?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Context: Phytochemical study and biological potential of Evax pygmaea (L.) Brot. (Asteraceae) are reported for the first time.
Objective: To identify the secondary metabolites of Evax pygmaea and to determine its antioxidant, antibacterial and cytotoxic activities.
Materials and methods: Dried aerial parts (1 kg) were macerated in 70% MeOH (5 L) during 72 h. The concentrated hydromethanolic extract was subjected to extractions with chloroform (3 × 300 mL), ethyl acetate (3 × 300 mL) and n-butanol (3 × 300 mL), successively. VLC of combined ethyl acetate (EAEP) and n-butanol (BEP) fractions was followed by column purifications. Antioxidant activity was investigated using DPPH, CUPRAC, and metal chelating, β-carotene/linoleic acid and ABTS assays. Agar method was used in the antibacterial study. Cytotoxic activity was determined by Brine shrimp lethality test in DMSO and ethanol, at varying concentrations (2, 1 and 0.2%) and (1, 0.2 and 0.1%) successively.
Results: Quercetin (1), isorhamnetin 3-O-β-d-xyloside (2), isorhamnetin 3-O-β-d-glucoside (3), quercetin 3-O-β-d-glucoside (4), quercetin 7-O-β-D-glucoside (5), patuletin 3-O-β-d-glucoside (6) were isolated from for the first time from Evax genus. The EAEP was the most active in ABTS (IC50: <3.125 μg/mL) assay whereas the BEEP exhibited the highest activity in the β-carotene/linoleic acid assay (IC50: <3.125 μg/mL). The EAEP and BEP exhibited good antibacterial activity (MIC: 40–80 µg/mL). The plant did not show any toxicity (LD50>80 µg/mL).
Discussion and conclusions: Six flavonoids were isolated for the first time from Evax pygmaea which exhibited good antioxidant and antibacterial activities.
Introduction
Medicinal plants have been widely used for therapeutic purposes since ancient times. The beneficial effects of fruits and vegetables are generally attributed to the presence of phenolic compounds such as phenolic acids, flavonoids and tannins, nitrogen compounds such as alkaloids and amines, as well as vitamins, terpenoids and other metabolites, which have a high antioxidant activity (Cai et al. Citation2004; Djeridane et al. Citation2006; Yang et al. Citation2009).
Asteraceae (Compositae) is the richest vascular plant family in the world, with 1600–1700 genera and 24,000–30,000 species (Funk et al. Citation2005), it is among the principal families whose species accumulate secondary metabolites with a vast array of important biological activities. Flavonoids are one of these metabolites. Numerous studies have been conducted to prove flavonoids efficacy as antimycotic, antibacterial, antiviral, antiinflammatory, antioxidant, immune modulator, enzyme inhibitor, mutagenic and toxic agents (Havsteen Citation2002), with antimutagenic and anticancer properties (Harborne Citation1998).
The genus Evax (syn. Filago) (Asteraceae) is mainly distributed in the Mediterranean area, it is represented by eight species, five of them are found in Algeria (Quezel and Santa Citation1963). This genus is not well documented. One paper reported the essential oil composition of E. pygmaea (L.) Brot. (syn. Filago pygmaea L.), growing wild in Tunisia (Boussaada et al. Citation2008a) which was characterized by sesquiterpenes (61.9%), diterpenes (33.5%) and monoterpenes (3.4). α-Calacorene (12.2%), cadina-1,4-diene (4.4%), elemol (8.8%), α-cadinol (7.4%), cedrol (5.6%), T-cadinol (4.3%), T-muurolol (4.9%), α-muurolol (3.5%) were the most important components. The second paper described the antimicrobial and antioxidant activities by DPPH and ABTS assays of methanol extracts of the same species collected from Tunisia (Boussaada et al. Citation2008b).
We report here, for the first time, the phytochemical study, the antioxidant activity, by the use of five methods, and the antibacterial and cytotoxic activities of E. pygmaea growing at Constantine (Eastern Algerian) which is known as an antimicrobial.
Materials and methods
Chemicals and drugs
NMR spectra were recorded on a Bruker AVANCE DRX 500 NMR spectrometer (Billerica, MA). Thin layer chromatography (TLC) was carried out on precoated silica gel 60 F254 (Merck, Kenilworth, NJ), column chromatography (CC) was performed on a polyamide SC6 and silica gel 60 spots were observed under UV light at 254 and 365 nm or visualized by heating after spraying with H2SO4 50%. Polyamide SC6 was purchased from Fluka (Buchs, Switzerland).
Both antioxidant and cytotoxic activity measurements were carried out on a 96-well microplate reader, butylatedhydroxylanisole (BHA), butylatedhydroxyltoluene (BHT), 1,1-diphenyl-2-picrylhydrazyl (DPPH•), ascorbic acid, 2,2′-azinobis(3-ethyl-benzothiazoline-6-suphonic acid) diammonium salt (ABTS•+), β-carotene-linoleic acid were obtained from Sigma Chemical Co. (Sigma-Aldrich GmbH, Steinheim, Germany). All other chemicals and solvents used were of analytical grade.
Plant material
The aerial parts of E. pygmaea were collected on May 2014 at Constantine (North Eastern Algerian) and identified by Professor Gérard De Bélair, Faculty of Sciences University of Annaba, Algeria. A voucher specimen (LOST.Ep.05.14) was deposited at the Herbarium of the Laboratory of Therapeutic Substances (LOST), University des frères Mentouri-Constantine.
Extraction and isolation of compounds
Air-dried and powdered aerial parts (1.5 kg) of Evax pygmaea, were macerated at room temperature with MeOH–H2O (70:30, v/v) for 24 h, three times. After filtration and concentration, the residue was dissolved in water. The resulting solution was extracted successively with CHCl3, EtOAc and n-butanol. Concentration in vacuo led to the following fractions: CHCl3 (2 g), EtOAc (4.7 g) and n-butanol (10 g). Ethyl acetate (EAEP) and n-butanol (BEP) fractions were combined because they are similar. This mixture (10 g) was column chromatographed on polyamide SC6 with a gradient of toluene–MeOH with increasing polarity; six main fractions (F3, F5, F7, F8, F10, F11) were collected. Fraction F5 (154 mg), obtained from toluene 88% was further purified on a silica gel CC eluted with an isocratic system (EtOAc:MeOH:H2O, 20:0.25:0.25) and yielded a yellow precipitate which was identified as compound 2 (15 mg), the fractions F3 (100 mg), F7 (400 mg), F8 (289 mg), F10 (230 mg) and F11 (320 mg), obtained from toluene 92, 85, 80, 75 and 70%, respectively, yielded the compounds 1, 3, 4, 5 and 6 successively as yellow precipitates (20, 15, 10, 30 and 10 mg, respectively).
The structures of these compounds were established by chemical and spectral analysis UV, NMR and acid hydrolysis as well as by comparing their spectroscopic data with those reported in the literature.
Acid hydrolysis
The pure compounds were treated with 4 M HCl at 100 °C for 1 h. The hydrolysates were extracted with EtOAc and the aglycones were identified by their UV spectra in methanol and by comparison of their Rf with authentic samples. Sugars were identified in the aqueous residue by comparison with authentic samples on silica gel TLC impregnated with 0.2 M NaH2PO4, solvent Me2CO–H2O (9:1), revealed with aniline malonate. The optical rotation of each purified sugar was measured and compared with authentic samples.
Antioxidant activity
DPPH free radical scavenging assay
The free radical-scavenging activity was determined spectrophotometrically by the DPPH assay (Blois Citation1958; Lakhal et al. Citation2011). In its radical form, DPPH absorbs at 517 nm, but upon reduction by an antioxidant or a radical species its absorbance decreases. Briefly, a 0.1 mM solution of DPPH• in methanol was prepared and 4 mL of this solution was added to 1 mL of sample solutions in methanol at different concentrations. Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicated higher free radical-scavenging activity. BHT and BHA were used as antioxidant standards for comparison of the activity. The scavenging capability of DPPH radical was calculated using the following equation. The results were given as IC50 (µg/mL) corresponding the concentration of 50% inhibition.
where Acontrol and Asample are the absorbances of the reference and sample obtained from the UV–visible spectrophotometer, respectively.
Metal chelating activity assay
The metal chelating activity by the ferrene–Fe2+ complexation assay measured spectrophotometrically (Decker and Welch Citation1990; Labed et al. Citation2016) with slight modifications. The extracts solution (80 μL dissolved in ethanol in different concentrations) were added to 40 μL 0.2 mM FeCl2. The reaction was initiated after addition of 80 μL 0.5 mM ferene. The mixture was shaken then left at room temperature for 10 min. At the equilibrium, the absorbance was measured at 593 nm. The activity was calculated by the use of the following equation. The results were given as IC50 value (mg/mL) (50% inhibition):
ABTS cation radical decolourization assay
The ABTS•+ scavenging activity was determined according to the method of Re et al. (Citation1999). The ABTS•+ was generated by the reaction between 7 mM ABTS in water and 2.45 mM potassium persulfate, stored in the dark at room temperature for 12 h. The ABTS•+ solution was diluted to get an absorbance of 0.703 ± 0.025 at 734 nm with ethanol which was used as a control. BHT and BHA were used as antioxidant standards and the activity was calculated by the use of the following equation. The results were given as IC50 (µg/mL)
β-Carotene/linoleic acid assay
The bleaching activity of the fractions was evaluated by the β-carotene-linoleic acid test system (Miller Citation1971; Ferhat et al. Citation2017). β-Carotene (0.5 mg) in 1 mL of chloroform and 25 μL of linoleic acid were dissolved in 200 mg of Tween 40 emulsifier mixture. After evaporation of chloroform under vacuum, 100 mL of distilled water saturated with oxygen, were added by vigorous shaking. Four millilitres of this mixture were transferred into different test tubes containing different concentrations of the sample. As soon as the emulsion was added to each tube, the zero time absorbance was measured at 470 nm using spectrophotometer. The emulsion system was incubated for 2 h at 50 °C. Ethanol was used as a control whereas BHA and BHT were used as antioxidant standards. The results were given as 50% inhibition concentration (IC50).
The bleaching rate (R) of β-carotene was calculated according to the following equation:
where ln is the natural log, a is the absorbance at time zero and b is the absorbance at time t (120 min). The antioxidant activity was calculated in terms of percent inhibition relative to the control, using following equation:
Cupric reducing antioxidant capacity (CUPRAC) assay
The cupric reducing capacity of the fractions was determined by the CUPRAC method (Apak et al. Citation2004; Bensouici et al. Citation2016). One millilitres of copper (II) chloride solution (0.01 M prepared from CuCl2·2H2O), 1 mL of ammonium acetate buffer at pH 7.0 and 1 mL of neocaproin solution (0.0075 M) were mixed to 0.5 mL of plant extract or standard of different concentrations solution. The final volume of the mixture was adjusted to 4.1 mL by adding 0.6 mL of distilled water. The resulting mixture was incubated for 1 h at room temperature, and then the absorbance of the solution was measured at 450 nm by the use of a spectrophotometer against blank and BHT and BHA as standards.
The results were given as A0.5 (µg/mL) corresponding the concentration indicating 0.50 absorbance intensity.
Polyphenol content
The total polyphenolics was determined by the Folin–Ciocalteu method, with slight modifications. Mixture of extract (0.5 mL) and 5 mL of Folin–Ciocalteu reagent (1 mol) was neutralized with 4 mL saturated sodium carbonate (75 g/L), and kept at room temperature for 2 h. The absorbance was measured at 760 nm by the use of a spectrophotometer. The total polyphenolics were expressed as gallic acid equivalents (GAE) (Singleton et al. Citation1999).
Determination of the antibacterial activity
Susceptibility of the bacterial strains namely Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 43300, Pseudomonas aeruginosa ATCC 27853, Klebsiella pneumonia, Salmonella heilberg, Shigella sonnei and Enterobarter aerogenes to the BEP, EAEP and CEP fractions of Evax pygmaea were investigated using the agar method (NCCLS Citation2007). The reference strains were obtained from the Pasteur Institute (Algiers). The other strains were clinically obtained from the laboratory of bacteriology, Benbadis Hospital, Constantine, using conventional methods (clinical isolation). Gentamycin was used as reference. The activity was estimated visually by the presence or absence of colonies. MIC values were recorded as the lowest concentrations of compounds showing no growth. This test was performed in triplicate.
Cytotoxic activity
Brine shrimp lethality test
Cytotoxicity of the BEP, EAEP and CEP fractions of E. pygmaea was evaluated using Brine shrimp lethality assay (Meyer et al. Citation1982). Each extract (100 μL) was incubated, for 24 h under lighting, with 10 larvae of Artemia adjusted until 5 mL of 70% salty sea water (five tubes were prepared for each extract). Control larvae were placed in DMSO and ethanol, at varying concentrations (2, 1 and 0.2%) and (1, 0.2 and 0.1%) successively. After 24 h, the tubes were examined against a lighted background and the average number of larvae that survived in each tube was determined.
The concentration at 50% mortality of the larvae (LC50) was determined using the microplate reader.
Statistical analyses
All data on activity tests were the averages of triplicate analyses. The data were recorded as means ± standard error meaning. Significant differences between means were determined by Student’s t-test; p values <0.05 were regarded as significant.
Results and discussion
Identification of compounds 1–6
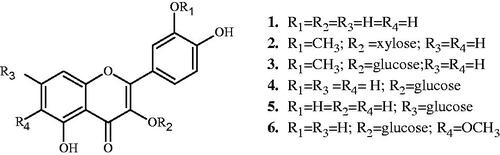
Six known flavonoids, quercetin (1) (Bencheraiet et al. Citation2011), isorhamnetin 3-O-β-d-xyloside (2) (Agrawal and Bansal Citation1989), isorhamnetin 3-O-β-d-glucoside (3) (Benmekhbi et al. Citation2008), quercetin 3-O-β-d-glucoside (4) (Touafek et al. Citation2011), quercetin 7-O-β-d-glucoside (5) (Sikorska & Matławska Citation2000), patuletin 3-O-β-d-glucoside (6) (Nacer et al. Citation2006) (), were isolated from the BEP and EAEP fractions of Evax pygmaea and identified by the use of UV, NMR and acid hydrolysis.
Quercetin (1)
C15H10O7, yellow powder, UV (MeOH, λmax, nm): 257, 375; +NaOH: 271, 425; +AlCl3: 270, 452; +AlCl3/HCl: 267, 426; NaOAc: 274, 394. +H3BO3: 269, 365. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.74 (1H, d, J = 2.0, H-2′), 7.65 (1H, dd, J = 8.5; 2.0, H-6′), 6.92 (1H, d, J = 8.5, H-5′), 6.41 (1H, d, J = 2.1, H-8), 6.21 (1H, d, J = 2.1, H-6).
Isorhamnetin 3-O-β-d-xyloside (2)
C21H20O11, yellow powder, UV (MeOH, λmax, nm): 253, 345; +NaOH: 271, 335, 415, +AlCl3: 268, 401; +AlCl3/HCl: 268, 402; NaOAc: 275, 395. +H3BO3: 270, 346. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.86 (1H, d, J = 2.0, H-2′), 7.57 (1H, dd, J = 8.4; 2.0, H-6′), 6.93 (1H, d, J = 8.4, H-5′), 6.44 (1H, d, J = 2.1, H-8), 6.19 (1H, d, J = 2.1, H-6), 5.37 (1H, d, J = 7.25, H-1″ xylose), 2.70–3.70 (sugar protons), 3.84 (3H, s, OCH3).
Isorhamnetin 3-O-β-d-glucoside (3)
C22H22O12, yellow powder, UV (MeOH, λmax, nm): 254, 355; +NaOH: 271, 330, 413, +AlCl3: 268, 403; +AlCl3/HCl: 269, 400; NaOAc: 274, 396. +H3BO3: 269, 365. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.95 (1H, d, J = 2.0, H-2′), 7.61 (1H, dd, J = 8.5; 2.0, H-6′), 6.93 (1H, d, J = 8.5, H-5'), 6.43 (1H, d, J = 2.1, H-8), 6.23 (1H, d, J = 2.1, H-6), 5.44 (1H, d, J = 7.4, H-1″ glucose), 3.20–3.90 (sugar protons), 3.76 (3H, s, OCH3).
Quercetin 3-O-β-d-glucoside (4)
C21H20O12, yellow powder, UV (MeOH, λmax, nm): 256, 357; +NaOH: 273, 328, 409, +AlCl3: 269, 435; +AlCl3/HCl: 269, 403; NaOAc: 273, 392. +H3BO3: 264, 380. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.74 (1H, d, J = 2.0, H-2′), 7.61 (1H, dd, J = 8.5; 2.0, H-6′), 6.92 (1H, d, J = 8.5, H-5′), 6.41 (1H, d, J = 1.8, H-8), 6.23 (1H, d, J = 1.8, H-6), 5.27 (1H, d, J = 7.6, H-1″ glucose), 3.20–3.79 (sugar protons).
Quercetin 7-O-β-d-glucoside (5)
C21H20O12, yellow powder, UV (MeOH, λmax, nm): 256, 372; +NaOH: 271, 425; +AlCl3: 271, 461; +AlCl3/HCl: 267, 429; NaOAc: 259, 406. +H3BO3: 261, 390. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.79 (1H, d, J = 2.1, H-2′), 7.69 (1H, dd, J = 8.4; 2.1, H-6′), 6.91 (1H, d, J = 8.4, H-5′), 6.78 (1H, d, J = 2.1, H-8), 6.49 (1H, d, J = 2.1, H-6), 5.08 (1H, d, J = 7.4, H-1″ glucose), 3.40–3.75 (sugar protons).
Patuletin 3-O-β-d-glucoside (6)
C22H22O13, yellow powder, UV (MeOH, λmax, nm): 263, 360; +NaOH: 269, 408; +AlCl3: 276, 456; +AlCl3/HCl: 273, 395; NaOAc: 261, 366. +H3BO3: 271, 381. 1H NMR (500 MHz, CD3OD, δ, ppm, J/Hz): 7.80 (1H, d, J = 2.1, H-2′), 7.69 (1H, dd, J = 8.5; 2.1, H-6′), 6.90 (1H, d, J = 8.5, H-5′), 6.69 (1H, s, H-8), 5.08 (1H, d, J = 7.2, H-1″ glucose), 3.10-4.10 (sugar protons), 3.71 (3H, s, OCH3).
Antioxidant properties
The BEP, EAEP and CEP fractions of Evax pygmaea were evaluated for their antioxidant activity. The results showed that the EAEP was the most active in DPPH (IC50: 6.91 ± 0.09 µg/mL), ABTS (IC50: <3.125 μg/mL) and CUPRAC (A0.50: 6.26 ± 0.04 μg/mL); this can be due to its polyphenol content which is higher than that of BEP (). The results obtained with the DPPH assay may be explained by the flavonoid content with isorhamnetin and quercetin major squeletons (). In their study of the structure–activity relationships, Muhammad et al. (Citation2016) showed that the position of –OH and –OCH3 has a great influence on the antioxidant activity.
Table 1. Total polyphenolic content of ethyl acetate (EAEP) and n-butanol (BEP) fractions of Evax pygmaeaTable Footnotea.
In the metal chelating inhibition, the CEP was the highest (>50% at 100 µg/mL), however, the BEP exhibited the best activity in the β-carotene/linoleic acid decolourization assay (IC50: <3.125 μg/mL) ().
Table 2. Antioxidant activity of E. pygmaea fractions by the DPPH, ABTS, CUPRAC, metal chelating and β-carotene assaysTable Footnotea.
Antibacterial activity
The EAEP and BEP fractions displayed the highest activity against Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 43300 and Pseudomonas aeruginosa ATCC 27853, with 40 µg/mL MIC’s value (). However, the CEP fraction inhibited the growth of almost tested microorganisms with 80 µg/mL MIC’s value except against Klebsiella pneumoniae (MIC 40 µg/mL).
Table 3. Antibacterial activity (MICs) of the chloroform (CEP), ethyl acetate (EAEP) and n-butanol (BEP) fractions of Evax pygmaea.
Cytotoxic activity
Cytotoxicity analysis, determined by Brine shrimp lethality test, revealed that this plant was not toxic as their LD50 values were all greater than 80 μg/mL ().
Table 4. Cytotoxic activity of extracts of E. pygmaea.
Conclusions
Six flavonoids have been isolated, for the first time, from the ethyl acetate and n-butanol fractions of E. pygmaea. These fractions showed a good antioxidant activity with the five used methods namely, β-carotene-linoleic acid, DPPH• and ABTS•+ scavenging, CUPRAC and metal chelating assays. This result could be in part related to the isolated flavonoids from which some possess a quercetin squeleton. The fractions showed a good antibacterial activity against tested microorganisms which confirms its use as an antimicrobial. The brine shrimp lethality test revealed that this plant was not toxic. The results of this study indicated that E. pygmaea could be an important dietary source of phenolic compounds with high antioxidant capacity.
Acknowledgements
The authors are grateful to the Groupe Isolement et Structure of the Institut de Chimie Moléculaire de Reims (ICMR), France for NMR spectra.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Agrawal PK, Bansal MC. 1989. Flavonoid glycosides. In: Agrawal PK, editor. Carbon-13 NMR of flavonoids. Amsterdam (The Netherlands): Elsevier; p. 283–364.
- Apak R, Güçlü K, Özyürek M, Karademir SE. 2004. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine CUPRAC method. J Agric Food Chem. 52:7970–7981.
- Bencheraiet R, Kherrab H, Kabouche A, Kabouche Z, Jay M. 2011. Flavonols and antioxidant activity of Ammi visnaga L. (Apiaceae). Rec Nat Prod. 5:52–55.
- Benmekhbi L, Kabouche A, Kabouche Z, Ait-Kaki B, Touzani R, Bruneau C. 2008. Five glycosylated, flavonoids from the antibacterial butanolic extract of Pituranthos scoparius (Apiaceae). Chem Nat Compd. 44:639–641.
- Bensouici C, Kabouche A, Karioti A, Ozturk M, Duru ME, Bilia AR, Kabouche Z. 2016. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharm Biol. 54:174–179.
- Blois MS. 1958. Antioxidant determinations by the use of a stable free radical. Nature. 181:1199–1200.
- Boussaada O, Ammar S, Saidana D, Chraeif I, Mahjoub MA, Helal AN, Mighri Z. 2008a. Volatile constituents of Evax pygmaea from Tunisia. J Essent Oil Bear Plant. 11:171–178.
- Boussaada O, Chriaa J, Nabli R, Ammar S, Saidana D, Mahjoub MA, Chraeif I, Helal AN, Mighri Z. 2008b. Antimicrobial and antioxidant activities of methanol extracts of Evax pygmaea (Asteraceae) growing wild in Tunisia. World J Microbiol Biotechnol. 24:1289–1296.
- Cai Y, Luo Q, Sun M, Corke H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74:2157–2184.
- Decker EA, Welch B. 1990. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 38:674–677.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. 2006. Antioxidant activity, of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97:654–660.
- Ferhat M, Erol E, Beladjila KA, Çetintaş Y, Duru ME, Öztürk M, Kabouche A, Kabouche Z. 2017. Antioxidant, anticholinesterase and antibacterial activities of Stachys guyoniana and Mentha aquatica. Pharm Biol. 55:324–329.
- Funk VA, Bayer RJ, Keeley S, Chan R, Watson L, Gemeinholzer B, Schilling E, Panero JL, Baldwin BG, Garcia JN. 2005. Everywhere but Antarctica: using a super tree to understand the diversity and distribution of the Compositae. Biol Skr. 55:343–374.
- Harborne JB. 1998. Phytochemical methods—a guide to modern techniques of plant analysis. III ed. UK: Chapman Hall; p. 94.
- Havsteen BH. 2002. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 96:67–202.
- Labed A, Ferhat M, Labed-Zouad I, Kaplaner E, Zerizer S, Voutquenne-Nazabadioko L, Alabdul Magid A, Semra Z, Kabouche A, Kabouche Z, et al. 2016. Compounds from the pods of Astragalus armatus with antioxidant, anticholinesterase, antibacterial and phagocytic activities. Pharm Biol. 54:3026–3032.
- Lakhal H, Boudiar T, Kabouche A, Laggoune S, Kabouche Z, Topçu G. 2011. Flavonoids and antioxidant activity of Stachys ocymastrum. Chem Nat Compd. 46:964–966.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 45:31–34.
- Miller HE. 1971. A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc. 45:91.
- Muhammad A, Tel-Çayan G, Mehmet Öztürk M, Duru ME, Nadeem S, Anis A, Weng Ng S, Raza Shah M. 2016. Phytochemicals from Dodonaea viscosa and their antioxidant and anticholinesterase activities with structure–activity relationships. Pharm Biol. 54:1649–1655.
- Nacer A, Bernard A, Boustie J, Touzani R, Kabouche Z. 2006. Aglycone flavonoids of Centaurea tougourensis from Algeria. Chem Nat Compd. 42:190–191.
- NCCLS (National Committee for Clinical Laboratory Standards). 2007. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A6. Wayne (PA): National Committee for Clinical Laboratory Standards.
- Quezel P, Santa S. 1963. Nouvelle flore de l’Algérie et des Régions Désertiques Méridionales. Paris: Tome II, CNRS.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity aplying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237.
- Sikorska M, Matławska I. 2000. Quercetin and its glycosides in the flowers of Asclepias syriaca L. Acta Pol Pharm. 57:321–324.
- Singleton VL, Orthofer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and the oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 299:152–178.
- Touafek O, Kabouche Z, Brouard I, Bermejo JB. 2011. Flavonoids of Campanula alata and their antioxidant activity. Chem Nat Compd. 46:968–970.
- Yang J, Martinson TE, Liu RH. 2009. Phytochemical profiles and antioxidant activities of wine grapes. Food Chem. 116:332–339.