Abstract
Context: Cudrania tricuspidata Bureau (Moraceae) is an important source of traditional Korean and Chinese medicines used to treat neuritis and inflammation.
Objective: The anti-neuroinflammatory effects of cudraflavanone A isolated from a chloroform fraction of C. tricuspidata were investigated in LPS-induced BV2 cells.
Materials and methods: Cudraflavanone A was isolated from the root of C. tricuspidata, and its structure was determined by MS and NMR data. Cytotoxicity of the compound was examined by MTT assay, indicating no cytotoxicity at 5–40 μM of cudraflavanone A. NO concentration was measured by the Griess reaction, and the levels of PGE2, cytokines and COX-2 enzyme activity were measured by each ELISA kit. The mRNA levels of cytokines were analysed by quantitative-PCR. The expression of iNOS, COX-2, HO-1, NF-κB, MAPKs and Nrf2 was detected by Western blot.
Results: Cudraflavanone A had no major effect on cell viability at 40 μM indicating 91.5% viability. It reduced the production of NO (IC50 = 22.2 μM), PGE2 (IC50 = 20.6 μM), IL-1β (IC50 = 24.7 μM) and TNF-α (IC50 = 33.0 μM) in LPS-stimulated BV2 cells. It also suppressed iNOS protein, IL-1β and TNF-α mRNA expression. These effects were associated with the inactivation of NF-κB, JNK and p38 MAPK pathways. This compound mediated its anti-neuroinflammatory effects by inducing HO-1 protein expression via increased nuclear translocation of Nrf2.
Discussion and conclusions: The present study suggests a potent effect of cudraflavanone A to prevent neuroinflammatory diseases. Further investigation is necessary to elucidate specific molecular mechanism of cudraflavanone A.
Introduction
Neuroinflammation is generally associated with neurodegenerative disorders of the central nervous system (CNS) (Groh and Martini Citation2017). Microglia are the resident macrophages of the CNS, and play significant roles in homeostasis and neuroinflammatory pathologies in the brain (Cianciulli et al. Citation2016). The activation of microglial cells is known to play a significant role in neuroinflammation through the excessive production of pro-inflammatory mediators (Okorji et al. Citation2016). Under inflammatory conditions, microglial cells may get activated and cause abnormal regulation of pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2 and pro-inflammatory cytokines as like interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α (Streit et al. Citation2004; More et al. Citation2013). This series of inflammatory reactions is related to the activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) pathways (Baliga et al. Citation2012). These pathways are activated by various stimuli, and result in the up-regulation of NF-κB and MAPK target genes, as observed with pro-inflammatory mediators (Lawrence Citation2009; Velagapudi et al. Citation2014). Therefore, the regulation of inflammation in the nervous system mediated by microglial cell is related to the various pathogenesis of neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), frontotemporal dementia and amyotrophic lateral sclerosis (Catorce and Gevorkian Citation2016).
Heme oxygenase (HO)-1 could be induced in response to immunological activities as like inflammation or oxidative stress. HO-1 is regulated by the nuclear transcription factor erythroid-2 related factor 2 (Nrf2) pathway. Several reports have demonstrated that the activation of HO-1 signalling results in the inhibition of inflammatory responses (Matz et al. Citation1996; Lin et al. Citation2014). Therefore, HO-1 is one of the potential targets for the treatment of various inflammatory diseases.
Cudrania tricuspidata Bureau (Moraceae) is a deciduous plant used as a traditional medicinal herb for the treatment of various disorders (Zhang Citation1985; Xin et al. Citation2017), as this plant is a rich source of bioactive compounds with antioxidant, neuroprotective and anti-inflammatory effects (Lee et al. Citation2005; Park et al. Citation2006; Kwon et al. Citation2014). Recent studies have highlighted the various components isolated from C. tricuspidata with anti-inflammatory effects (Jo et al. Citation2017; Tuan Anh et al. Citation2017). Our previous report showed that the compounds isolated from this plant display anti-neuroinflammatory effects (Yoon et al. Citation2016). Cudraflavanone A is one of the components contained in C. tricuspidata, and it has been reported that cudraflavanone A inhibits topoisomerase I and protein kinase C (PKC) activity leading to inducing the apoptotic cell death of human cancer cells (Rho et al. Citation2007), and vascular smooth muscle cells growth via an Akt-dependent pathway (Han et al. Citation2007). However, the anti-neuroinflammatory effects of cudraflavanone A on microglial cells have not been investigated yet. Therefore, as a part of our ongoing searching for novel compounds from natural products to treat, the present study describes the isolation of cudraflavanone A from C. tricuspidata and its potent effect as an anti-neuroinflammatory agent in BV2 cells.
Materials and methods
Plant materials
The root barks of C. tricuspidata were purchased in May 2014 from Daerim Korean crude drug store, Kumsan, Chungnam Province, Korea, and identified by Dr. Kyu-Kwan Jang, Botanical Garden, Wonkwang University. A voucher specimen (WP-2014-12) was deposited at the Herbarium of the College of Pharmacy, Wonkwang University (Iksan, Korea). Cudraflavanone A was isolated from the chloroform fraction of the methanol extract of C. tricuspidata by various chromatographic methods. The structure of the compound was determined by mass spectrometry (MS) and nuclear magnetic resonance (NMR) analysis (Quang et al. Citation2015).
Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), foetal bovine serum (FBS), and other tissue culture reagents were purchased from Gibco BRL Co. (Grand Island, NY). All other chemicals, including lipopolysaccharide (LPS), cobalt protoporphyrin IX chloride (CoPP) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), were obtained from Sigma-Aldrich (St. Louis, MO). Primary antibodies such as anti-iNOS, anti-COX-2, anti-inhibitor kappa B (IкB)-α, anti-p-IкB-α, anti-p50, anti-p65, anti-actin and anti-proliferating cell nuclear antigen (PCNA) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). Anti-p-extracellular signal-regulated kinase (ERK), anti-ERK, anti-p-c-Jun N-terminal kinase (JNK), anti-JNK, anti-p-p38, anti-p38, anti-p-protein kinase B (Akt) and anti-Akt were obtained from Cell Signaling Technology (Danvers, MA). Anti-HO-1 antibody was gained from Merck Millipore (Darmstadt, Germany), and anti-Nrf2 antibody was purchased from Abcam (Cambridge, MA). Anti-mouse, anti-goat and anti-rabbit secondary antibodies were supplied by Merck Millipore (Darmstadt, Germany). Tin protoporphyrin IX (SnPP IX), an inhibitor of HO activity, was obtained from Porphyrin Products (Logan, UT). The enzyme-linked immunosorbent assay (ELISA) kit for PGE2 was purchased from R&D Systems, Inc. (Minneapolis, MN).
Cell culture
BV2 cells were maintained at 5 × 106 cells/dish and 5 × 105 cells/mL in a 100 mm dish in diameter in DMEM supplemented with 10% (v/v) heat-inactivated FBS, penicillin G (100 units/mL), streptomycin (100 μg/mL) and l-glutamine (2 mM), and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Cell viability analysis with MTT assay
Cell viability was determined by using the MTT assay as previously described (Ngan et al. Citation2017). The assay was conducted three times, independently.
Western blot analysis
The details of Western blot analysis are described in the previous report (Ngan et al. Citation2017). Briefly, BV2 cells were harvested and pelleted by centrifugation at 16,000×g for 15 min. The cells were washed with phosphate-buffered saline (PBS) and lysed with 20 mM Tris–HCl buffer (pH 7.4) containing a protease inhibitor mixture (0.1 mM phenylmethylsulphonylfluoride (PMSF), 5 mg/mL aprotinin, 5 mg/mL pepstatin A and 1 mg/mL chymostatin). An equal amount of protein from each sample was resolved using 7.5% and 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Determination of nitrite (NO production)
The nitrite concentration in the medium, an indicator of NO production, was measured by the Griess reaction, as previously described (Ngan et al. Citation2017).
PGE2 assay
The level of PGE2 present in each sample was determined using a commercially available kit from R&D Systems (Minneapolis, MN). Three independent assays were performed according to the manufacturer's instructions.
Assays for IL-1β, IL-6 and TNF-α
The culture media were collected to determine the levels of IL-1β, IL-6 and TNF-α present in each sample using appropriate ELISA kits (R&D Systems, Inc., Minneapolis, MN), as per the manufacturer’s instructions.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the cells using Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer’s recommendations, and spectrophotometrically quantified (at 260 nm wavelength). Total RNA (1 mg) was reverse transcribed using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Carlsbad, CA). The cDNA was amplified with SYBR Premix Ex Taq kit (TaKaRa Bio, Shiga, Japan) using a StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA). Briefly, each 20 mL of the reaction mixture contained 10 mL of SYBR Green PCR Master Mix, 0.8 mM of each primer, and diethyl pyrocarbonate (DEPC) treated water. The primer sequences were designed using PrimerQuest (Integrated DNA Technologies, Cambridge, MA). The sequences of primers used in this experiment are shown in . The optimum conditions for PCR amplification of cDNA were established by following the manufacturer’s instructions. The data were analysed using StepOne software (Applied Biosystems, Foster City, CA) and the cycle number at the linear amplification threshold (Ct) values for the endogenous control gene (GAPDH) and the target gene was recorded. Relative gene expression (target gene expression normalized to the expression of the endogenous control gene) was calculated by using the comparative Ct method (2–ΔΔCt). The analysis was conducted three times, independently.
Table 1. Primers used for qPCR.
Assay for COX colorimetric inhibitor screening
The COX inhibition assay was performed with colorimetric COX (Ovine) inhibitor screening assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions. The assay was conducted three times, independently.
Analysis of DNA binding activity of Nrf2
The DNA-binding activity of Nrf2 in nuclear extracts was measured using Nrf2 transcription factor (CAYMAN, Ann Arbor, MI) assay kits, according to the manufacturer’s instructions. Three independent replicates were performed.
Statistical analysis
The data are expressed as the mean ± standard deviation (SD) of at least three independent experiments. To compare three or more groups, one-way analysis of variance (ANOVA) was used, followed by Tukey’s multiple comparison tests. The statistical analysis was performed with GraphPad Prism software, version 3.03 (GraphPad Software Inc., San Diego, CA).
Results
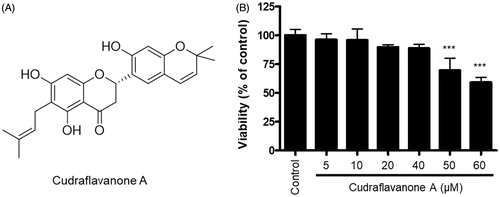
Structure and effects of cudraflavanone A on BV2 cell viability
The structure of cudraflavanone A was identified in the previous study (Quang et al. Citation2015), as shown in . To evaluate the cytotoxic effects of cudraflavanone A, we determined the cell viability of BV2 cells following treatment with cudraflavanone A for 24 h at concentrations ranging from 5 to 60 μM. An MTT assay was conducted to determine optical density. Results of the MTT assay showed that cudraflavanone A at 5–40 μM concentration had no effect on BV2 cell viability ().
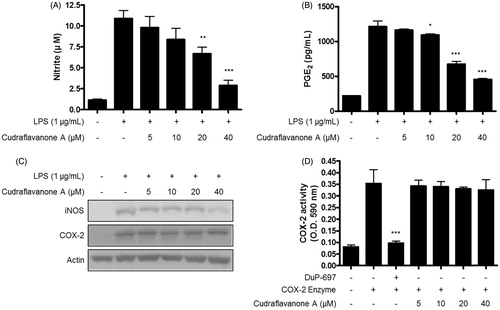
Cudraflavanone A inhibited LPS-induced production of NO and PGE2, expression of iNOS protein, but had no effect on COX-2 in BV2 cells
The inhibitory effects of cudraflavanone A on LPS-induced production of NO and PGE2 were tested. BV2 cells were pretreated with or without cudraflavanone A at a nontoxic concentration range for 3 h, and stimulated by LPS (1 μg/mL) for 24 h. As a result, cudraflavanone A significantly inhibited NO and PGE2 production manner, with IC50 values 22.2 and 20.6 μM for NO and PGE2, respectively (). Continuously, the effects of cudraflavanone A on LPS-induced expression of iNOS and COX-2 protein in BV2 cells were investigated by Western blot analysis. Cudraflavanone A suppressed iNOS expression, however, it had no effect on COX-2 expression (). In addition, COX enzyme activity assay was conducted to determine the effect of cudraflavanone A on COX enzyme activity, and found that cudraflavanone A showed no effect on COX enzyme activity ().
Figure 2. Effects of cudraflavanone A on LPS-induced NO, PGE2 production, iNOS, COX-2 protein expression and COX-2 enzyme activity in BV2 cells. (A–C) Cells were pretreated with/without the indicated concentrations of cudraflavanone A for 3 h and then stimulated with LPS (1 µg/mL) for 24 h. Nitrite levels (A) were determined using the Griess reaction and PGE2 (B) was quantified by ELISA. *p < 0.05, **p < 0.01, and ***p < 0.001 in comparison with LPS-treated group. (C) iNOS and COX-2 protein expression was determined by Western blot analysis. Representative blots from three independent experiments are shown. (D) COX-2 enzyme was treated with the indicated concentrations of cudraflavanone A for 5 min. DuP-697 was used as positive COX-2 inhibitor controls. ***p < 0.001 in comparison with COX-2 enzyme treated group. Values shown are means ± SD of three independent experiments.
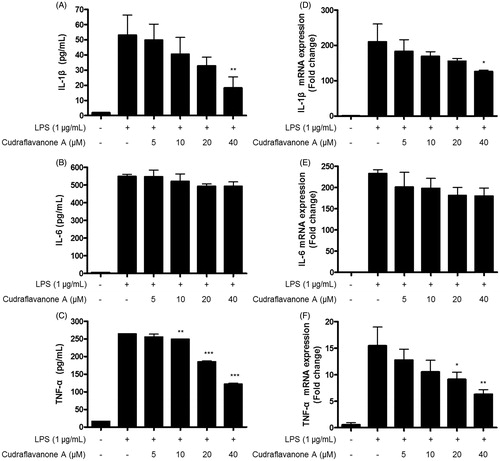
Cudraflavanone A inhibited the LPS-induced production of pro-inflammatory cytokines and the expression of those mRNA in BV2 cells
Continuously, effects of cudraflavanone A on LPS-induced pro-inflammatory cytokines, as like IL-1β, IL-6 and TNF-α were examined by ELISA kits and qRT-PCR in BV2 cells. The production of IL-1β, IL-6 and TNF-α, and their corresponding mRNA expression was increased in response to LPS. However, pretreatment of cudraflavanone A decreased the production of these molecules. The reported IC50 values were 24.7 and 33.0 μM for IL-1β () and TNF-α (), respectively. The mRNAs expression of IL-1β and TNF-α was also repressed by cudraflavanone A (). However, cudraflavanone A failed to affect the protein and mRNA levels of IL-6 ().
Figure 3. Effects of cudraflavanone A on IL-1β (A, D), IL-6 (B, E) and TNF-α (C, F) in LPS-stimulated BV2 cells. Cells were pretreated with/without the indicated concentrations of cudraflavanone A for 3 h and then stimulated with LPS (1 μg/mL) for 24 h (A–C) and 6 h (D–F), respectively. The concentration of cytokines was measured by ELISA, and mRNA expression was analysed by qPCR. *p < 0.005, **p < 0.01 and ***p < 0.001 in comparison with LPS-treated group.
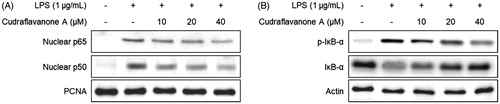
Cudraflavanone A inhibited LPS-induced activation of the NF-κB pathway in BV2 cells
To examine the effect of cudraflavanone A on LPS-induced activation of the NF-κB pathway, BV2 cells were pretreated with the indicated concentrations of cudraflavanone A for 3 h, and then stimulated with LPS (1 μg/mL) for 1 h. The cytosolic and nuclear fractions were extracted from the cell lysate, and the protein levels of p65 and p50 were increased in the nuclear fraction of the LPS-treated group. However, the pretreatment of cudraflavanone A prevented the expression of NF-κB subunits in the nuclear fraction (). The phosphorylation and degradation of IκB-α in the cytosolic fraction were also increased upon LPS stimulation, but cudraflavanone A inhibited these responses (). Therefore, it was inferred that cudraflavanone A exhibited anti-neuroinflammatory effects via suppressing the activation of NF-κB pathway.
Figure 4. Effect of cudraflavanone A on LPS-induced activation of NF-κB in BV2 cells. Cells were pretreated with the indicated concentrations of cudraflavanone A for 3 h and then stimulated with LPS (1 μg/mL) for 1 h. Nuclear and cytosolic extracts were isolated and the levels of p65 and p50 in the nuclear fraction, and p-IκB-α and IκB-α in the cytosolic fraction were determined by Western blot analysis. PCNA and actin were used as internal controls. The experiment was repeated three times, and similar results were obtained.
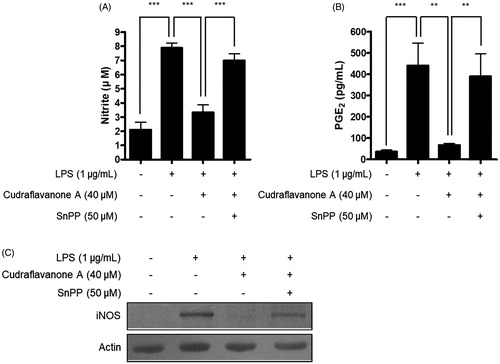
Cudraflavanone A inhibited LPS-induced activation of the MAPK pathways in BV2 cells
It was further investigated whether cudraflavanone A affects the activation of MAPKs pathways. BV2 cells were pretreated with the indicated concentrations of cudraflavanone A for 3 h, and then stimulated with LPS (1 μg/mL) for 30 min. The phosphorylation of ERK, JNK and p38 MAPKs was increased in LPS only treated cells. However, the pretreatment of cudraflavanone A decreased the phosphorylation of JNK and p38 MAPKs (), but it had no effect on ERK MAPK (). These results demonstrate that cudraflavanone A showed anti-neuroinflammatory effects through the JNK and p38 MAPK pathways.
Figure 5. Effects of cudraflavanone A on LPS-induced activation of MAPK pathways in BV2 cells. Lysates were prepared from cells pretreated with/without the indicated concentrations of cudraflavanone A for 3 h and then with LPS (1 μg/mL) for 30 min. The phosphorylated and total forms of p38, ERK and JNK were determined by Western blot analysis. Actin was used as internal controls. The experiment was repeated three times, and similar results were obtained.
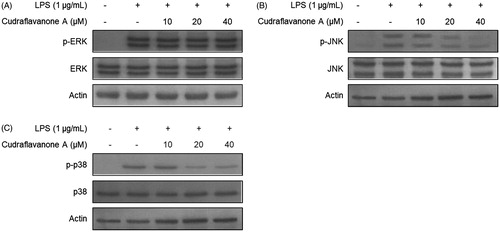
Cudraflavanone A induced HO-1 protein expression and Nrf2 translocation into the nucleus in BV2 cells
Continuously, the correlation between HO-1 expression induced by cudraflavanone A and anti-inflammatory effects of cudraflavanone A was examined. First, whether cudraflavanone A could induce HO-1 protein expression was evaluated by Western blot analysis. Cudraflavanone A induced HO-1 protein expression (), and this effect was regulated by cudraflavanone A-mediated nuclear translocation of Nrf2 protein (). In addition, the influence of SnPP, the established selective HO-1 inhibitor, on the anti-neuroinflammatory effects of cudraflavanone A was examined in LPS-treated BV2 cells. Cudraflavanone A decreased LPS-induced production of NO and PGE2, but these effects were partially reversed by pretreatment of SnPP (). Furthermore, SnPP restored the expression of iNOS protein inhibited by cudraflavanone A (). These results indicate that cudraflavanone A exhibited its anti-neuroinflammatory effects through the expression and activity of HO-1 protein.
Figure 6. Effects of cudraflavanone A on the HO-1 expression (A), Nrf2 nuclear translocation (B) and Nrf2 DNA binding activity (C) in BV2 cells. (A) Cells were treated with cudraflavanone A for 12 h at various concentrations and Western blot analysis for HO-1 expression was performed. The HO-1 inducer CoPP, was used as a positive control. (B) Cells were treated with 40 μM cudraflavanone A for 0.5, 1 and 1.5 h, and the levels of Nrf2 protein in each fraction were determined by Western blot analysis. (C) Cells were treated with 40 μM cudraflavanone A for 0.5, 1 and 1.5 h. The degree of Nrf2 DNA binding activity was determined by ELISA kit. **p < 0.01 in comparison with 0 h treated group. The experiments were repeated three times and similar results were obtained.
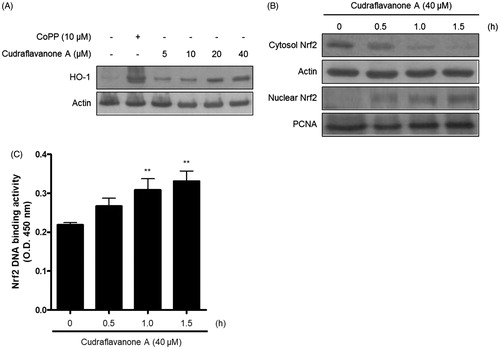
Figure 7. Effects of SnPP on the inhibitory actions of cudraflavanone A on the NO, PGE production and iNOS protein expression. Cells were pretreated with 50 μM of SnPP and incubated with or without cudraflavanone A (40 μM) for 24 h. (A) Nitrite levels were determined using the Griess reaction and (B) PGE2 was quantified by ELISA. **p < 0.01 and ***p < 0.001. (C) iNOS protein expression was determined by Western blot analysis, and representative blots from three independent experiments are shown.
Discussion
This study demonstrated that cudraflavanone A appeared to have anti-neuroinflammatory effect against LPS-induced inflammation in BV2 microglial cells. The anti-neuroinflammatory activity of cudraflavanone A was related to the inactivation of NF-κB, and the JNK and p38 MAPK pathways. Moreover, cudraflavanone A exerted its anti-neuroinflammatory effects by inducing HO-1 protein expression and Nrf2 pathway activation.
Activated microglial cells induce the production of pro-inflammatory mediators such as NO, PGE2 and pro-inflammatory cytokines as like IL-1β, IL-6 and TNF-α (Zhang et al. Citation2013). These mediators aggravate and maintain the inflammatory responses (Du et al. Citation2014). Therefore, the substances which inhibit the release of pro-inflammatory mediators may be valuable for the treatment of neuroinflammatory diseases. In this study, cudraflavanone A was treated in LPS-treated BV2 microglial cells, and it resulted in decreased NO and PGE2 production, and inhibition of iNOS protein expression (). Cudraflavanone A reduced LPS-induced production and expression of IL-1β and TNF-α (), but failed to affect IL-6 protein and mRNA expression ().
Interestingly, cudraflavanone A repressed the production of PGE2, but had no effect on COX-2 protein expression and activity. Generally, COX-2 is an important mediator of inflammatory reactions, and COX-2 inhibition has been shown to exert anti-inflammatory (Sano et al. Citation1992; Yan et al. Citation2016), and renoprotective effects (Komers et al. Citation2007; Quilley et al. Citation2011). However, the long-term treatment with COX-2 inhibitor may induce numerous side effects, including the significant cardiovascular concerns (Jia et al. Citation2014). Thus, specific agent that targets PGE2 synthases may serve as the most promising anti-inflammatory drugs (Trebino et al. Citation2003; Kudo and Murakami Citation2005). In particular, microsomal PGE synthase-1 (mPGES-1) is an inducible enzyme essential for the production of pro-inflammatory PGE2 from PGH2. Under inflammatory conditions, COX-2 converts arachidonic acid to PGH2, and eventually mPGES-1 converts PGH2 into PGE2. Thus, the function of mPGES-1 and COX-2 is coupled to produce PGE2 (Oshima et al. Citation2009). However, studies have shown that mPGES-1 and COX-2 are not always coupled together (Candelario-Jalil et al. Citation2007; Shie et al. Citation2015). Therefore, the specific inhibition of mPGES-1 expression or enzyme activity observed in our study may be associated with the suppression of PGE2 production by cudraflavanone A.
The genes involved in the production of pro-inflammatory mediators may be regulated by the NF-κB pathway. Under an inactivation state, NF-κB components exist in the cytoplasm with the IκB protein. Upon activation of cells by various stimuli as like LPS, cytokines or chemokines, IκB undergoes phosphorylation and degradation, leading to the translocation of NF-κB components into the nucleus. The translocated NF-κB subunits activate the transcription of pro-inflammatory mediators. Therefore, the inhibition of NF-κB activity may be one of the targets for the treatment of neuroinflammation (Kim et al. Citation2013). In this investigation, cudraflavanone A attenuated LPS-induced activation of the NF-κB signalling pathway, thereby inhibiting the translocation of NF-κB subunits (p65 and p50), and IκB phosphorylation and degradation (). These results suggest that cudraflavanone A could exhibit the anti-neuroinflammatory effects through the inactivation of NF-κB signalling in LPS-stimulated microglial cells.
MAPK pathways consist of three major types, ERK, JNK and p38 MAPKs. These enzymes are related to the extensive activation of pro-inflammatory reactions as well as the NF-κB pathway (Gonzalez-Scarano and Baltuch Citation1999). In addition, MAPKs are associated with LPS-induced production of pro-inflammatory mediators. Several studies have demonstrated the regulatory role of p38 and JNK MAPKs in inflammatory responses (Da Silva et al. Citation1997; Guo et al. Citation2017; Jiang et al. Citation2017). Results from this study show that cudraflavanone A inhibited the phosphorylation of p38 and JNK MAPKs (), and that the p38 and JNK MAPK pathways may be one of the targets for the treatment of neuroinflammation by cudraflavanone A.
The enzyme HO-1 catalyses the degradation of heme, which produces carbon monoxide (CO), ferrous ion and biliverdin. CO can specifically regulate the production of pro-inflammatory cytokines and mediators, and modulate the inflammatory reactions (Ryter and Choi Citation2016). Nrf2 is the transcription factor that binds to antioxidant response element (ARE)-binding site, activates HO-1 gene, and induces HO-1 expression, leading to anti-inflammatory reactions (Okorji et al. Citation2016; Yan et al. Citation2016). Hence, it was investigated whether cudraflavanone A induces HO-1 protein expression and Nrf2 translocation into the nucleus. Treatment of cudraflavanone A induced HO-1 protein expression (), and increased the nuclear translocation of Nrf2 in a time-dependent manner (). In addition, it was examined whether the anti-neuroinflammatory effect of cudraflavanone A is regulated by HO-1 activity. The pretreatment of cells with SnPP, the established HO activity inhibitor, partially reversed the inhibitory effects of cudraflavanone A on NO and PGE2 production, and iNOS protein expression (). These results demonstrated that HO-1 expression correlated with the anti-inflammatory effect of cudraflavanone A.
Conclusions
In this study, cudraflavanone A exerted its anti-neuroinflammatory effects by inhibiting the production of NO and PGE2, and expression of iNOS protein. This effect was mediated through the inactivation of NF-κB, as well as the p38 and JNK MAPK pathways in BV2 microglial cells. In addition, cudraflavanone A induced HO-1 expression via Nrf2 translocation into the nucleus, and HO-1 expression was associated with the anti-neuroinflammatory effect of cudraflavanone A. However, cudraflavanone A had no effect on COX-2 protein expression and activity. Taken together, cudraflavanone A may serve as a potent substrate for the development of therapeutic agents to treat neurodegenerative diseases. Therefore, further studies should identify the molecular mechanisms underlying the effects of cudraflavanone A in neuroinflammatory reactions, such as mPGES-1 signalling and upstream pathways of NF-κB or MAPK.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Baliga MS, Joseph N, Venkataranganna MV, Saxena A, Ponemone V, Fayad R. 2012. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: preclinical and clinical observations. Food Funct. 3:1109–1117.
- Candelario-Jalil E, de Oliveira AC, Graf S, Bhatia HS, Hüll M, Muñoz E, Fiebich BL. 2007. Resveratrol potently reduces prostaglandin E2 production and free radical formation in lipopolysaccharide-activated primary rat microglia. J Neuroinflamm. 4:25.
- Catorce MN, Gevorkian G. 2016. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. 14:155–164.
- Cianciulli A, Calvello R, Porro C, Trotta T, Salvatore R, Panaro MA. 2016. PI3k/Akt signaling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol. 36:282–290.
- Da Silva J, Pierrat B, Mary JL, Lesslauer WJ. 1997. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J Biol Chem. 272:28373–28380.
- Du RW, Du RH, Bu WG. 2014. β-Arrestin 2 mediates the anti-inflammatory effects of fluoxetine in lipopolysaccharide-stimulated microglial cells. J Neuroimmune Pharmacol. 9:582–590.
- Gonzalez-Scarano F, Baltuch G. 1999. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 22:219–240.
- Groh J, Martini R. 2017. Neuroinflammation as modifier of genetically caused neurological disorders of the central nervous system: understanding pathogenesis and chances for treatment. Glia. 65:1407–1422.
- Guo T, Lin Q, Li X, Nie Y, Wang L, Shi L, Xu W, Hu T, Guo T, Luo FJ. 2017. Octacosanol attenuates inflammation in both RAW264.7 macrophages and a mouse model of colitis. J Agric Food Chem. 65:3647–3658.
- Han HJ, Kim TJ, Jin YR, Hong SS, Hwang JH, Hwang BY, Lee KH, Park TK, Yun YP. 2007. Cudraflavanone A, a flavonoid isolated from the root bark of Cudrania tricuspidata, inhibits vascular smooth muscle cell growth via an Akt-dependent pathway. Planta Med. 73:1163–1168.
- Jia Z, Sun Y, Liu S, Liu Y, Yang T. 2014. COX-2 but not mPGES-1 contributes to renal PGE2 induction and diabetic proteinuria in mice with type-1 diabetes. PLoS One. 9:e93182.
- Jiang F, Guan H, Liu D, Wu X, Fan M, Han J. 2017. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 8:1313–1322.
- Jo YH, Kim SB, Liu Q, Hwang BY, Lee MK. 2017. Prenylated xanthones from the roots of Cudrania tricuspidata as inhibitors of lipopolysaccharide-stimulated nitric oxide production. Arch Pharm Life. 350:e1600263.
- Kim BW, Koppula S, Hong SS, Jeon SB, Kwon JH, Hwang BY, Park EJ, Choi DK. 2013. Regulation of microglia activity by glaucocalyxin-A: attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-κB and p38 MAPK signaling pathways. PLoS One. 8:e55792.
- Komers R, Lindsley JN, Oyama TT, Anderson S. 2007. Cyclo-oxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clin Exp Pharmacol Physiol. 34:36–41.
- Kudo I, Murakami M. 2005. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J Biochem Mol Biol. 38:633–638.
- Kwon J, Hiep NT, Kim DW, Hwang BY, Lee HJ, Mar W, Lee D. 2014. Neuroprotective xanthones from the root bark of Cudrania tricuspidata. J Nat Prod. 77:1893–1901.
- Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 1:a001651.
- Lee BW, Lee JH, Lee ST, Lee HS, Lee WS, Jeong TS, Park KH. 2005. Antioxidant and cytotoxic activities of xanthones from Cudrania tricuspidata. Bioorg Med Chem Lett. 15:5548–5552.
- Lin HY, Huang BR, Yeh WL, Lee CH, Huang SS, Lai CH, Lin H, Lu DY. 2014. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol Aging. 35:191–202.
- Matz P, Turner C, Weinstein PR, Massa SM, Panter SS, Sharp FR. 1996. Heme-oxygenase-1 induction in glia throughout rat brain following experimental subarachnoid hemorrhage. Brain Res. 713:211–222.
- More SV, Kumar H, Kim IS, Koppulla S, Kim BW, Choi DK. 2013. Strategic selection of neuroinflammatory models in Parkinson’s disease: evidence from experimental studies. CNS Neurol Disord Drug Targets. 12:680–697.
- Ngan NT, Quang TH, Kim KW, Kim HJ, Sohn JH, Kang DG, Lee HS, Kim YC, Oh H. 2017. Anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungal strain Penicillium sp. SF-5629. Arch Pharm Res. 40:328–337.
- Okorji UP, Velagapudi R, El-Bakoush A, Fiebich BL, Olajide OA. 2016. Antimalarial drug artemether inhibits neuroinflammation in BV2 microglia through Nrf2-dependent mechanisms. Mol Neurobiol. 53:6426–6443.
- Oshima H, Oguma K, Du YC, Oshima M. 2009. Prostaglandin E2, Wnt, and BMP in gastric tumor mouse models. Cancer Sci. 100:1779–1785.
- Park KH, Park YD, Han JM, Im KR, Lee BW, Jeong IY, Jeong TS, Lee WS. 2006. Anti-atherosclerotic and anti-inflammatory activities of catecholic xanthones and flavonoids isolated from Cudrania tricuspidata. Bioorg Med Chem Lett. 16:5580–5583.
- Quang TH, Ngan NT, Yoon CS, Cho KH, Kang DG, Lee HS, Kim YC, Oh H. 2015. Protein tyrosine phosphatase 1B inhibitors from the roots of Cudrania tricuspidata. Molecules (Basel, Switzerland). 20:11173–11183.
- Quilley J, Santos M, Pedraza P. 2011. Renal protective effect of chronic inhibition of COX-2 with SC-58236 in streptozotocin-diabetic rats. Am J Physiol Heart Circ Physiol. 300:H2316–H2322.
- Rho YH, Lee BW, Park KH, Bae YS. 2007. Cudraflavanone A purified from Cudrania tricuspidata induces apoptotic cell death of human leukemia U937 cells, at least in part, through the inhibition of DNA topoisomerase I and protein kinase C activity. Anti-Cancer Drugs. 18:1023–1028.
- Ryter SW, Choi AM. 2016. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 167:7–34.
- Sano H, Hla T, Maier JA, Crofford LJ, Case JP, Maciag T, Wilder RL. 1992. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Investig. 89:97–108.
- Shie PH, Huang SS, Deng JS, Huang GJ. 2015. Spiranthes sinensis suppresses production of pro-inflammatory mediators by down-regulating the NF-κB signaling pathway and up-regulating HO-1/Nrf2 anti-oxidant protein. Am J Chin Med. 43:969–989.
- Streit WJ, Mrak RE, Griffin WS. 2004. Microglia and neuroinflammation: a pathological perspective. J Neuroinflamm. 1:14.
- Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, et al. 2003. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci USA. 100:9044–9049.
- Tuan Anh HL, Tuan DT, Trang DT, Tai BH, Nhiem NX, Yen PH, Kiem PV, Minh CV, Duc TM, Kang HK, et al. 2017. Prenylated isoflavones from Cudrania tricuspidata inhibit NO production in RAW 264.7 macrophages and suppress HL-60 cells proliferation. J Asian Nat Prod Res. 19:510–518.
- Velagapudi R, Aderogba M, Olajide OA. 2014. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim Biophys Acta. 1840:3311–3319.
- Xin LT, Yue SJ, Fan YC, Wu JS, Yan D, Guan HS, Wang CY. 2017. Cudrania tricuspidata: an updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 7:31807.
- Yan T, Yu X, Sun X, Meng D, Jia JM. 2016. A new steroidal saponin, furotrilliumoside from Trillium tschonoskii inhibits lipopolysaccharide-induced inflammation in Raw264.7 cells by targeting PI3K/Akt, MARK and Nrf2/HO-1 pathways. Fitoterapia. 115:37–45.
- Yoon CS, Kim DC, Quang TH, Seo J, Kang DG, Lee HS, Oh H, Kim YC. 2016. A prenylated xanthone, cudratricusxanthone A, isolated from Cudrania tricuspidata inhibits lipopolysaccharide-induced neuroinflammation through inhibition of NF-κB and p38 MAPK pathways in BV2 microglia. Molecules. 21:E1240.
- Zhang F, Wang H, Wu Q, Lu Y, Nie J, Xie X, Shi J. 2013. Resveratrol protects cortical neurons against microglia-mediated neuroinflammation. Phytother Res: PTR. 27:344–349.
- Zhang YD. 1985. In the dictionary of Chinese drugs; Shanghai science and technological Shanghai: China, vol. 2. Japan: Shougakukan; p. 2383.