elevated level of TG, TC and LDL-C, as well as increase the HDL-C level. TAE also ameliorated dyslipidaemia by inhibiting the overproduction of intestinal apoB48. The underlying mechanism might be attributed to downregulating MTP expression level and the TNF-α/p38MAPK signalling pathway.
Dyslipidaemia is mainly resulting from a sedentary life style and fat-rich diets (Naser et al. Citation2008). Hyperlipidaemia is known to elevate levels of lipid peroxidation, and the products such as MDA has been associated with the deposition of LDL on vascular wall (Delgado Roche et al. Citation2012). High TC/HDL ratio, the atherogenic index (AI), indicates a higher risk of atherosclerosis development (Xia et al. Citation2011). Therefore, our study applied HFD feeding to induce dyslipidaemia in rats. TAE exerted the beneficial effects to mitigate dislipidaemia and the antioxidant properties in the HFD-induced hyperlipidaemia rats. These findings further support the antihyperlipidaemic activities of TAE from C. paliurus and its potential effect in the treatment of cardiovascular disorder.
Apolipoproteins are the primary organizing protein components of the lipoproteins. ApoB48, in response to dietary lipids, is mainly synthesized in enterocytes and involved in chylomicron assembly (Pal et al. Citation2005). ApoB48 also has been used as a measure of intestinal TRL particles (Zilversmit Citation1995). A high concentration of plasma apoB48 is associated with plaques formation that causes vascular diseases and is believed to be a reliable indicator of cardiovascular diseases (Mori et al. Citation2013). Lipid-lowering agents including simvastatin and natural products like green tea polyphenols have been reported to reduce the level of plasma intestinal-derived apoB48 (Borthwick et al. Citation2014). Our previous work also revealed C. paliurus leaves extract remarkably decreased plasma total- and TRL-apoB48 levels (Ma et al. Citation2015). In the present study, TAE also improved the elevation of serum apoB48 level, as well as down-regulated the expression level of intestinal apoB48. The antihyperlipidaemic efficacy might be attributed to the TAE inhibitory effect on the intestinal apoB48 overproduction and secretion.
De novo synthesized apoB48 molecules are either secreted within chylomicrons or intracellularly degraded by proteases (Chen et al. Citation1987; Welty et al. Citation1999). MTP facilitates the translocation of newly synthesized apoB48 to the endoplasmic reticulum, thereby protects apoB from intracellular degradation in the intestine (Hussain et al. Citation2003). In our study, the decreased expression of MTP might be owing to the down-regulatory effect of TAE on intestinal apoB48 secretion.
Inflammation is an important aetiology underlying lipid metabolism disorders (Chen et al. Citation2009). TNF-a, an inflammatory cytokine, is one of the agonists of the MAPK pathway and plays a potential role in the metabolism of apoB48 (Yun et al. Citation2009; Qin et al. Citation2007, Citation2010). It is demonstrated that TNF-α stimulation on intestinal apoB48 production and MTP expression might be mediated via the p38 MAPK pathway (Qin et al. Citation2007). Moreover, MAPKs was reported to interfere with apoB48 production and MTP activity by regulating the expressions of CD36 (Yun et al. Citation2009), a transmembrane protein involved in lipid absorption (Tran et al. Citation2011). In the present study, TAE inhibited TNF-α expression and p38 phosphorylation in hyperlipidaemia rats, which was consistent with the previous report (Ma et al. Citation2015). We speculated that TAE inhibitory effect on intestinal apoB48 overproduction might be partially mediated by the TNF-α/p38 MAPK signalling pathway. Further investigation is warranted to delineate the underlying mechanism(s) associated with the regulation effect of CD36 on apoB48 metabolism.
The main components of TAE were identified according to the retention times and quasi-molecular ion peaks compared with authentic reference compounds. The results revealed the presence of dammarane-, ursane- and oleanane-type triterpenic acids, including arjunolic acid, 2α, 3α, 23-trihydroxyursa-12, 20(30)-dien-28-oic acid, cyclocaric acid B, pterocaryoside B, 2α, 3α, 23-trihydroxyurs-12-en-28-oic acid, 3β, 23-dihydroxy-12-ene-28-ursolic acid, hederagenin and oleanolic acid, in the TAE fraction. Arjunolic acid and 3β, 23-dihydroxy-12-ene-28-ursolic acid possess an inhibitory activity of cholesterol acyltransferase, and exerted antihyperlipdemic effect (Kim et al. Citation2005; Khaliq et al. Citation2013; Han et al. Citation2014). In addition, oleanolic acid has been reported to decrease serum lipids and reduce hepatic lipid accumulation (Wang et al. Citation2013b). Moreover, 3β, 19α-dihydroxyurs-12, 20(21)-diene-28-oic acid can reduce the elevated lipid level in streptozotocin-induced diabetic mice (Gutiérrez et al. Citation2009). Echinocystic acid showed inhibitory effect on cholesterol acyltransferase activity (Han et al. Citation2014). Our results further support that triterpenic acids can ameliorate hyperlipidaemia. Taken together, it is reasonable to speculate that the triterpenic acids might be responsible for the lipid-lowering effects of C. paliurus leaves. Further studies are necessary to investigate the structure-activity relationships and the antihyperlipidaemic mechanism(s).
Conclusion
The triterpenic acid-enriched fraction of C. paliurus leaves could mitigate dyslipidaemia by decreasing the atherogenic lipid levels in serum and liver, and increasing the antioxidant enzyme activities. The underlying antihyperlipidaemic effect may be partially mediated by inhibiting the production of intestinal apoB48 through the TNF-α/p38 MAPK signalling pathway.
Zhiqi_Yin_et_al_Supplemental_content.zip
Download ()Acknowledgements
The authors thank Prof. Minjian Qin (China Pharmaceutical University) for authenticating the plant material.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Borthwick F, Mangat R, Warnakula S, Jacome-Sosa M, Vine DF, Proctor SD. 2014. Simvastatin treatment upregulates intestinal lipid secretion pathways in a rodent model of the metabolic syndrome. Atherosclerosis. 232:141–148.
- Chen MQ, Liang JY, Jiao ZH, Guo YH, Meng GZ, Shen XQ, Li F, Zhang RS, Chen LX GB. 2002. Clinical observation on regulation action of Green Qian Willow tea on blood lipid level. Chin J Pr Chin Mod Med. 2:863–865.
- Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, Cai SJ, Deslypere JP, Rosseneu M, et al. 1987. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 238:363–366.
- Chen X, Xun K, Chen L, Wang Y. 2009. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 27:407–416.
- Cohn JS, Johnson EJ, Millar JS, Cohn SD, Milne RW, Marcel Y, Russell R, Schaefer E. 1993. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postprandial increases in the plasma concentration of TRL triglycerides and retinyl esters. J Lipid Res. 34:2033–2040.
- Cui B, Li S. 2012. Chemical constituents from leaves of Cyclocarya paliurus. Chin Tradit Herb Drugs. 43:2132–2136.
- Delgado Roche L, Acosta Medina E, Fraga Perez A, Becquer Viart MA, Soto Lopez Y, Falcon Cama V, Vazquez Lopez AM, Martinez-Sanchez G, Fernandez-Sanchez E. 2012. Lipofundin-induced hyperlipidemia promotes oxidative stress and atherosclerotic lesions in New Zealand white rabbits. Int J Vas Med. 2012:898769.
- Dong CJ, Xie MY, Nie SP, Xie JH, Jia SH, Huang P. 2007. Study on antioxidant activity of Cyclocarya paliurus (Bata1.) Iljinskaya extracts in vitro. Food Sci. 28:31–34.
- Glass CK, Witztum JL. 2001. Atherosclerosis. the road ahead. Cell. 104:503–516.
- Gutiérrez RMP, Solis RV, Baez EG, Navarro YG. 2009. Hypoglycemic activity of constituents from Astianthus viminalis in normal and streptozotocin-induced diabetic mice. J Nat Med. 63:393–401.
- Han L, Lai P, Du JR. 2014. Deciphering molecular mechanism underlying hypolipidemic activity of echinocystic acid. Exid-Based Compl Alt. 2014:823154.
- Hirunpanich V, Utaipat A, Morales NP, Bunyapraphatsara N, Sato H, Herunsale A, Suthisisang C. 2006. Hypocholesterolemic and antioxidant effects of aqueous extracts from the dried calyx of Hibiscus sabdariffa L. in hypercholesterolemic rats. J Ethnopharmacol. 103:252–260.
- Huang MQ, Shang GXC, Xu MS, Wang WJ, Shen YG, Jiang Y, Chen Q, Chen TT. 2011. Studies on hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja polysaccharide. Acta Agr U Jiangxi. 33:157–161.
- Hussain MM, Shi J, Dreizen P. 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 44:22–32.
- Jiang C, Yao N, Wang Q, Zhang J, Sun Y, Xiao N, Liu K, Huang F, Fang S, Shang X, et al. 2014. Cyclocarya paliurus extract modulates adipokine expression and improves insulin sensitivity by inhibition of inflammation in mice. J Ethnopharmacol. 153:344–351.
- Khaliq F, Parveen A, Singh S, Hussain ME, Fahim M. 2013. Terminalia arjuna improves cardiovascular autonomic neuropathy in streptozotocin-induced diabetic rats. Cardiovas Toxicol. 13:68–76.
- Kim DH, Han KM, Chung IS, Kim DK, Kim SH, Kwon BM, Jeong TS, Park MH, Ahn EM, Baek NI. 2005. Triterpenoids from the flower of Campsis grandiflora K. Schum. as human Acyl-CoA: cholesterol acyltransferase inhibitors. Arch Pharm Res. 28:550–556.
- Kurihara H, Asami S, Shibata H, Fukami H, Tanaka T. 2003. Hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol Pharm Bull. 26:383–385.
- Ma Y, Jiang C, Yao N, Li Y, Wang Q, Fang S, Shang X, Zhao M, Che C, Ni Y, et al. 2015. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J Ethnopharmacol. 166:286–296.
- Masuda D, Sakai N, Sugimoto T, Kitazume-Taneike R, Yamashita T, Kawase R, Nakaoka H, Inagaki M, Nakatani K, Yuasa-Kawase M. 2011. Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb. 18:1062–1070.
- Mori K, Ishida T, Yasuda T, Monguchi T, Sasaki M, Kondo K, Hasokawa M, Nakajima H, Haraguchi Y, Sun L, et al. 2013. Fasting serum concentration of apolipoprotein B48 represents residual risks in patients with new-onset and chronic coronary artery disease. Clin Chim Acta. 421:51–56.
- Naser A, Jafar S, Kumar GV, Hadi HS, Saeed D, Mohamadreza S, Hossein MA. 2008. Cardiac risk factor changes through an intensive multifactorial life style modification program in CHD patients: results from a two year follow up. J Biol Sci. 8:248–257.
- Natarajan P, Ray KK, Cannon CP. 2010. High-density lipoprotein and coronary heart disease: current and future therapies. J Am Coll Cardiol. 55:1283–1299.
- Pal S, Ho SS, Takechi R. 2005. Red wine polyphenolics suppress the secretion of ApoB48 from human intestinal CaCo-2 cells. J Agr Food Chem. 53:2767–2772.
- Pang X, Yao M, Lu Y, Gong Q. 2002. Effect of soy isoflavones on malondialdehyde and superoxide dismutase of blood and liver in hypercholesterolemia rats. Chin J New Drugs Clin Remedies. 21:257–260.
- Paraschos S, Magiatis P, Mitakou S, Petraki K, Kalliaropoulos A, Maragkoudakis P, Mentis A, Sgouras D, Skaltsounis AL. 2007. In vitro and in vivo activities of Chios mastic gum extracts and constituents against Helicobacter pylori. Antimicrob Agents Chemother. 51:551–559.
- Qin B, Dawson H, Anderson RA. 2010. Elevation of tumor necrosis factor-alpha induces the overproduction of postprandial intestinal apolipoprotein B48-containing very low-density lipoprotein particles: evidence for related gene expression of inflammatory, insulin and lipoprotein signaling in enterocytes. Exp Biol Med (Maywood). 235:199–205.
- Qin B, Polansky MM, Sato Y, Adeli K, Anderson RA. 2009. Cinnamon extract inhibits the postprandial overproduction of apolipoprotein B48-containing lipoproteins in fructose-fed animals. J Nutr Biochem. 20:901–908.
- Qin B, Qiu W, Avramoglu RK, Adeli K. 2007. Tumor necrosis factor-alpha induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes. 56:450–461.
- Ren L. 1994. Fundamental research and clinical observations of Cyclocarya paliurus. Jiangxi U Tradit Chin Med. 25:64–65.
- Ros E. 2000. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis. 151:357–379.
- Shu RG, Xu CR, Li LN, Yu ZL. 1995. Cyclocariosides II and III: Two secodammarane triterpenoid saponins from Cyclocarya paliurus. Planta Med. 61:551–553.
- Somova L, Nadar A, Rammanan P, Shode F. 2003. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine. 10:115–121.
- Tran TT, Poirier H, Clement L, Nassir F, Pelsers MM, Petit V, Degrace P, Monnot MC, Glatz JFC, Abumrad NA. 2011. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 286:25201–25210.
- Wang Q, Jiang C, Fang S, Wang J, Ji Y, Shang X, Ni Y, Yin Z, Zhang J. 2013a. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J Ethnopharmacol. 150:1119–1127.
- Wang X, Liu R, Zhang W, Zhang X, Liao N, Wang Z, Li W, Qin X, Hai C. 2013b. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol. 376:70–80.
- Welty FK, Lichtenstein AH, Barrett PHR, Dolnikowski GG, Schaefer EJ. 1999. Human apolipoprotein (Apo) B-48 and ApoB-100 kinetics with stable isotopes. Arterioscl Throm Vas. 19:2966–2974.
- Xia W, Sun C, Zhao Y, Wu L. 2011. Hypolipidemic and antioxidant activities of Sanchi (Radix Notoginseng) in rats fed with a high fat diet. Phytomedicine. 18:516–520.
- Xie MY, Li L. 2001. Review in studies on chemical constituents and bioactivities of Cyclocarya paliurus. Chin Tradit Herb Drugs. 32:365–366.
- Xie MY, Xie JH. 2008. Review about the research on Cyclocarya paliurus (Batal.) Iljinskaja. J Food Sci Biotechnol. 27:113–121.
- Yi X, Xie MY, Wen HL, Deng ZY. 2001. Hypoglycemic effects of Cyclocarya Paliurus (Batal.) Iljinsk. on alloxan diabetic mice. Nat Prod Res Dev. 13:52–57.
- Yun MR, Im DS, Lee SJ, Park HM, Bae SS, Lee WS, Kim CD. 2009. 4-Hydroxynonenal enhances CD36 expression on murine macrophages via p38 MAPK-mediated activation of 5-lipoxygenase. Free Radic Biol Med. 46:692–698.
- Zilversmit DB. 1995. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich remnant lipoproteins. Clin Chem. 41:153–158.