Abstract
Context: Medicinal plants have attracted global attention for their hidden therapeutic potential. Clinacanthus nutans (Burm.f) Lindau (Acanthaceae) (CN) is endemic in Southeast Asia. CN contains phytochemicals common to medicinal plants, such as flavonoids. Traditionally, CN has been used for a broad range of human ailments including snake bites and cancer.
Objectives: This article compiles the ethnomedicinal uses of CN and its phytochemistry, and thus provides a phytochemical library of CN. It also discusses the known pharmacological and biological effects of CN to enable better investigation of CN.
Methods: This literature review was limited to articles and websites published in the English language. MEDLINE and Google Scholar databases were searched from December 2014 to September 2016 using the following keywords: "Clinacanthus nutans" and "Belalai gajah". The results were reviewed to identify relevant articles. Information from relevant selected studies was systematically analyzed from contemporary ethnopharmacological sources, evaluated against scientific literature, and extracted into tables.
Results: The literature search yielded 124 articles which were then further scrutinized revealing the promising biological activities of CN, including antimicrobial, antiproliferative, antitumorigenic and anti-inflammatory effects. Few articles discussed the mechanisms for these pharmacological activities. Furthermore, CN was beneficial in small-scale clinical trials for genital Herpes and aphthous stomatitis.
Conclusion: Despite the rich ethnomedicinal knowledge behind the traditional uses of CN, the current scientific evidence to support these claims remains scant. More research is still needed to validate these medicinal claims, beginning by increasing the understanding of the biological actions of this plant.
Introduction
Natural product drug discovery is a vibrant research area traversing nearly all scientific fields. Natural products, such as medicinal plants, serve as a rich potential source of new therapeutic compounds (Ramesh et al. Citation2014). In many countries, traditional medicinal plants have been used to treat a plethora of ailments, and this precious ethnomedicinal knowledge has been passed on over many generations. For as long as they have inhabited the earth, humans have used plants as a medicinal source. Plant-based drug development has grown more sophisticated, with modern chemists using compounds isolated from plants as structural leads to generate novel compounds with additional benefits, such as lower toxicity or higher efficacy in drug-resistant diseases.
Most medicinal plants have been studied for a range of biological activities including anticarcinogenic, anti-inflammatory and antimicrobial activities. These activities were further evaluated to identify potential therapeutic benefits for various human ailments such as cancers, autoimmune diseases and chronic infections. Continuing the quest for plant-based natural products is critical because plants contain many potentially novel therapeutic compounds. Clinacanthus nutans (Burm.f) Lindau (Acanthaceae) (CN) has been selected for the focus of this review as the plant has garnered much attention from social media about its potential therapeutic benefits. CN is one of many medicinal plants traditionally used to treat various diseases and injuries such as skin rashes, burns, fever and snakebite (Aslam et al. Citation2015). Some cancer patients have claimed that CN leaf consumption has helped in treating their cancer and improving their health, although there is a lack of clinical studies to support these claims (Shim et al. Citation2013).
To undertake a systematic and extensive review, literature was searched from various computerized databases (MEDLINE and Google Scholar) up to September 2016 as available on PubMED. Moreover, this literature review was limited to websites and articles published in the English language. The following keywords were used: “Clinacanthus nutans”, “Sabah Snake Grass” and “Belalai gajah”. The results were reviewed to identify relevant articles. Contemporary sources of knowledge were also used to compare the ethnopharmacological information against the scientific literature available. Data from the selected studies was extracted systematically into tables for analysis. A total of 124 articles were found using the search method described above revealing promising biological activities of CN, including antimicrobial, antiproliferative, antitumorigenic and anti-inflammatory effects.
Clinacanthus nutans botany
CN is known in different countries by various vernacular names listed in , such as belalai gajah in Brunei Darussalam and Malaysia. The plant is native to the tropical regions of Southeast Asia, particularly Thailand, and also grows in southern China and some temperate regions (Hanelt Citation2001). It is suggested in the Plant List A (Citation2013) that the plant Justicia nutans Burm is synonymous with CN. Clinacanthus siamensis has also been identified as another name for CN; however, at least two studies and ‘The Plant List B’ (Citation2013) has made a distinction between C. siamensis and CN (The Plant List B Citation2013; Kunsorn et al. Citation2013; Fong et al. Citation2014). Moreover, it is also confused with Andrographis paniculata (Burm. f.) Nees (Indian Snake grass), in the same family, because of their similar names (Fong et al. Citation2014).
Table 1. Vernacular names of Clinacanthus nutans.
The Acanthaceae family includes approximately 250 genera and 2500 species of dicotyledonous flowering plants that are mostly herbs and shrubs (Mohlenbrock Citation2008). CN is a shrub that can grow up to 1 to 3 m tall and has pubescent branches. Its stems are cylindrical, striated and smooth, while its leaves are lanceolate, long, narrow and oppositely arranged, and measure approximately 0.5–4 cm in width and 2.5–13 cm in length (). The leaf has a pointed apex, and its margin is either exsculptate-dentate or subentire. The leaf base is rounded and pubescent on the nerves, and the length of the petiole is approximately 3–15 mm (Mat Ali et al. Citation2010).
CN has flowers that are arranged in a simple inflorescence (a cluster of flowers) called cyme at the tip of the branches, and each cyme has 5 to 8 flowers (). The flower has a dark red corolla with a green base that measures approximately 4–6 cm. The lower lip of the flower has yellow streaks and is positioned upwards. The upper lip of the flower is located in the throat and is triangular with 2 stamens. The CN plant has a compacted 2-celled ovary with 2 ovules in each cell, and its style is filiform and shortly bidentate. Its capsule has an oblong shape and is basally contracted into a 4-seeded short, solid stalk and the seed is approximately 2 mm in diameter (Deng et al. Citation2002). The plant can be propagated through stem cutting (Mat et al. Citation2010).
Because CN leaves are widely sold as commercial products with the plant often harvested before it can flower, it has been suggested that the extensive propagation and harvest of the plant for commercial uses may hinder the plant from developing its sexual reproduction to maturity, thus resulting in a lack of flowers (Fong et al. Citation2014). Therefore, CN flowers are rare, and there is little information on how long it takes for the plant to flower and to fruit.
Ethnomedicinal uses of CN
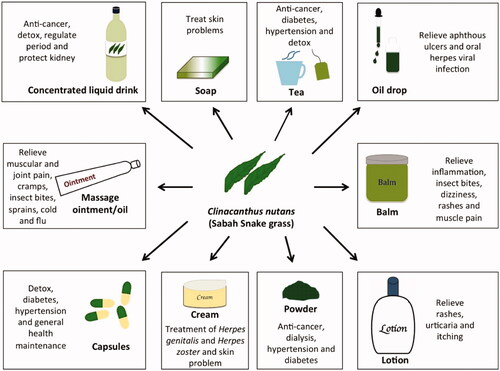
CN is commonly used in traditional medicine in the Southeast Asian region, particularly in Thailand and Malaysia. Many traditional therapeutic uses for CN have been reported (), but clinical and scientific data support only a few of these. In general, only the leaves of CN are used in traditional medicine. The use of CN to treat Herpes virus infections is supported by the results of various scientific studies and clinical trials (Sangkitporn et al. Citation1993, Citation1995; Yoosook et al. Citation1999; Lipipun et al. Citation2011; Kongkaew & Chaiyakunapruk Citation2011; Kunsorn et al. Citation2013). CN leaves were initially consumed for general health in various countries in Southeast Asia (Shim et al. Citation2013; Siew et al. Citation2014). However, CN is gaining popularity in Malaysia and Singapore because of claims of its anticancer properties (). This has led to the availability of a wide variety of commercial products, including teas, drinks and powders ().
Table 2. Traditional and modern uses of Clinacanthus nutans.
Phytochemical review of CN
Dampawan et al. (Citation1977) isolated lupeol and β-sitosterol crystals using ether and light petroleum solvent system, after having extracted the stem of CN in a Soxhlet apparatus with light petroleum. X-ray diffraction of these crystals revealed discrete molecules without short intermolecular contacts. Teshima et al. (Citation1998) isolated sulfur-containing glucosides, clinacoside A and clinacoside B, from the colorless butanol soluble fraction of the methanol extract prepared from the CN stem and leaves. These phytoconstituents were identified using chemical and spectroscopic methods.
Based on reports that plant extracts had activity against the Herpes simplex virus (HSV), from pharmacological and clinical studies (Jayavasu et al. Citation2013), Janwitayanuchit et al. (Citation2003) synthesized monoglycosyl diglycerides and investigated their structure–activity relationships. In this study, the fatty acyl and sugar moieties were identified as being critical for HSV inhibition and 1,2-O-dilinolenoyl-3-O-β-d-glucopyranosyl-sn-glycerol had the highest anti-HSV activity.
Sakdarat et al. (Citation2006) extracted CN with chloroform and chromatographically separated different varieties of chlorophyll A and chlorophyll B (Sakdarat et al. Citation2006) and a total of eight compounds were isolated. Those related to chlorophyll A were 132-hydroxy-(132-S)-phaeophytin A, 132-hydroxy-(132-R)-phaeophytin A, purpurin-18-phytyl ester and phaeophorbide A. Those related to chlorophyll B were 132-hydroxy-(132-S)-chlorophyll B, 132-hydroxy-(132-R)-chlorophyll B, 132-hydroxy-(132-S)-phaeophytin B and 132-hydroxy-(132-R)-phaeophytin B. Additionally, trigalactosyl and digalactosyl diglyceride compounds were isolated from CN leaves and had anti-HSV activity.
Under similar conditions, the same group of researchers (Sakdarat et al. Citation2009) isolated three more chlorophyll derivatives of pheophytins, 132-hydroxy-(132-R)-pheophytin B, 132-hydroxy-(132-S)-pheophytin a and 132-hydroxy-(132-R)-pheophytin A. These compounds were characterized using 1H NMR and 13C NMR spectroscopy and had anti-HSV activity.
Using GC-MS, Yong et al. (Citation2013) identified 14 chemical constituents (n-pentadecanol; eicosane; 1-nonadecene; heptadecane; dibutylphthalate; n-tetracosanol-1; heneicosane; behenic alcohol; 1-heptacosanol; 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester; nonadecyl heptafluorobutyrate; eicosayl trifluoroacetate; 1,2-benzenedicarboxylic acid, dinonyl ester; and phthalic acid dodecyl nonylester) from chloroform, methanol and aqueous leaf extracts of CN. The 1,2-benzenedicarboxylic acid mono (2-ethylhexyl) ester was the major chemical constituent, with a relative peak area of approximately 28.6% while those of the others were each less than 2%. Chelyn et al. (Citation2014) identified C-glycosidic flavones, including isovitexin, vitexin, isoorientin, orientin and shaftoside, in an ethanol extract of CN leaves. These flavones were optimized and validated with an HPLC method for quantification and quality control of herbal materials.
Tu et al. (Citation2014) isolated phytochemicals containing sulfur, clinamides A, B and C and 2-cis-entadamide A, from ethanol extracts of the aerial parts of CN. Clinamide A, a pale yellow oil, was assigned the molecular formula, C6H11NO4S by 1H-NMR spectroscopy and exhibited a methylsulfonyl signal and two methylene signals, including a trans-disubstituted double bond. Clinamide B was assigned a molecular formula of C8H13NO4S by HRESIMS (high-resolution electrospray ionization mass spectrometry), and its 13C-NMR spectrum was similar to that of entadamide C, but with an additional acetyl group. The partial NMR spectrum of clinamide C, a pale yellow oil, also indicated a close structural similarity to entadamide A and HRESIMS determined its molecular formula C12H22N2O4S2. HRESIMS assigned the molecular formula C6H11NO2S to both 2-cis-entadamide A and entadamide A, indicating that they are geometric isomers.
Yang et al. (Citation2013) isolated saponins, phenolics, flavonoids, diterpenes and phytosterols from methanol extracts of CN leaves with a 15% w/w yield and with approximately 1.77 mg gallic acid equivalents per g of total phenolic content.
Use of CN leaves for various pharmacological purposes has increased exponentially because of information on the internet. Plant harvesting and preparation of its parts prior to extraction are of paramount importance, influencing the quantity and quality of the phytoconstituents extracted. Raya et al. (Citation2015) assessed effects of storage duration on the phytochemical content of CN stems and leaves at different stages of harvesting. Phenolic content was 26% and 90% higher in younger leaves and stems, respectively, compared with their mature counterparts. Moreover, parts of mature plants had lower contents of phytochemicals, chlorophyll, and ascorbic acid compared with those from young plants. Also, prolonged storage reduced levels of these CN constituents. After storage for 4 d, the contents of total phenolics and chlorophyll were reduced to 50% and 25%, respectively, of the amounts in freshly prepared CN parts. Such evidence demands that fresh plant parts be used to avoid phytochemical loss and, thereby, optimize efficacy.
Huang et al. (Citation2015) extracted dried aerial parts of CN using ethanol, purified the crude extract and identified compounds by HPLC with tandem mass spectrometry (LC/MS/MS). 1H-NMR analysis provided additional confirmation of compound identity. Flavonoids such as shaftoside, apigenin 6,8-C-α-l-pyranarabinoside, orientin, isoorientin, vitexin and isovitexin were observed in this study.
Several parameters such as solvent characteristics, prior plant preparation and thermal degradation influence the phytochemicals extracted from the plant. The extraction technique is a key factor determining the amounts and the natures of the phytoconstituents obtained. Mustapa et al. (Citation2015) compared extraction efficiencies with microwave-assisted extraction (MAE), pressurized microwave-assisted extraction (P-MAE), supercritical carbon dioxide extraction (SFE) and the Soxhlet method. They reported on yields, extraction times and recovery of phytoconstituents, specifically, phenols, flavonoids, phytosterols and β-sitosterol. While MAE resulted in the highest yields of polyphenol and flavonoids, SFE was the best method for extracting phytosterols and β-sitosterol. P-MAE resulted in slightly improved yields of polyphenol and flavonoids. Overall, the study concluded that MAE was the most efficient extraction technique for CN, giving high extraction efficiency and better selectivity, compared with the other techniques, for compounds of nutraceutical interest, including those with anti-inflammatory, antioxidant and antimicrobial activities (Mustapa, Martin, Mato et al. Citation2015). The study further explored the influence of ethanol concentration and applied microwave energy and solvent-to-feed ratio on CN extraction using a microwave-assisted technique. Microwave pretreatment improved extraction rates by a factor of 2-5 fold, with water: ethanol (1:1) solvent (Mustapa, Martin, Gallego et al. 2015). shows the chemical structures discussed in the phytochemistry section.
Table 3. Chemical structures of compounds isolated from Clinacanthus nutans.
Metabolomics generate metabolic fingerprints of an organism by identifying and quantifying its metabolites, which enhances the understanding of chemical variability among various organisms. Khoo et al. (Citation2015) used nuclear magnetic resonance (NMR) to analyze the metabolite profile of CN leaves and stems, which were stratified based on two techniques: firstly, drying including air, oven, and freeze; secondly, extraction including soaking and sonication methods. Compared to leaves, stems contained a higher amount of terpenoids and phenolic compounds, correspondingly the activity levels of total phenolic content, α-glucosidase inhibition and antioxidant were higher as well, confirming to the well-established compound-activity correlation. Drying and extraction methods affect the yield of various phytoconstituents in extracts and thus its pharmacological activity. Partial least-squares analysis (PLS) biplot model analysis of the NMR revealed the superiority of oven and air drying methods over freeze drying and soaking methods for their yield of terpenoids, phenolic compounds, and glucosides, which were further confirmed by their corresponding better biological activities.
Huang et al. (Citation2016) successfully attempted the purification and analysis of a novel polysaccharide-peptide complex from the leaves of CN, which showed significant promising results for gastric cancer cells SGC-7901 inhibition. The monosaccharide analysis, FTIR, 1H NMR and methylation analysis revealed the presence of CN polysaccharide which includes l-rhamnose and a backbone 1-6 linked Galp residues, while atomic force microscopy (AFM) displayed the entangled and branched structure of the compound. Identification of compounds will lead to the determination of the structure-activity relationship to develop it into a lead molecule. Multi-targeted therapy by plant extract as a single herb regimen either as the adjuvant or main treatment therapy calls for the systematic investigation of purified compounds of their beneficial therapeutic activities and mechanism to optimize them toward clinical studies.
Pharmacological activities
CN is a medicinal plant with promising therapeutic potential. Many investigators have reported antioxidative (Pannangpetch et al. Citation2007; Arullappan et al. Citation2014), antiproliferative (Yong et al. Citation2013; Ghasemzadeh et al. Citation2014), antitumorigenic (Huang et al. Citation2015), antibacterial (Chomnawang et al. Citation2009; Arullappan et al. Citation2014), antiviral (Sangkitporn et al. Citation1993; Kunsorn et al. Citation2013) and anti-inflammatory (Sriwanthana et al. Citation1996; Wanikiat et al. Citation2008) effects of CN leaf extracts, as summarized in .
Table 4. In vitro and in vivo studies supporting pharmacological activities of Clinacanthus nutans.
Antioxidant activity
Antioxidants are substances that neutralize potentially damaging oxidizing agents or free radicals, which are thought to cause chronic health problems in diseases such as cancer, cardiac disease, and aging-related disorders. Pannangpetch et al. (Citation2007) reported that CN extracts significantly reduced oxidative free-radical production by phorbol 12-myristate 13-acetate (PMA)-stimulated rat macrophages. Furthermore, the extract showed a substantial inhibitory effect (98%) on haemolysis in a 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH)-induced cell lysis model. AAPH causes lysis of red blood cells through oxidation of lipids and proteins in the blood cell membranes. Subsequent studies demonstrated in vitro antioxidant effects of CN based on various criteria, as summarized in . Collectively, data from these studies showed clearly that CN extracts could have antioxidant properties.
Antiproliferative and cytotoxic activity
Antiproliferative compounds are used to inhibit the growth of cells and can be potentially used in the treatment of cancer. CN leaf extracts were reported to inhibit proliferation of a number of cell types including HeLa (Yong et al. Citation2013; Arullappan et al. Citation2014; Ghasemzadeh et al. Citation2014) cell lines. The effects of CN extracts on these cell lines are summarized in . All these studies used the MTT assay as a standard measure of cell proliferation. This is a colorimetric assay measuring cellular metabolic activity. Few studies have tested the antiproliferative effects of CN on primary cell types, although one study tested CN extracts on primary human gingival fibroblasts and detected no antiproliferative activity (Roeslan et al. Citation2012).
Antitumorigenic activity
While antiproliferative compounds exert their anticancer effects through the inhibition of cell proliferation (Zulkipli et al. Citation2015), compounds with antitumorigenic activities may have a myriad of effects, which may prevent the development, maturation or spread of cancerous cells. Only one study has shown the potential antitumorigenic activity of CN. A CN ethanol extract, compared with a control treatment, significantly reduced tumour growth in a mouse HepA hepatoma model (Huang et al. Citation2015). The antitumorigenic effect in this model was significantly greater than that of fluorouracil, an established chemotherapeutic drug. Western blotting analysis showed that high levels of the pro-apoptotic mediator, Bax and apoptotic executioner protein, caspase 3 in tumours extracted from CN-treated tumour-bearing HepA mice. This suggests that CN could induce apoptosis in cancer cells as a mechanism to halt cell proliferation during tumour growth. However, more conclusive data obtained in multiple types of tumour models will be needed to prove that CN has antitumorigenic activity.
Antimicrobial activity
With the rise of antibiotic-resistant strains of bacteria in the clinical environment, scientists have turned to natural compounds in medicinal plants to identify potential new antibacterial compounds. The antibacterial effects of CN extracts have been tested on microbial strains (Yang et al. Citation2013; Arullappan et al. Citation2014). Several studies have reported that CN extracts inhibited bacterial growth and survival, while other studies have reported no antibacterial activities of CN extracts against similar species of bacteria (). Overall, these mixed findings suggest that the antimicrobial effects of CN extracts may be selective for only certain microorganisms. However, the exact mode of action of CN on bacteria killing is yet to be defined.
Antiviral activity
One of the most common ethnomedicinal use for CN is for treating Herpes infections (Sangkitporn et al. Citation1993, Citation1995). It is not surprising that there has been much interest in identifying a potential anti-Herpes agent from CN. The early work by Jayavasu et al. (Citation2013) showed that CN leaf extracts inhibited plaque formation by HSV-2 in a baby hamster kidney cell line, suggesting that these extracts may contain antiviral components. The majority of work on the antiviral effects of CN has focused on infections with HSV, the causative agent for genital Herpes in cell lines and HSV-infected animals ().
CN extract-based topical formulations were shown to be effective against the development and progression of skin lesions in a mouse model of cutaneous HSV-1 infection (Lipipun et al. Citation2011). Recently, Pongmuangmul et al. (Citation2016) showed that purified monogalactosyl diglyceride (MGDG) and digalactosyl diglyceride (DGDG) from CN leaves resulted in 100% inhibition of HSV viral plaque formation. Glycoglycerolipids, such as MGDG, have previously been shown to exert anti-HSV effects (Janwitayanuchit et al. Citation2003). Although the mechanism of anti-HSV activity of these glycoglycerolipids is still not clear, the known antiviral effects of monoglycerides against enveloped viruses such as HSV (Thormar et al. Citation1994) makes these glycoglycerolipids potential drug candidates against HSV. Interestingly, work by several groups showed that compounds from CN leaf extracts were able to inhibit dengue virus activity (Sittiso et al. Citation2010; Tu et al. Citation2014). CN extracts were also shown to be effective against a fish virus, the yellow-head virus, in CHSE-214-infected cells (Direkbusarakom et al. Citation1998). This suggests that CN-derived products could be effective not only against viruses other than HSV but also against viruses from various animal hosts.
Anti-inflammatory activity and immune-modulatory effects
Extracts from CN leaves have been used to reduce symptoms of inflammation in insect bites, Herpes infection and allergic responses in traditional medicine. A few reports have also described the effects of CN extracts on the immune system.
CN extracts at low doses increased peripheral blood mononuclear cells (PBMC) proliferation, suggesting potential mitogenic properties (Sriwanthana et al. Citation1996). Interestingly, levels of interleukin-4 IL-4, an anti-inflammatory cytokine, were elevated only at higher CN doses, suggesting that inflammatory effects could be dampened with such doses. However, in a hepatocarcinoma (HepA) tumour model in mice, IL-4 induction by CD4 + T-helper 1 lymphocytes (Th1 cells) was not affected by treatment with a CN ethanol extract, compared with vehicle (Huang et al. Citation2015). Th1 cells primarily secrete IL-2 and interferon (IFN)-γ, which can suppress tumour growth by promoting CD8 + cytotoxic T lymphocyte (CTL) function (Dunn et al. Citation2006). Indeed, treatment with a CN ethanol extract-induced IL-2 and IFN-γ release and promoted CD8 + CTL infiltration into the tumour tissue in the HepA tumour-bearing mice (Huang et al. Citation2015). This suggests that CN could modulate the adaptive immune system by skewing the immune system toward a Th1-biased response, which would favour tumour suppression. In contrast, CN was also shown to act on the innate immune system. In two rat models of inflammation, ethyl phenylpropionate-induced ear oedema, and carrageenan-induced hind paw oedema, CN extracts significantly reduced oedema in the ears and paws, respectively (Wanikiat et al. Citation2008). The same study also reported attenuation of N-formyl-methionyl-leucyl-phenylalanine (fMLP)-mediated migration (chemotaxis and chemokinesis) and function (myeloperoxidase production and elastase release) in neutrophils treated with CN extract. These studies suggest that bioactive compounds found in CN leaves may have multiple effects on the immune system and any resulting inflammation, depending on the model system used. Additional studies are summarized in , and key studies have been highlighted here.
Toxicity studies
The purpose of toxicity studies as per Barle et al. (Citation2012) is, ‘to determine the effect of an action on a biological system which can be used later to extrapolate the doses and effects on humans’. This data is essential to identify the optimal therapeutic dose and the highest dose up to which the extract can be given, above which lethality would be expected. The toxicity studies can be acute, sub-acute and chronic, where the former is the most commonly used type to evaluate the dose to be used for testing the dose required for preliminary testing.
Sub-chronic toxicity study for 90 days of ethanol CN extract exhibited similar food consumption in control and test yet body weight was significantly lower in male rats with 1 g/kg bw. Although toxicity was not observed at the given dose, platelet and creatinine levels were altered in different ways, with the former being higher than control (Chavalittumrong et al. Citation1995).
Sub-acute toxicity study for 14 days of methanol CN extract with a maximum 0.9 g/kg dose did not display any toxic effect in rats. Compounds administered by an oral route of administration are, in general, metabolized by the liver and eliminated by the kidney, thus are investigated during oral toxicological experiments. While AST and ALT are markers for hepatocyte integrity, blood urea and creatinine are biochemical indicators for renal function. Consecutive administration of the intervention substance in rats is equivalent to less than 7 days human consumption (World Health Organization Citation2004). ‘Acceptable daily intake is the level that is harmless to humans based on the non-observable adverse effect level value obtained from animal study’, as mentioned by Food and Agricultural Materials Inspection Center was calculated to be 9 mg/kg in humans (P’ng et al. Citation2013; FAMIC Citation2014).
The mice were orally administered 1000 mg/kg of methanol CN crude extract for 14 days and were observed to have normal behaviour related to central nervous system despite having significantly elevated levels of acetylcholinesterase enzyme in liver, heart and kidney but not in the brain (Lau et al. Citation2014). It would be beneficial to elucidate the compounds responsible and the mechanism of the elevated enzyme levels in various organs.
Farsi investigated the correlation between dose and exposure period and included male and female rats. The longer treatment duration, induced decreased ALP at 2000 mg/kg oral dose of aqueous CN, yet within the physiological range and is clinically insignificant. There was an increase in body weight on day 42. However, the contrary was true on day 77 in rats administered with 500 mg/kg. Nevertheless, significant changes were not observed in the group administered with 2000 mg/kg. Mutagenicity, the tendency of a test compound to induce DNA changes, was measured by the bacteria reverse mutation test and mutagenicity was not observed with CN (Farsi et al. Citation2016).
Methanol, ethanol and aqueous extracts of CN extracts were evaluated for toxicity in rats or mice to enrich the understanding of CN safety profile (summarized in ). The animal toxicity experiments provide only preliminary information and must be followed by a battery of tests in animals. Subsequently, chronic and toxicokinetic assessments must be carried out to confirm the safety profile of CN.
Clinical trials
In addition to in vitro work, small-scale clinical trials performed using a topical formulation of a CN extract showed significant resolution of clinical symptoms of genital Herpes (Sangkitporn et al. Citation1993) and Herpes zoster (Sangkitporn et al. Citation1995; Charuwichitratana et al. Citation1996), as compared with patients treated with a placebo. A meta analysis of patients with hepatitis genitalis and Herpes zoster infections indicated that treatment with creams containing CN extracts resulted in faster healing of infection-induced lesions (Kongkaew & Chaiyakunapruk Citation2011).
The review mentions about its three limitations, namely: inability to perform a test of publication bias, the influence of small study effect and overestimate of clinical trials effect due to non-blinding. Due to these limitations, the results are to be interpreted with caution and summons for well-designed, robust, systematic, randomized controlled trials, which is either double or triple blinded. However, use of acyclovir with CN with its potential synergistic effect beckons for use as an adjuvant therapy in H. genitalis treatment. Patients with minor recurrent aphthous stomatitis, which is benign mouth ulcer, were recruited for the randomized, double-blind controlled trial to assess the efficacy of CN (Buajeeb & Kraivaphan Citation1994). Three arms were recording the duration of ulcers and pain, with CN, triamcinolone acetonide, and placebo, wherein the interventions were given in Orabase®, an oral pain reliever. CN was better than placebo in shortening the ulcer duration than placebo, albeit triamcinolone acetonide was the best. Despite this evidence, the precise molecular mechanism of how CN extracts kill HSV is not known and warrants further elucidation in this area.
Future directions
CN has been demonstrated to have pharmacological effects on both host cells and microbes. The potential use of CN as an anti-HSV agent is promising. Future studies should investigate the antiviral activity of CN against other types of viruses, especially those endemic to regions where CN is abundant. Studies on the molecular mechanism of how CN extracts kill HSV are also needed.
Research to date has shown the antibacterial activity of CN only in vitro, in minimum inhibitory concentration assays, and the mechanism of this antibacterial activity is still unknown. Also, effects of CN in vivo in animal infection models have not yet been demonstrated. The lack of observed toxicities when various oral doses of CN leaf methanol extracts were given to Sprague–Dawley rats (P’ng et al. Citation2013; Farsi et al. Citation2016), and Balb/c mice (Lau et al. Citation2014) suggested that testing CN in more in vivo studies is feasible.
The anti-inflammatory effects of CN suggest that it has the potential to modulate the immune system. However, there is little current evidence addressing this and studies measuring cytokine production in immune cells have conflicting results. Therefore, more investigations would be required to establish the mechanisms by which CN dampens inflammatory activity in immune cells.
The mechanism for anti-proliferation by CN also remains to be determined. A link between antioxidant activity and anticancer effects has been suggested, particularly for phenolic compounds, such as flavonoids, from medicinal plants (Kumar & Pandey Citation2013; Roleira et al. Citation2015). Therefore, it is possible that phenolic compounds from CN could possess such biological activities. Although in vivo studies showed that CN has potential as an anticancer agent, more evidence from additional in vivo tumor models will be required conclusively to prove the biological relevance of the anticancer properties of CN.
Most of the studies investigating the antiproliferative effects of CN extracts on cells have focused on using the MTT colorimetric assay. Alternative lines of investigation for future studies could include investigating whether the antiproliferative effects of CN involve a cell division blockade or initiation of a cell death pathway (Zulkipli et al. Citation2015). Interestingly, in a recent study, high levels of the pro-apoptotic mediator, Bax, and apoptotic executioner protein, caspase 3, were detected by western blotting in tumor tissue from HepA tumor-bearing mice treated with a CN extract (Huang et al. Citation2015). Such data suggest that CN might induce apoptosis in cancer cells as a mechanism to halt tumor growth. The reported potent antiproliferative and antioxidant activities suggest that CN could be a good source of anticancer therapies.
There is a discrepancy in the procurement and processing of CN leaves used for various purposes. It is interesting to note that most CN users prefer fresh leaves while most researchers have experimented with dried plant parts. Thus, more experimentation by researchers on the effects of storage conditions on extract efficacy is needed. Many websites including Singapore Sabah Snake Grass (Citation2011) have described a regimen of treatment for cancer and diabetes with fresh CN leaves (Roosita et al. Citation2008; Singapore Sabah Snake Grass Citation2011; Ching et al. Citation2013; Globinmed Citation2015). Standardization of the chemical constituents present in the leaves, their interaction with chemical constituents present in other plants and the efficacy of CN-derived preparations for the different stages of cancer have yet to be confirmed. Moreover, researchers also need to consider the selection of plant parts as well as the method of extraction and suitable solvents, to isolate the chemical constituents present in CN fresh leaves. Further research on the harvesting of CN parts and their proper storage will be needed to minimize loss of essential phytochemicals. These isolated compounds should be analyzed to compare their efficacy with the CN preparations used traditionally. Although it has been claimed that ingestion of CN prevents cancer, its prophylactic action has not been demonstrated. These measures would be beneficial to the community of CN users. If a potent activity is identified, the common process of drug discovery may then be applied such as what has been done for Viscum album mistletoe plant (Lim et al. Citation2016). Any potential drug arising from CN extracts would need to be investigated to determine the best formulation, dosage and delivery route. Once the mechanisms of the antitumor and other pharmacological properties of CN are better understood, it will be possible to identify molecular targets for upstream drug discovery research.
Disclosure statement
This work was financially supported by grants from the Universiti Brunei Darussalam [UBD/PNC2/2/RG/1(322)], and the Islamic Educational, Scientific and Cultural Organization (ISESCO) [I.C.P.S.R/3.8.2.1/11].
Additional information
Funding
References
- Abdul Rahim MH, Zakaria ZA, Mohd Sani MH, Omar MH, Yakob Y, Cheema MS, Ching SM, Ahmad Z, Abdul Kadir A. 2016. Methanolic Extract of Clinacanthus nutans Exerts Antinociceptive Activity via the Opioid/Nitric Oxide-Mediated, but cGMP-Independent, Pathways. Evidence-Based Complement Altern Med. 2016:1–11.
- Anti-cancer and Anti-inflammation Herbs Sabah Snake Grass. 2009. [cited 2015 Jun 17]. Available from: http://sennyong.blogspot.com/2009/11/anti-cancer-herbs-and-anti-inflammation.html
- Arullappan S, Rajamanickam P, Thevar N, Kodimani C. 2014. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop J Pharm Res. 13:1455–1461.
- Aslam MS, Ahmad MS, Mamat ASOH. 2015. A review on the phytochemical constituents and pharmacological activities of Clinacanthus nutans. Int J Pharm Pharm Sci. 7:2–5.
- Barle E, Looser R, Erne M, Bechter R. 2012. The value of acute toxicity testing of pharmaceuticals for estimation of human response. Regul Toxicol Pharmacol. 62:412–418.
- Buajeeb W, Kraivaphan P. 1994. Clinical evaluation of Clinacanthus nutans Lindau in orabase in the treatment of recurrent aphthous stomatitis. Mahidol Dent J. 14:10–16.
- Cancer cure. 2015. [cited 2015 Jun 17]. Available from: http://yourhealth.asiaone.com/content/can-sabah-snake-grass-cure-cancer.
- Charuwichitratana S, Wongrattanapasson N, Timpatanapong P, Bunjob M. 1996. Herpes zoster: treatment with Clinacanthus nutans cream. Int J Dermatol. 35:665–666.
- Chavalittumrong P, Attawish A, Rugsamon P, Chuntapet P. 1995. Toxicological study of Clinacanthus nutans (Burm.f.) Lindau. Bull Dep Med Sci. 37:323–338.
- Cheeptham N, Towers GHN. 2002. Light-mediated activities of some Thai medicinal plant teas. Fitoterapia. 73:651–662.
- Chelyn JL, Omar MH, Mohd Yousof NSA, Ranggasamy R, Wasiman MI, Ismail Z. 2014. Analysis of flavone C-glycosides in the leaves of Clinacanthus nutans (Burm. f.) Lindau by HPTLC and HPLC-UV/DAD. Sci World J. 2014:1–6.
- Ching SM, Zakaria ZA, Paimin F, Jalalian M. 2013. Complementary alternative medicine use among patients with type 2 diabetes mellitus in the primary care setting: a cross-sectional study in Malaysia. BMC Complement Altern Med. 13:148.
- Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritsanapan W. 2005. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol. 101:330–333.
- Chomnawang MT, Surassmo S, Wongsariya K, Bunyapraphatsara N. 2009. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia. 80:102–104.
- Chompuki L, Sriwanthana B, Chavalittumrong P, Chompuk L. 1996. Effect of Clinacanthus nutans on Human Cell-mediated In Vitro Immune Response. Thail J Pharm Sci. 20:261–267.
- Chotchoungchatchai S, Saralamp P, Jenjittikul T, Pornsiripongse S, Prathanturarug S. 2012. Medicinal plants used with Thai Traditional Medicine in modern healthcare services: A case study in Kabchoeng Hospital, Surin Province, Thailand. J Ethnopharmacol. 141:193–205.
- Dampawan P, Huntrakul C, Reutrakul V. 1977. Constituents of Clinacanthus nutans and the crystal structure of LUP-20 (29)-ene-3-one. J Sci Soc Thail. 3:14–26.
- Deng Y, Hu J, Daniel TF, Wood JRI, Wood JRI, Burman NL. 2002. Flora of China. In: Zhengyi W, Raven PH, Deyuan H, editors. Beijing: Science Press; St. Louis: Missouri Botanical Garden.
- Direkbusarakom S, Ruangpan L, Ezura Y, Yoshimizu M. 1998. Protective efficacy of Clinacanthus nutans on yellow-head disease in black tiger shrimp (Penaeus monodon). Fish Pathol. 33:401–404.
- Dunn GP, Koebel CM, Schreiber RD. 2006. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 6:836–848.
- FAMIC. 2014. How are MRLs established? [Internet]. [cited 2016 May 28]. Available from: http://www.acis.famic.go.jp/eng/chishiki/04.htm.
- Farsi E, Esmailli K, Shafaei A, Moradi Khaniabadi P, Al Hindi B, Khadeer Ahamed MB, Sandai D, Abdul Sattar M, Ismail Z, Abdul Majid AMS, et al. 2016. Mutagenicity and preclinical safety assessment of the aqueous extract of Clinacanthus nutans leaves. Drug Chem Toxicol. 545:1–13.
- Fong SY, Piva T, Urban S, Huynh T. 2014. Genetic homogeneity of vegetatively propagated Clinacanthus nutans (Acanthaceae). J Med Plant Res. 8:903–914.
- Ghasemzadeh A, Nasiri A, Jaafar HZE, Baghdadi A, Ahmad I. 2014. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah Snake Grass (Clinacanthus nutans L.) in relation to plant age. Molecules. 19:17632–17648.
- Globinmed. 2015. [cited 2015 Jun 22]. Available from: http://www.globinmed.com/index.php?option=com_content&view=article&id=79320:clinacanthus-nutans-burmf-lindau.
- Hanelt P. editor. 2001. Research I of PG and CP. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops (Except Ornamentals). Berlin, Heidelberg: Springer-Verlag; p. 1912–1918.
- Hariana AH. 2013. Ki Tajam/Dandang Gendis. In: 262 Tumbuh Obat dan Khasiatnya. Jakarta: Penebar Swadaya Grup; p. 183?185.
- Huang D, Guo W, Gao J, Chen J, Olatunji J. 2015. Clinacanthus nutans (Burm. f.) Lindau ethanol extract inhibits hepatoma in mice through upregulation of the immune response. Molecules. 20:17405–17428.
- Huang D, Li Y, Cui F, Chen J, Sun J. 2016. Purification and characterization of a novel polysaccharide-peptide complex from Clinacanthus nutans Lindau leaves. Carbohydr Polym. 137:701–708.
- Janwitayanuchit W, Suwanborirux K, Patarapanich C, Pummangura S, Lipipun V, Vilaivan T. 2003. Synthesis and anti-Herpes simplex viral activity of monoglycosyl diglycerides. Phytochemistry. 64:1253–1264.
- Jayavasu C, Dechatiwongse T, Balachandra K. 1992. Virucidal activity of Clinacanthus nutans Lindau extracts against herpes simplex virus type 2?: In vitro study. Bull Dep Med Serv. 34.
- Jayavasu C, Dechatiwongse T, Balachandra K, Chavalittumrong P, Jongtrakulsiri S. 2013. The virucidal activity of Clinacanthus nutans lindau extracts against Herpes simplex virus type-2: an in vitro study. Bull Dep Med Sci. 4:153–158.
- Khoo LW, Mediani A, Zolkeflee NKZ, Leong SW, Ismail IS, Khatib A, Shaari K, Abas F. 2015. Phytochemical diversity of Clinacanthus nutans extracts and their bioactivity correlations elucidated by NMR based metabolomics. Phytochem Lett. 14:123–133.
- Kongkaew C, Chaiyakunapruk N. 2011. Efficacy of Clinacanthus nutans extracts in patients with Herpes infection: systematic review and meta-analysis of randomised clinical trials. Complement Ther Med. 19:47–53. [Internet]. [cited 2014 Nov 25]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/21296267.
- Kumar S, Pandey AK. 2013. Chemistry and biological activities of flavonoids: an overview. Sci World J. 2013. doi: 10.1155/2013/162750
- Kunsorn P, Ruangrungsi N, Lipipun V, Khanboon A, Rungsihirunrat K. 2013. The identities and anti-Herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac J Trop Biomed. 3:284–290.
- Lau K, Lee S, Chin J. 2014. Effect of the methanol leaves extract of Clinacanthus nutans on the activity of acetylcholinesterase in male mice. J Acute Dis. 3:22–25.
- Liew S-Y, Stanbridge EJ, Yusoff K, Shafee N. 2012. Hypoxia affects cellular responses to plant extracts. J Ethnopharmacol. 144:453–456.
- Lim YC, Rajabalaya R, Lee S, Tennakoon K, Le Q-V, Idris A, Zulkipli I, Keasberry N, David S. 2016. Parasitic mistletoes of the genera Scurrula and Viscum: from bench to bedside. Molecules. 21:1048.
- Lipipun V, Kurokawa M, Suttisri R, Taweechotipatr P, Pramyothin P, Hattori M, Shiraki K. 2003. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antiviral Res. 60:175–180.
- Lipipun V, Sasivimolphan P, Yoshida Y, Daikoku T, Sritularak B, Ritthidej G, Likhitwitayawuid K, Pramyothin P, Hattori M, Shiraki K. 2011. Topical cream-based oxyresveratrol in the treatment of cutaneous HSV-1 infection in mice. Antiviral Res. 91:154–160.
- Mai CW, Yap KSI, Kho MT, Ismail NH, Yusoff K, Shaari K, Chin SY, Lim ESH. 2016. Mechanisms Underlying the Anti-Inflammatory Effects of Clinacanthus nutans Lindau Extracts: Inhibition of Cytokine Production and Toll-Like Receptor-4 Activation. Front Pharmacol. 7:1–11.
- Mat Ali R, Abu Samah Z, Mustapha NM, Hussein NN, Ali RM, Samah ZA, Mustapha NM, Hussein NN. 2010. ASEAN Herbal and Medicinal Plants. [Internet]. Jakarta: ASEAN; [cited 2015 Aug 25]. Available from: http://www.asean.org/archive/publications/aseanherbal2010.pdf.
- Mohlenbrock RH. 2008. Acanthaceae to Myricaceae: water willows to wax myrtles. 1st ed. Carbondale, Illinois, US: Southern Illinois University Press.
- Mustapa AN, Martin A, Gallego JR, Mato RB, Cocero MJ. 2015. Microwave-assisted extraction of polyphenols from Clinacanthus nutans Lindau medicinal plant: energy perspective and kinetics modeling. Chem Eng Process Process Intensif. 97:66–74.
- Mustapa AN, Martin A, Mato RB, Cocero MJ. 2015. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind Crops Prod. 74:83–94.
- P’ng XW, Akowuah GA, Chin JH. 2013. Evaluation of the sub–acute oral toxic effect of methanol extract of Clinacanthus nutans leaves in rats. J Acute Dis. 2:29–32.
- Pannangpetch P, Laupattarakasem P, Kukongviriyapan V, Kukongviriyapan U, Kongyingyoes B, Aromdee C. 2007. Antioxidant activity and protective effect against oxidative hemolysis of Clinacanthus nutans (Burm. f) Lindau. Songklanakarin J Sci Technol. 29:1–9.
- Pongmuangmul S, Phumiamorn S, Sanguansermsri P, Wongkattiya N, Fraser IH, Sanguansermsri D. 2016. Monogalactosyl diglyceride and digalactosyl diglyceride from Clinacanthus nutans, a traditional Thai herbal medicine, exhibits anti-Herpes simplex virus activities. Asian Pac J Trop Biomed 6:192–197.
- Quattrocchi U. 2012. Clinacanthus Nees Acanthaceae. In: CRC World Dict Med Poisonous Plants Common Names, Sci Names, Eponyms, Synonyms, Etymology. CRC Press; p. 1020.
- Ramesh S, Rajan R, Santhanam R. 2014. Freshwater phytopharmaceutical compounds. Boca Raton, Florida: CRC Press; Taylor & Francis.
- Rathnasamy S, Mohamed K Bin, Sulaiman SF, Akinboro A. 2013. Evaluation of cytotoxic, mutagenic and antimutagenic potential of leaf extracts of three medicinal plants using Allium cepa chromosome assay. Int Curr Pharm J. 2:131–140.
- Raya KB, Ahmad SH, Farhana SF, Mohammad M, Tajidin NE, Parvez A. 2015. Changes in phytochemical contents in different parts of Clinacanthus nutans (Burm. f.) Lindau due to storage duration. Bragantia. 74:445–452.
- Roeslan MO, Ayudhya TDN, Kootongkaew S. 2012. Characteristics of Clinacanthus nutans extraction from Thailand and Indonesia (preliminary study). Sci-Health. 2.
- Roleira FMF, Tavares-da-Silva EJ, Varela CL, Costa SC, Silva T, Garrido J, Borges F. 2015. Plant derived and dietary phenolic antioxidants: anticancer properties. Food Chem. 183:235–258.
- Roosita K, Kusharto CM, Sekiyama M, Fachrurozi Y, Ohtsuka R. 2008. Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J Ethnopharmacol. 115:72–81.
- Sakdarat S, Shuyprom A, Na ATD, Waterman PG, Karagianis G. 2006. Chemical composition investigation of the Clinacanthus nutans Lindau leaves. Thai J Phytopharm. 13:13–24.
- Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S. 2009. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorganic Med Chem. 17:1857–1860.
- Sangkitporn S, Chaiwat S, Balachandra K, Na-Ayudhaya TD, Bunjob M, Jayavasu C. 1995. Treatment of Herpes zoster with Clinacanthus nutans (bi phaya yaw) extract. J Med Assoc Thai. 78:624–627.
- Sangkitporn S, Polchan K, Thawatsupa P, Bunchob M, Chawalitthumrong P. 1993. Treatment of recurrent genital Herpes simplex virus infection with Clinacanthus nutans extract. Bull Dep Med Serv. 18:226–231.
- Sarega N, Imam MU, Ooi D, Chan KW, Md Esa N, Zawawi N, Ismail M. 2016a. Phenolic Rich Extract from Clinacanthus nutans Attenuates Hyperlipidemia-Associated Oxidative Stress in Rats. Oxid Med Cell Longev. 2016:1–16.
- Sarega N, Imam MU, Md Esa N, Zawawi N, Ismail M. 2016b. Effects of phenolic-rich extracts of Clinacanthus nutans on high fat and high cholesterol diet-induced insulin resistance. BMC Complement Altern Med. 16 (1):1–11.
- Shim SY, Aziana I, Khoo BY. 2013. Perspective and insight on Clinacanthus nutans Lindau in traditional medicine. Int J Integr Biol. 14:7–9.
- Siew YY, Zareisedehizadeh S, Seetoh WG, Neo SY, Tan CH, Koh HL. 2014. Ethnobotanical survey of usage of fresh medicinal plants in Singapore. J Ethnopharmacol. 155:1450–1466.
- Singapore Sabah Snake Grass. 2011. [cited 2015 Jun 17]. Available from: http://sabahsnakegrasssingapore.blogspot.com/.
- Sittiso S, Ekalaksananan T, Pientong C, Sakdarat S, Charoensri N, Kongyingyoes B. 2010. Effects of compounds from Clinacanthus nutans on dengue virus type 2 infection. Srinagarind Med J. 25:272–275.
- Sriwanthana B, Chavalittumrong P, Chompuk L. 1996. Effect of Clinacanthus nutans on kuman cell-mediated in vitro immune response. Thail J Pharm Sci. 20:261–267.
- Sabah Snake Grass. 2009. [cited 2015 Jun 17]. Available from: http://sennyong.blogspot.com/2009/11/anti-cancer-herbs-and-anti-inflammation.html.
- Teshima K-I, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S, Picheansoonthon C, Yamasaki K. 1998. Sulfur-containing glucosides from Clinacanthus nutans. Phytochemistry. 48:831–835.
- The Plant List A. 2013. [cited 2016 Jan 14]. Available from: http://www.theplantlist.org/tpl1.1/record/tro-100366425.
- The Plant List B. 2013. [cited 2016 Jan 14]. Available from: http://www.theplantlist.org/tpl1.1/record/kew-2727902.
- Thormar H, Isaacs CE, Kim KS, Brown HR. 1994. Inactivation of visna virus and other enveloped viruses by free fatty acids and monoglycerides. Ann N Y Acad Sci. 24:465–471.
- Tsai H-D, Wu J-S, Kao M-H, Chen J-J, Sun GY, Ong W-Y, Lin T-N. 2016. Clinacanthus nutans Protects Cortical Neurons Against Hypoxia-Induced Toxicity by Downregulating HDAC1/6. NeuroMolecular Med. 18:274–282.
- Tu S-F, Liu R, Cheng Y-B, Hsu Y-M, Du Y-C, El-Shazly M, Wu Y-C, Chang F-R. 2014. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules. 19:20382–20390.
- Uawonggul N, Chaveerach A, Thammasirirak S, Arkaravichien T, Chuachan C, Daduang S. 2006. Screening of plants acting against Heterometrus laoticus scorpion venom activity on fibroblast cell lysis. J Ethnopharmacol. 103:201–207.
- Wanikiat P, Panthong A, Sujayanon P, Yoosook C, Rossi AG, Reutrakul V. 2008. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barleria lupulina and Clinacanthus nutans extracts. J Ethnopharmacol. 116:234–244.
- Watson RR, Preedy VR. 2008. Clinacanthus nutans. In: Bot Med Clin Pract. CABI; p. 819.
- World Health Organization. 2004. IPCS Harmonization Project-IPCS Risk assessment terminology. Geneva: World Health Organization.
- Yang HS, Peng TW, Madhacan P, Abdul Shukkoor MS, Akowuah GA. 2013. Phytochemical analysis and antibacterial activity of methanolic extract of Clinacanthus nutans leaf. Int J Drug Dev Res. 5:349–355.
- Ying NL. 2013. Establishment of Axenic Explants and Callus Culture of Clinacanthus nutans (Rumput Belalai Gajah). [PhD Thesis]: University Malaysia Sarawak.
- Yong YK, Tan JJ, Teh SS, Mah SH, Ee GCL, Chiong HS, Ahmad Z. 2013. Clinacanthus nutans extracts are antioxidant with antiproliferative effect on cultured human cancer cell lines. Evid Based Complement Alternat Med. 2013:462751.
- Yoosook C, Bunyapraphatsara N, Boonyakiat Y, Kantasuk C. 2000. Anti-herpes simplex virus activities of crude water extracts of Thai medicinal plants. Phytomedicine. 6:411–419.
- Yoosook C, Panpisutchai Y, Chaichana S, Santisuk T, Reutrakul V. 1999. Evaluation of anti-HSV-2 activities of Barleria lupulina and Clinacanthus nutans. J Ethnopharmacol. 67:179–187.
- Yuann J-MP, Wang J-S, Jian H-L, Lin C-C, Liang J-Y. 2012. Effects of Clinacanthus nutans (Burm. f) Lindau leaf extracts on protection of plasmid DNA from riboflavin photoreaction. MC-Transaction Biotechnol. 4:45–58.
- Zulkipli I, Rajabalaya R, David S, Idris A. 2015. Medicinal plants: a potential source of compounds for targeting cell division. Drug Target Insights 9:919.