Abstract
Context: Geniposide (genipin-1-O-β-d-glucoside) is a major bioactive ingredient in the fruits of gardenia [Gardenia jasminoides J. Ellis (Rubiaceae)], a traditional herbal medicine in Asian countries.
Objective: This work assesses the skin anti-photoaging potential of geniposide in human dermal fibroblasts under UV-B irradiation.
Materials and methods: The anti-photoaging property of geniposide, at varying concentrations (5, 12 and 30 μM) treated for 30 min prior to UV-B irradiation, was evaluated by analysing reactive oxygen species (ROS), promatrix metalloproteinase-2 (proMMP-2), glutathione (GSH), superoxide dismutase (SOD), nuclear factor erythroid 2-related factor 2 (Nrf2) and cellular viability.
Results: Geniposide suppressed the ROS elevation under UV-B irradiation, which was revealed using three ROS-sensitive fluorescent dyes. The use of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), dihydroethidium (DHE) and dihydrorhodamine 123 (DHR-123) elicited the IC50 values of 10.5, 9.8 and 21.0 μM, respectively. Geniposide attenuated proMMP-2 at activity and protein levels that were elevated under UV-B-irradiation. Geniposide at 5, 12 and 30 μM augmented the UV-B-reduced total GSH content to 1.9 ± 0.1-, 2.2 ± 0.2- and 4.1 ± 0.2-fold, respectively. Geniposide at 5, 12 and 30 μM upregulated total SOD activity to 2.3 ± 0.1-, 2.5 ± 0.3- and 3.3 ± 0.3-fold, respectively, under UV-B irradiation. The UV-B-reduced Nrf2 levels were also upregulated by geniposide treatment. Geniposide, at the concentrations used, was unable to interfere with cellular viabilities under UV-B irradiation.
Discussion and conclusions: After the skin anti-photoaging potential of geniposide may be further verified, it can be utilized as a safer resource in the manufacture of effective anti-aging cosmetics.
Introduction
Ultraviolet (UV) radiation gives rise to harmful phenomena in human skin, which include tanning, sunburn, immune suppression, cancer, photoaging, etc. (Afnan et al. Citation2012). Photoaging is regarded as an extrinsic aging caused by environmental factors, including UV radiation, and basically characterized by wrinkles and dryness (Anandan et al. Citation2013). Among the three subtypes of UV radiation, UV-C radiation (wavelength, 100–280 nm) is almost completely absorbed by the ozone layer and has no harmful effect, UV-B radiation (280–315 nm) causes adverse effects on the superficial layer of the skin and is the most threatening environmental factor to photoaging, and UV-A radiation (315–400 nm) has a minor influence on the skin. Chronic exposure to UV-B radiation typically brings about the enhanced generation of reactive oxygen species (ROS) in the skin, which leads to oxidative stress and photodamages to macromolecules, such as proteins and nucleic acids (Jo et al. Citation2012). It also causes adverse changes in the extracellular matrix that lead to skin photoaging due to the enhanced production of matrix metalloproteinases (MMPs), including MMP-1, MMP-2 and MMP-9, which is possibly mediated by augmented ROS production (Ho et al. Citation2005).
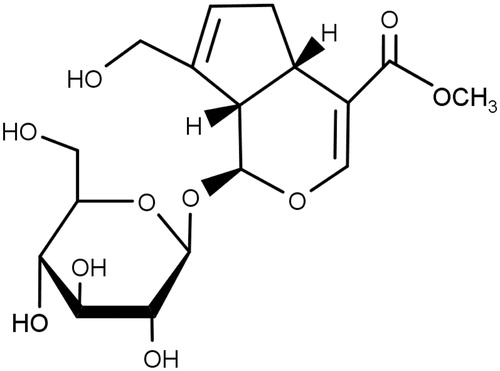
The fruits of gardenia [Gardenia jasminoides J. Ellis, (Rubiaceae)] have been included in traditional formulations for the treatment of inflammation, jaundice, headache, oedema, fever, hepatic disorders and hypertension (Aburada et al. Citation1976; Tseng et al. Citation1995). Their main ingredients include iridoid glycosides, such as geniposide (), gardenoide, genipin-1-O-β-gentiobioside, geniposidic acid, acetylgeniposide and gardoside (Bergonzi et al. Citation2012). Besides them, they contain crocin, crocin-3, crocetin, imperatorin, isoimperatorin, 5-hydroxy-7,3′,4′,5′-tetrainethoxyflavone and so on (Chen et al. Citation2007). Geniposide has been shown to have various biological and pharmacological activities, including neuroprotective (Chen et al. Citation2015), antidepressant (Cai et al. Citation2015), antinociceptive (Gong et al. Citation2014), anti-allergic (Sung et al. Citation2014), anti-angiogenic (Koo et al. Citation2004), anti-inflammatory (Chen et al. Citation2015), antidiabetic (Yao et al. Citation2015), antithrombotic (Suzuki et al. Citation2001) and hepatoprotective (Wang et al. Citation2015) activities.
At present, geniposide has been found to exert some of its known pharmacological effects through its antioxidant activity. It inhibits hypoxia/reoxygenation-induced myocardial apoptosis by reversing mitochondrial dysfunction through attenuating oxidative stress products, including ROS/reactive nitrogen species and malondialdehyde, and enhancing antioxidant enzymes (Jiang et al. Citation2016). Geniposide can protect cultured primary cortical neurons from amyloid-β-mediated mitochondrial dysfunction by reducing ROS production, recovering mitochondrial membrane potential and inhibiting apoptosis (Zhao et al. Citation2016). It diminishes amyloid-β-induced inflammation and oxidative stress by targeting receptor for advanced glycation end products (RAGE)-dependent signalling in vivo and in vitro (Lv et al. Citation2015). However, the beneficial effects of geniposide in human skin have not been clearly established. In the present work, the protective properties of geniposide against UV-B-induced photooxidative stress have been ascertained in human dermal fibroblasts, implying its potential use as a natural resource in the manufacture of anti-photoaging cosmetics.
Materials and methods
Chemicals
Geniposide (purity ≥98%), bovine serum albumin (BSA), Bradford’s reagent, 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT), 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), dihydrorhodamine 123 (DHR-123), dihydroethidium (DHE), 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), glutathione (GSH), glutathione reductase (GR), catalase, xanthine, xanthine oxidase, cytochrome c and NADPH were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). Cell lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM 1,2-diaminocyclohexane-N,N,Nv,Nv-tetraacetic acid, 2 mM dithiothreitol, 10% glycerol, 1% Triton X-100] was obtained from Promega Korea (Seoul, Korea). All other chemicals used were of the highest grade commercially available.
Cell culture
A human dermal fibroblast cell line, CCD986Sk (ATCC, Manassas, VA), was grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% heat-inactivated foetal bovine serum (FBS), 100 μg/mL streptomycin and 100 units/mL penicillin in a humidified atmosphere with 5% CO2 at 37 °C.
UV-B irradiation
As a source of UV-B radiation, an UV lamp (peak, 312 nm; model VL-6M, Vilber Lourmat, Marine, France) was used along with a radiometer (model VLX-3W, Vilber Lourmat, Marine, France) and a sensor (bandwidth, 280–320 nm; model CX-312, Vilber Lourmat, Marine, France). Fibroblasts were irradiated with solar simulated UV-B radiation at the intensity of 70 mJ/cm2 which was chosen to induce photooxidative stress based on the preliminary work.
Preparation of cellular lysate
After adherent cells were twice washed with phosphate-buffered saline and stored on ice for 5 min, they were collected using a cell scraper. After centrifugation at 5000×g for 10 min, the cell pellets were resuspended in cell lysis buffer and stored for 30 min on ice. Cellular lysate was taken after centrifugation at 5000×g for 15 min.
Protein contents in cellular lysates were quantitated according to the Bradford protein assay (Bradford Citation1976) using BSA as a reference protein.
Quantitation of intracellular ROS
An ROS-sensitive probe DCFH-DA, which generates the fluorescent 2′,7′-dichlorofluorescein (DCF; λexcitation = 485 nm, λemission = 530 nm) upon enzymatic reduction and subsequent oxidation by ROS, was used (Royall and Ischiropoulos Citation1993). Two other ROS probes, such as DHE and DHR-123, were also used in a similar manner, which produce fluorescent 2-hydroxyethidium (2-HE; λexcitation = 480 nm, λemission = 525 nm) and rhodamine 123 (RH-123; λexcitation = 500 nm, λemission = 535 nm), respectively, upon reaction with ROS. After cells were treated with geniposide and/or 20 μM DCFH-DA, 5 μM DHE or 5 μM DHR-123 for 30 min at 37 °C, they were twice washed with 1 mL FBS-free DMEM. The cells, resuspended in 1 mL FBS-free DMEM, were irradiated. The ROS levels were quantitated by monitoring fluorescence using multi-mode microplate reader (Synergy™ Mx, BioTek Instruments, Winooki, VT).
MTT assay
Cell viabilities were determined using MTT assay based on metabolic activity (Freshney Citation1994). The amount of formazan, produced from reduction of MTT by the mitochondria of living cells, was quantitated by monitoring absorbance at 540 nm.
Gelatin zymography
The gelatinolytic activity of promatrix metalloproteinase-2 (proMMP-2) in conditioned media was determined using zymographic analysis (Kleiner and Stetler-Stevenson Citation1994). After SDS-PAGE gel was stained with 0.1% Coomassie Brilliant Blue R-250, proMMP-2 activities were convinced as clear zones against a blue background. The proMMP-2 band was confirmed in accordance with its molecular mass, 72 kDa, which was identified by protein molecular weight markers.
Western blotting analysis
To quantitate proMMP-2 and nuclear factor erythroid 2-related factor 2 (Nrf2) in cellular lysates, western blotting analysis was performed using anti-MMP-2 (ALX-210-753, Enzo Life Sciences, Farmingdale, NY) and anti-Nrf2 (ab31163, Abcam, Cambridge, MA) antibodies as primary antibodies. GAPDH, used as an internal loading control, was detected using anti-GAPDH antibody (LF-PA0212, AbFrontier, Seoul, Korea). In brief, cellular lysates were separated on 10% (w/v) SDS-PAGE and electrotransferred to PVDF membranes. The blotted membrane was blocked with blocking buffer (2% BSA in 1× TBS-Tween 20), probed with primary antibodies overnight at 4 °C, incubated with secondary antibody (goat anti-rabbit IgG-pAb-HRP-conjugate; ADI-SAB-300, Enzo Life Sciences, Farmingdale, NY) for 1 h at room temperature, and developed using an enhanced West-save upTM (AbFrontier, Seoul, Korea).
Quantitation of total GSH
Total GSH in cellular lysates was quantitated using an enzymatic recycling assay based upon GR (Nakagawa et al. Citation1990). The reaction mixture (200 μL), which contained 175 mM KH2PO4, 6.3 mM EDTA, 0.21 mM NADPH, 0.6 mM DTNB, 0.5 units/mL GR and cellular lysate, was incubated at 25 °C. Absorbance at 412 nm was monitored using a microplate reader. Total GSH content was reported as μg/mg protein.
Determination of SOD activity
Total superoxide dismutase (SOD) activity in cellular lysates was determined as the reduction of cytochrome c with xanthine/xanthine oxidase system (Lee et al. Citation2002). The reaction mixture (200 μL) contained 50 mM phosphate buffer (pH 7.4), 0.01 units/mL xanthine oxidase, 0.1 mM EDTA, 1 μM catalase, 0.05 mM xanthine, 20 μM cytochrome c and cellular lysate. A change in absorbance at 550 nm was monitored using a microplate reader.
Statistical analysis
The results were represented as mean ± SD. Differences between experimental groups were analysed using one-way ANOVA followed by post hoc Tukey’s HSD test for multiple comparisons. A p value <0.05 was considered statistically significant.
Results
Suppression of UV-B-induced ROS elevation
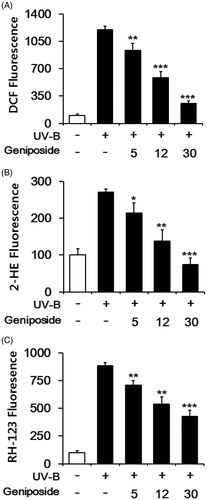
Fibroblasts were subjected to varying concentrations (0, 5, 12 or 30 μM) of geniposide prior to UV-B irradiation. When DCFH-DA was used as an ROS probe, the UV-B irradiation alone gave rise to about 12.3 ± 0.4-fold elevation in the ROS level over that in the non-irradiated control (). Geniposide at 5, 12 and 30 μM made the UV-B-induced ROS elevation reduce to 78.4 ± 6.8, 48.6 ± 6.0 and 21.6 ± 2.6% of the UV-B irradiation alone, respectively (). When DHE was used, the UV-B irradiation alone induced the ROS level to about 2.7 ± 0.1-fold over the non-irradiated control, and geniposide concentration-dependently attenuated the UV-B-induced ROS elevation (). When DHR-123 was used, geniposide similarly displayed an attenuation on the UV-B-induced ROS (). The use of DCFH-DA, DHE and DHR-123 gave rise to the IC50 values of 10.5, 9.8 and 21.0 μM, respectively. Collectively, geniposide suppresses the UV-B-induced ROS elevation in fibroblasts.
Figure 2. Attenuating effects of geniposide on the reactive oxygen species (ROS) elevation in human dermal fibroblasts under UV-B irradiation. Fibroblasts were subjected to the varying concentrations (0, 5, 12 or 30 μM) of geniposide for 30 min before the irradiation. The intracellular ROS levels were determined using DCFH-DA (A), DHE (B) and DHR-123 (C) in a microplate fluorometer. The intracellular ROS level was represented as DCF (A), 2-hydroethidium (2-HE, B) and rhodamine 123 (RH-123, C) fluorescence, expressed as % of the non-irradiated control. *p < 0.05; **p < 0.01; ***p < 0.001 versus the non-treated control (UV-B irradiation alone).
Non-cytotoxicity
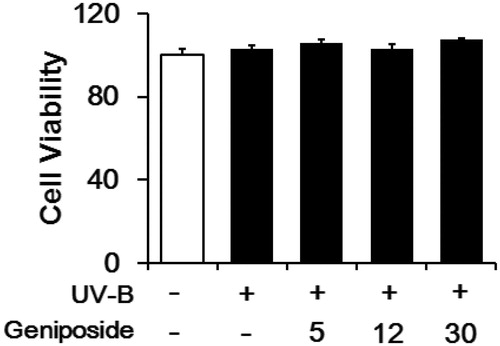
UV-B irradiation, under the experimental conditions used, was nontoxic to the viabilities of fibroblasts (). Geniposide was unable to interfere with the viability of fibroblasts under UV-B irradiation ().
Attenuation of UV-B-induced proMMP-2 elevation
As previously described (Kim et al. Citation2013), the UV-B irradiation enhanced the proMMP-2 gelatinolytic activity in cellular lysates to 5.5 ± 0.2-fold, compared with the non-irradiated control (). Geniposide at 5, 12 and 30 μM diminished the UV-B-induced proMMP-2 activity to 87.9 ± 3.8, 78.8 ± 5.3 and 69.7 ± 5.7% of the non-treated control, respectively ().
Figure 4. Attenuating effect of geniposide on the elevation of promatrix metalloproteinase-2 (proMMP-2) activity (A) and protein (B) levels in human dermal fibroblasts under UV-B irradiation. Fibroblasts were subjected to the varying concentrations (0, 5, 12 or 30 μM) of geniposide for 30 min before the irradiation. (A) The proMMP-2 gelatinolytic activity in conditioned medium was detected using gelatin zymography. (B) The proMMP-2 proteins in cellular lysates were determined using western blotting analysis with anti-MMP-2 antibodies. GAPDH was used as an internal loading control. A representative of the three independent results was shown. The band strength, represented as % of the non-irradiated control, was determined by densitometry using the ImageJ (version 1.48) software which is downloaded from the NIH website. (B) It was normalized to the corresponding GAPDH band. #p < 0.05 versus the non-irradiated control. (UV-B irradiation alone). *p < 0.05; **p < 0.01; ***p < 0.001.
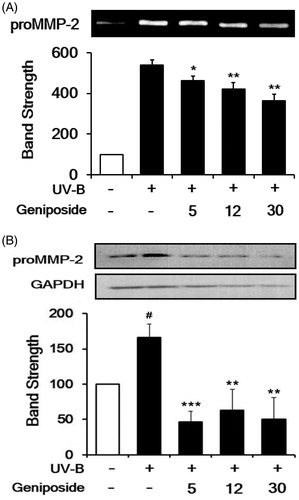
The UV-B irradiation alone significantly enhanced the proMMP-2 protein levels, compared to the non-irradiated control, and geniposide at 5, 12 and 30 μM markedly attenuated the UV-B-induced proMMP-2 protein levels (). In brief, geniposide down-regulates the UV-B-induced proMMP-2 at the protein level.
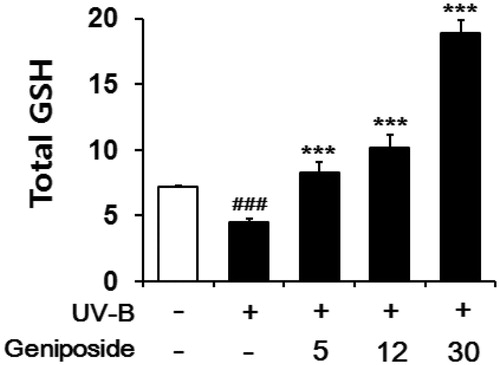
Enhancement of UV-B-reduced total GSH
In the irradiated fibroblasts, total GSH contents in cellular lysates were diminished to 64.4 ± 3.1% of the non-irradiated control (), which was consistent with the previous finding (Zhu and Bowden Citation2004). Geniposide at 5, 12 and 30 μM enhanced the total GSH levels by 1.9 ± 0.1-, 2.2 ± 0.2- and 4.1 ± 0.2-fold, respectively, compared to the irradiated fibroblasts that was not pretreated with geniposide (). Briefly, geniposide is capable of enhancing the UV-B-reduced total GSH levels in fibroblasts.
Figure 5. Enhancing effects of geniposide on total glutathione (GSH) levels in cellular lysates of human dermal fibroblasts under the irradiation with UV-B radiation. Fibroblasts were subjected to the varying concentrations (0, 5, 12 or 30 μM) of geniposide for 30 min before the irradiation. Total GSH content, expressed as μg/mg protein, was determined with enzymatic recycling assay using GR. ###p < 0.001 versus the non-irradiated control. ***p < 0.001 versus the non-treated control (UV-B irradiation alone).
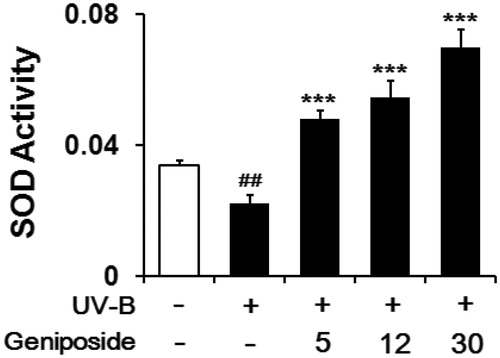
Enhancement of UV-B-reduced SOD activity
When fibroblasts were irradiated with UV-B light, total SOD activity in cellular lysates was diminished to 64.8 ± 7.4% of the non-irradiated control (). Geniposide at 5, 12 and 30 μM enhanced total SOD activity by 2.3 ± 0.1-, 2.5 ± 0.3- and 3.3 ± 0.3-fold, respectively, compared to the UV-B-irradiated fibroblasts without geniposide pretreatment (). Briefly, geniposide enhances the UV-B-reduced total SOD activity in fibroblasts.
Figure 6. Enhancing effects of geniposide on total superoxide dismutase (SOD) activity levels in cellular lysates of human dermal fibroblasts under the irradiation with UV-B radiation. Fibroblasts were subjected to the varying concentrations (0, 5, 12 or 30 μM) of geniposide for 30 min before the irradiation. Total SOD activity, expressed as Δ550/min/mg protein, was measured using a spectrophotometric assay. ##p < 0.01 versus the non-irradiated control. ***p < 0.001 versus the non-treated control (UV-B irradiation alone).
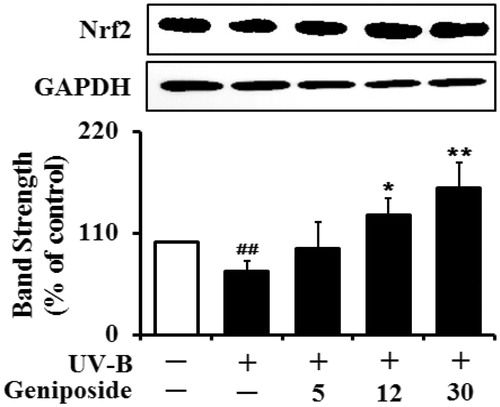
Enhancement of UV-B-reduced Nrf2 levels
The Nrf2 levels in the UV-B-irradiated cells were dropped to 71.4 ± 9.3% of the non-irradiated control (). Geniposide at 5, 12 and 30 μM could cause an enhancement in the UV-B-reduced Nrf2 levels by 1.3 ± 0.4-, 1.8 ± 0.3- and 2.3 ± 0.4-fold, respectively, compared to the UV-B irradiation only (). Taken together, geniposide has an up-regulating activity on the UV-B-reduced Nrf2 levels in fibroblasts.
Figure 7. Enhancing effect of geniposide on the Nrf2 levels in human dermal fibroblasts under UV-B irradiation. Fibroblasts were subjected to fresh media with the varying concentrations (0, 5, 12 or 30 μM) of geniposide for 30 min before the irradiation. The Nrf2 proteins were determined using western blotting analysis with anti-Nrf2 antibodies. GAPDH was used as a protein loading control. The relative band strength was determined with densitometry using ImageJ software. Data are presented as % of control versus the non-irradiated control. ##p < 0.01 versus the non-irradiated control; *p < 0.05; **p < 0.01 versus the non-treated control (UV-B irradiation alone).
Discussion
Gardenia fruits have been used for the treatment of diverse disorders, including inflammation, oedema and dermatitis, in traditional folk medicine. Geniposide, one of the main ingredients known to have both medicinal and nutritional value in gardenia fruits, has been assessed to be responsible for their diverse pharmacological actions. Geniposide protects against acute alcohol-induced liver injury through up-regulating the expression of the main antioxidant components, such as GSH, glutathione-S-transferase, GSH peroxidase, SOD and catalase, which thus ameliorates the alcohol-induced oxidative stress injury in the liver (Wang et al. Citation2015). It also enhances the levels of GSH and antioxidant enzymes, such as SOD and catalase, which are diminished in CCl4-treated mouse livers (Chen et al. Citation2016). Geniposide protects against the liver injury induced by tripterygium glycosides, commonly used as a basic medicine in the treatment of rheumatoid arthritis but with a high incidence of liver injury, in mice through the attenuation of oxidative stress and inflammation (Wang et al. Citation2016). It plays a protective role against lipopolysaccharide-induced acute lung injury by diminishing inflammatory responses via the activation of blocking nuclear factor-κB and mitogen-activated protein kinase signalling pathways (Xiaofeng et al. Citation2012). Geniposide suppresses the release of histamine from mast cells, which implies an anti-allergic effect and subsequently a therapeutic potential for atopic dermatitis (Sung et al. Citation2014). Penta-acetyl geniposide, an acetylated geniposide known to have hepatoprotective, anti-proliferative and apoptotic properties, exhibits an antimetastatic action in C6 glioma cells by mitigating MMP-2 levels (Huang et al. Citation2009). In the present work, we demonstrate that geniposide possesses radioprotective properties against UV-B irradiation by attenuating UV-B-induced ROS and proMMP-2 elevation, which proposes its skin anti-photoaging property. Our work further supports that the skin anti-photoaging property of geniposide might be based upon its up-regulating activity on antioxidant components, including total GSH and SOD activity tested in this work. This finding might correspond with the up-regulation of antioxidant components by geniposide in its hepatoprotective activity against the alcohol-induced oxidative stress injury in the liver (Wang et al. Citation2015). A recent report demonstrates that geniposide ameliorates 2,4,6-trinitrobenzene sulfonic acid-induced experimental rat colitis by reducing inflammation and modulating the disrupted epithelial barrier function via activating the AMP-activated protein kinase signalling pathway (Xu et al. Citation2017).
Diverse environmental factors, such as solar UV radiation and other external aggressors, can elicit an oxidative challenge that is detrimental to skin health. For example, continuing exposure to solar UV radiation leads to immune suppression, inflammation, photoaging and skin photocarcinogenesis. Since the levels of endogenous antioxidants decrease with age, thus resulting in a less protection and a greater potential for skin damage (Rodriguez et al. Citation2013), the improved strategies for skin photoprotection are needed, especially for elderly people. Pterostilbene, a natural phytoalexin, prevents an acute UV-B-induced increase in skin fold, thickness and redness, as well as photoaging-associated skin winkling and hyperplasia (Sirerol et al. Citation2015). It also prevents the chronic UV-B irradiation-induced skin carcinogenesis, which is associated with the maintenance of skin antioxidant defences, including GSH, catalase, SOD and GSH peroxidase, and the inhibition of UV-B-induced oxidative damage (Sirerol et al. Citation2015). These previous findings strongly suggest that certain compounds, either synthetic or natural, which are able to up-regulate the levels of antioxidant components, can act as effective skin anti-photoaging agents. Geniposide is been thought to be one candidate of such compounds, which can up-regulate at least two main antioxidant components, such as GSH and SOD.
Nrf2 is known as a redox-sensitive transcription factor which plays an important role in the protective process against UV-B-induced photoaging, originally based upon the fact that UV-B-irradiated nrf2–/– mice exhibit accelerated photoaging symptoms and decreased cutaneous GSH levels (Hirota et al. Citation2011). As a master regulator of the cellular antioxidant defence against environmental electrophilic insult, Nrf2 has emerged as a crucial determinant of cutaneous damage from solar UV, and the concept of pharmacological activation of Nrf2 has attracted considerable attention as a valuable approach to skin photoprotection (Tao et al. Citation2013). Nrf2 plays a protective role against UV-induced apoptosis in vitro and acute sunburn reactions in vivo, and prevents photoaging by preserving high levels of antioxidants, such as GSH, in the skin (Kim et al. Citation2015). In a human skin reconstruct exposed to solar simulated UV radiation, dihydrotanshinone, a phenanthrenequinone-based Nrf2 inducer, was found to cause an enhancement in Nrf2 and γ-glutamylcysteine synthetase levels together with the elevation of total GSH levels, and subsequently attenuate the occurrence of epidermal solar insult-markers, such as cleaved procaspase-3, pyknotic nuclei, eosinophilic cytoplasm and acellular cavities (Tao et al. Citation2013). Sulforaphane, an isothiocyanate derived from broccoli, induces the endogenous cellular defences regulated by Nrf2, including cytoprotective enzymes and GSH (Benedict et al. Citation2012). The apocarotenoid bixin, a natural food additive consumed worldwide, was previously shown to protect skin against solar UV-induced damage through the activation of Nrf2 (Tao et al. Citation2015). Geniposide is capable of up-regulating Nrf2 levels diminished under UV-B irradiation, suggesting that geniposide acts as an Nrf2 activator. Subsequently, geniposide causes enhancements in antioxidant components, which may result from its up-regulation of Nrf2. Currently, geniposide is assumed to have its defensive properties against photooxidative stress via the mediation of Nrf2.
Conclusions
Geniposide suppresses the UV-B-induced elevation of ROS and proMMP-2 in human dermal fibroblasts. On the contrary, it augments the UV-B-reduced total GSH and SOD activity levels under UV-B irradiation. Geniposide up-regulates the UV-B-reduced Nrf2, a major transcriptional regulator that mediates the expression of a variety of endogenous antioxidants and detoxifying genes. Collectively, geniposide plays an anti-photoaging role in human skin under UV-B irradiation, possibly via up-regulation of Nrf2. This finding implies geniposide and geniposide-containing mixtures can be utilized as a natural resource in the manufacture of anti-photoaging cosmetics.
Acknowledgements
The authors are very grateful to Ms Yuri Oh and Ms Su Hee Lee for their technical assistance.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for performing the work and writing the manuscript.
Additional information
Funding
References
- Aburada M, Sasaki H, Harada M. 1976. Pharmacological studies of gardeniae fructus. II. Contribution of the constituent crude drugs to choleretic activity of “Inchinko-to” in rats. Yakugaku Zasshi. 96:147–153.
- Afnan Q, Adil MD, Nissar-Ul A, Rafiq AR, Amir HF, Kaiser P, Gupta VK, Vishwakarma R, Tasduq SA. 2012. Glycyrrhizic acid (GA), a triterpenoid saponin glycoside alleviates ultraviolet-B irradiation-induced photoaging in human dermal fibroblasts. Phytomedicine. 19:658–664.
- Anandan R, Ganesan B, Obulesu T, Mathew S, Asha KK, Lakshmanan PT, Zynudheen AA. 2013. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young and aged rats. Cell Stress Chaperones. 18:121–125.
- Benedict AL, Knatko EV, Dinkova-Kostova AT. 2012. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis. 33:2457–2466.
- Bergonzi MC, Righeschi C, Isacchi B, Bilia AR. 2012. Identification and quantification of constituents of Gardenia jasminoides Ellis (Zhizi) by HPLC-DAD-ESI-MS. Food Chem. 134:1199–1204.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Cai L, Li R, Tang WJ, Meng G, Hu XY, Wu TN. 2015. Antidepressant-like effect of geniposide on chronic unpredictable mild stress-induced depressive rats by regulating the hypothalamus-pituitary-adrenal axis. Eur Neuropsychopharmacol. 25:1332–1341.
- Chen H, Xiao YQ, Li L, Zhang C. 2007. Studies on chemical constituents in fruit of Gardenia jasminoides. Zhongguo Zhong Yao Za Zhi. 32:1041–1043.
- Chen JY, Wu H, Li H, Hu SL, Dai MM, Chen J. 2015. Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 24:102–109.
- Chen P, Chen Y, Wang Y, Cai S, Deng L, Liu J, Zhang H. 2016. Comparative evaluation of hepatoprotective activities of geniposide, crocins and crocetin by CCl4-induced liver injury in mice. Biomol Ther (Seoul). 24:156–162.
- Chen Y, Zhang Y, Li L, Hölscher C. 2015. Neuroprotective effects of geniposide in the MPTP mouse model of Parkinson's disease. Eur J Pharmacol. 768:21–27.
- Freshney RI. 1994. Culture of animal cells: a manual of basic technique. 4th ed. New York: Wiley-Liss Press.
- Gong N, Fan H, Ma AN, Xiao Q, Wang YX. 2014. Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology. 84:31–45.
- Hirota A, Kawachi Y, Yamamoto M, Koga T, Hamada K, Otsuka F. 2011. Acceleration of UVB-induced photoageing in nrf2 gene-deficient mice. Exp Dermatol. 20:664–668.
- Ho JN, Lee YH, Park JS, Jun WJ, Kim HK, Hong BS, Shin DH, Cho HY. 2005. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm Bull. 28:1244–1248.
- Huang HP, Shih YW, Wu CH, Lai PJ, Hung CN, Wang CJ. 2009. Inhibitory effect of penta-acetyl geniposide on C6 glioma cells metastasis by inhibiting matrix metalloproteinase-2 expression involved in both the PI3K and ERK signaling pathways. Chem-Biol Interact. 181:8–14.
- Jiang YQ, Chang GL, Wang Y, Zhang DY, Cao L, Liu J. 2016. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell Physiol Biochem. 39:407–421.
- Jo WS, Yang KM, Park HS, Kim GY, Nam BH, Jeong MH, Choi YJ. 2012. Effect of microalgal extracts of Tetraselmis suecica against UVB-induced photoaging in human skin fibroblasts. Toxicol Res. 28:241–248.
- Kim M, Park YG, Lee HJ, Lim SJ, Nho CW. 2015. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-induced MMP expression and promote type I procollagen production via repression of MAPK/AP-1/NF-κB and activation of AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J Agric Food Chem. 63:5428–5438.
- Kim MS, Oh GH, Kim MJ, Hwang JK. 2013. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem Photobiol. 89:911–918.
- Kleiner DE, Stetler-Stevenson WG. 1994. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem. 218:325–329.
- Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ, Park EH. 2004. Geniposide, an anti-angiogenic compound from the fruits of Gardenia jasminoides. Planta Med. 70:467–469.
- Lee YY, Kim HG, Jung HI, Shin YH, Hong SM, Park EH, Sa JH, Lim CJ. 2002. Activities of antioxidant and redox enzymes in human normal hepatic and hepatoma cell lines. Mol Cells. 14:305–311.
- Lv C, Wang L, Liu X, Yan S, Yan SS, Wang Y, Zhang W. 2015. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology. 89:175–184.
- Nakagawa K, Saijo N, Tsuchida S, Sakai M, Tsunokawa Y, Yokota J, Muramatsu M, Sato K, Terada M, Tew KD. 1990. Glutathione-S-transferase pi as a determinant of drug resistance in transfectant cell lines. J Biol Chem. 265:4296–4301.
- Rodriguez KJ, Wong HK, Oddos T, Southall M, Frei B, Kaur S. 2013. A purified feverfew extract protects from oxidative damage by inducing DNA repair in skin cells via a PI3-kinase-dependent Nrf2/ARE pathway. J Dermatol Sci. 72:304–310.
- Royall JA, Ischiropoulos H. 1993. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 302:348–355.
- Sirerol JA, Feddi F, Mena S, Rodriguez ML, Sirera P, Aupí M, Pérez S, Asensi M, Ortega A, Estrela JM. 2015. Topical treatment with pterostilbene, a natural phytoalexin, effectively protects hairless mice against UVB radiation-induced skin damage and carcinogenesis. Free Rad Biol Med. 85:1–11.
- Sung YY, Lee AY, Kim HK. 2014. The Gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J Ethnopharmacol. 156:33–40.
- Suzuki Y, Kondo K, Ikeda Y, Umemura K. 2001. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. 67:807–810.
- Tao S, Justiniano R, Zhang DD, Wondrak GT. 2013. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 1:532–541.
- Tao S, Park SL, de la Vega MR, Zhang DD, Wondrak GT. 2015. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic Biol Med. 89:690–700.
- Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. 1995. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett. 97:61–67.
- Wang J, Miao M, Qu L, Cui Y, Zhang Y. 2016. Protective effects of geniposide against Tripterygium glycosides (TG)-induced liver injury and its mechanisms. J Toxicol Sci. 41:165–173.
- Wang J, Zhang Y, Liu R, Li X, Cui Y, Qu L. 2015. Geniposide protects against acute alcohol-induced liver injury in mice via up-regulating the expression of the main antioxidant enzymes. Can J Physiol Pharmacol. 93:261–267.
- Xiaofeng Y, Qinren C, Jingping H, Xiao C, Miaomiao W, Xiangru F, Xianxing X, Meixia H, Jing L, Jingyuan W, et al. 2012. Geniposide, an iridoid glucoside derived from Gardenia jasminoides, protects against lipopolysaccharide-induced acute lung injury in mice. Planta Med. 78:557–564.
- Xu B, Li YL, Xu M, Yu CC, Lian MQ, Tang ZY, Li CX, Lin Y. 2017. Geniposide ameliorates TNBS-induced experimental colitis in rats via reducing inflammatory cytokine release and restoring impaired intestinal barrier function. Acta Pharmacol Sin. 38:688–698.
- Yao DD, Yang L, Wang Y, Liu C, Wei YJ, Jia XB, Yin W, Shu L. 2015. Geniposide promotes beta-cell regeneration and survival through regulating β-catenin/TCF7L2 pathway. Cell Death Dis. 6:e1746.
- Zhao C, Lv C, Li H, Du S, Liu X, Li Z, Xin W, Zhang W. 2016. Geniposide protects primary cortical neurons against oligomeric Aβ1–42-induced neurotoxicity through a mitochondrial pathway. PLoS One. 11:e0152551.
- Zhu M, Bowden GT. 2004. Molecular mechanism(s) for UV-B irradiation-induced glutathione depletion in cultured human keratinocytes. Photochem Photobiol. 80:191–196.