Abstract
Context: Scutellaria baicalensis Georgi (Lamiaceae) is a popular medicinal plant. Its roots are used as the famous traditional Chinese medicine Huang-Qin, which is recorded in Chinese Pharmacopoeia, European Pharmacopoeia, and British Pharmacopoeia.
Objective: This review comprehensively summarizes research progress in phytochemistry, pharmacology, and flavonoid biosynthesis of S. baicalensis.
Methods: English and Chinese literature from 1973 to March 2018 was collected from databases including Web of Science, SciFinder, PubMed, Elsevier, Baidu Scholar (Chinese), and CNKI (Chinese). Scutellaria baicalensis, chemical constituents, phytochemistry, biological activities, and biosynthesis were used as the key words.
Results: A total of 126 small molecules (1–126) and 6 polysaccharides have been isolated from S. baicalensis. The small molecules can be classified into four structural types, namely, free flavonoids, flavonoid glycosides, phenylethanoid glycosides, and other small molecules. Extracts of S. baicalensis and its major chemical constituents have been reported to possess anti-viral, anti-tumor, anti-bacterial, antioxidant, anti-inflammatory, hepatoprotective, and neuroprotective activities. Key steps in the biosynthetic pathways of Scutellaria flavonoids have also been summarized.
Conclusions: This article could be helpful for researchers who are interested in the chemical constituents, bioactivities, biosynthesis, and clinical applications of S. baicalensis.
Introduction
The plants of genus Scutellaria L. (Lamiaceae) are perennial herbs with around 360 species in the world. Many of these species have medicinal uses (Cantor et al. Citation2009; Shang et al. Citation2010; Paton et al. Citation2016). Among them, the roots of Scutellaria baicalensis Georgi are used in China as Huang-Qin (Scutellariae Radix), one of the most popular traditional Chinese medicines (). Scutellaria baicalensis is widely distributed in North China, Japan, Korea, Mongolia, and Russia (Zhao et al. Citation2016a; Jiang et al. Citation2017). Due to its increasing demands in recent years, it is now cultivated on a large scale in Shandong, Hebei, Inner Mongonia, Shanxi, and Gansu provinces of China (Gu et al. Citation2013). It should be noted that the herb of an allied species, Scutellaria barbata D. Don, is used as the Chinese medicine Ban-Zhi-Lian.
Figure 1. Pictures of the plant (A), TCM crude drugs (B), and TCM prepared slices (C) of Scutellaria baicalensis.

In China, S. baicalensis has a medicinal history of at least 2000 years. Huang-Qin was first recorded in Shennong’s Classic of Materia Medica (Shennong Bencao Jing in Chinese) in around 200 AD. In ancient Chinese language, the character ‘Qin’ means ‘herb for hemostasis’, and ‘Huang’ means yellow color (Li and Li Citation2017). The Traditional Chinese Medicine (TCM) theory considers Huang-Qin has the functions of clearing heat, eliminating dampness, purging fire, detoxification, hemostasis, and preventing miscarriage. Huang-Qin is now listed officially in Chinese Pharmacopoeia (2015), European Pharmacopoeia (EP 9.0), and British Pharmacopoeia (BP 2018). It is the key component herb for many famous Chinese medicine patent drugs, such as Gegen Qinlian Pills (to treat diarrhea, dysentery, fever, and influenza), Lanqin Oral Liquid (to treat sore throat), Yinzhihuang Granules (to treat jaundice and hepatitis), and Xiongdan Huangqin Eye Drops (to treat conjunctivitis). Flavonoids are the major bioactive chemical constituents of Huang-Qin. Among them, baicalin has been developed into a new drug (Huangqingan Tablets, manufactured by a number of companies including Shanghai Hutchison Pharmaceuticals and Jingfukang Pharmaceutical Group Co. Ltd), and is used to treat acute and chronic hepatitis. The total flavonoids extract of the stems and leaves of S. baicalensis has also been developed into a new drug (Huangqin Jingye Jiedu Capsules), and is mainly used to treat sore throat.
Despite the popular clinical use of Huang-Qin, scientific evidences are not adequate to identify the effective chemical components responsible for the versatile biological activities. The quality control of Huang-Qin crude drugs and related patent drugs still needs to be improved, and the medicinal potential of many bioactive compounds of this plant has yet to be explored. A comprehensive review of S. baicalensis could be helpful for researchers, manufacturers, and policymakers to obtain a holistic view of this important herbal medicine.
Several review articles are available on the Scutellaria genus or S. baicalensis (Shang et al. Citation2010; Zhang et al. Citation2014; Zhao et al. Citation2016a; Karimov and Botirov Citation2017; Cheng et al. Citation2018). As an increasingly popular herbal medicine, important research progress has been made in recent years. Herein, we comprehensively summarized research literature on phytochemistry, pharmacology, and flavonoid biosynthesis of S. baicalensis. English and Chinese literature published during 1973 to March 2018 was collected from databases including Web of Science, PubMed, Elsevier, SciFinder, Baidu Scholar (Chinese), and CNKI (Chinese). Scutellaria baicalensis, chemical constituents, phytochemistry, biological activities, and biosynthesis were used as the key words.
Phytochemistry
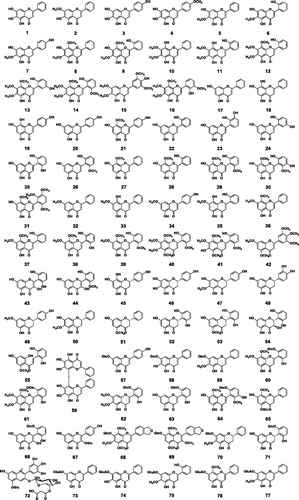
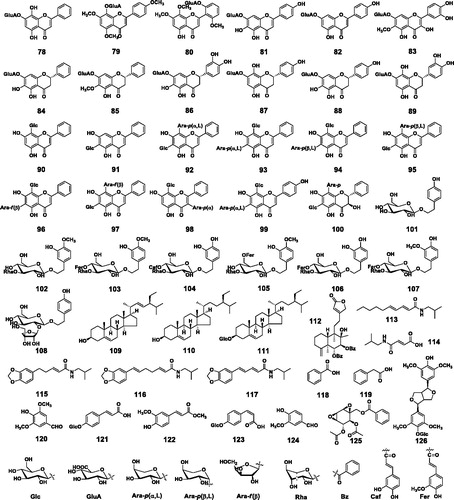
To date, a total of 126 small molecule compounds (1–126) and 6 polysaccharides have been isolated from S. baicalensis Georgi (; ). Most of these compounds were obtained from the roots (the Chinese medicine Huang-Qin). A few research groups studied chemical constituents of the aerial part (Ma Citation2013; Wang HW et al. Citation2016) and the hairy root cultures (Zhou et al. Citation1997). The small molecules can be classified into four structure types, i.e., free flavonoids, flavonoid glycosides, phenylethanoid glycosides, and other small molecules. Among them, flavonoids and their glycosides are the major compounds.
Figure 2. Chemical structures of compounds 1–126.
Table 1. Compounds 1-126 from Scutellaria baicalensis.
Free flavonoids (1–56)
A total of 56 free flavonoids have been isolated from S. baicalensis. They include 42 flavones (1–42), 2 flavonols (43–44), 9 flavanones (45–53), 1 flavonol (54), 1 chalcone (55), and 1 biflavonoid (56). The most abundant ones are baicalein (1), wogonin (27), and oroxylin A (5). Wogonin is the first free flavonoid isolated from S. baicalensis, and its structure was established in 1930 (Hattori Citation1930). Aside from the commonly seen C-5 and C-7 substituents, a number of Scutellaria flavonoids contain hydroxyl or methoxyl groups at C-6 and C-8, which are rare for plants. The regio-specific hydroxylation at C-6 and C-8 of flavones are catalyzed by two novel CYP450 enzymes (Zhao Q et al. Citation2018).
Flavonoid glycosides (57-100)
Baicalin (74) is the most abundant compound of S. baicalensis. As the first pure compound reported from this plant, baicalin was originally reported by G. Bargellini in 1919 (Azimova and Vinogradova Citation2013), and its structure was established in 1923 (Shibata et al. Citation1923). Today, 44 flavonoid glycosides have been reported from S. baicalensis. They can be classified into O-glucosides (57–72), O-glucuronides (73–89), and C-glycosides (90–100).
For most of the O-glucosides, the glucosyl residues are substituted at 7-OH or 2′-OH. Wogonin 5-O-β-d-glucoside (65), kaempferol 3-O-β-d-glucoside (67), and 72 are exceptions. Compound 72 is an acylated anthocyanin containing two glucosyl residues at C-3 and C-5, and contributes to the blue (or purple) color of the flowers (Oszmiański et al. Citation2004).
While glucuronides are not as prevalent as glucosides in plant secondary metabolites, S. baicalensis contains at least 17 O-glucuronides. Baicalin (74) and wogonoside (76) are the most abundant ones. For majority of these compounds, the glucuronyl group is linked to 7-OH, except for 79 (8-OH) and 80 (2′-OH).
The first two C-glycosides were reported from S. baicalensis in 1994 (Miyaichi and Tomimori Citation1994). Up to now, 11 C-glycosides have been isolated from this plant. Most of them are glycosides of chrysin, though it is not the most abundant free flavonoid in S. baicalensis. Aside from two mono-C-glucosides, majority of the other compounds are 6,8-di-C-glycosides, containing one glucosyl residue and one arabinosyl residue. Interestingly, the arabinosyl residue in these compounds occurred as both furano- and pyrano- forms, and in different configurations (α-l, β-l). Their structures were mainly determined by NMR spectroscopic analysis. Unlike O-glycosides, the sugar residues are not easily hydrolyzed to identify their forms and stereo-configurations. Structures for some C-glycosides need to be further confirmed.
Phenylethanoid glycosides (101–108)
A total of nine phenylethanoid glycosides have been reported from S. baicalensis. The aglycones are usually conjugated with a glucosyl group, which are further substituted with a rhamnosyl residue (Rha), or acylated with a caffeoyl (Caf) or feruloyl (Fer) group.
Other small molecules (109–126)
The other types of small molecules isolated from S. baicalensis include three steroids (109–111), one diterpene (112), five amides (113-117), and nine phenolic compounds (118–126). The amides are conjugates of isobutyl amine and organic acids, and were isolated from a water extract by Xu et al. (Citation2016).
Polysaccharides
Olennikov and colleagues isolated five polysaccharides from the aerial part of S. baicalensis. They were named as WSPS′-1, WSPS′-2, WSPS′-3, WSPS′-4, and WSPS′-5. Among them, WSPS′-1, WSPS′-2, and WSPS′-3 are composed of arabinose, galactose and glucose, whereas WSPS′-4 and WSPS′-5 are composed of glucose (Olennikov et al. Citation2008a, Citation2008b). The same research group also obtained a homopolysaccharide SbRP-1′′ from the roots of S. baicalensis. SbRP-1′′ is a slightly branched glucan. The main chain is composed of α-(1 → 4)-glucopyranose units, 8.3% of which are substituted with an α-glucopyranose unit at C-6 (Olennikov et al. Citation2011).
Qualitative and quantitative analyses
With the rapid development of mass spectrometry techniques, liquid chromatography coupled with mass spectrometry (LC/MS) has been widely used to characterize the chemical constituents in herbal extracts. A number of reports are available on chemical analysis of S. baicalensis to characterize tens of compounds within 1 h (Han et al. Citation2007; Liu GZ et al. Citation2009). Wang et al. (Citation2013) depleted high-abundance flavonoids from an ethanol extract of S. baicalensis, and characterized 117 low-abundance compounds by LC/MS. Recently, our group established a targeted post-acquisition data processing strategy, key ion filtering (KIF), and tentatively characterized 132 compounds in Huang-Qin by ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry analysis (UHPLC/Orbitrap-MS) (Qiao et al. Citation2016). Among these compounds, 59 were reported in this herb for the first time.
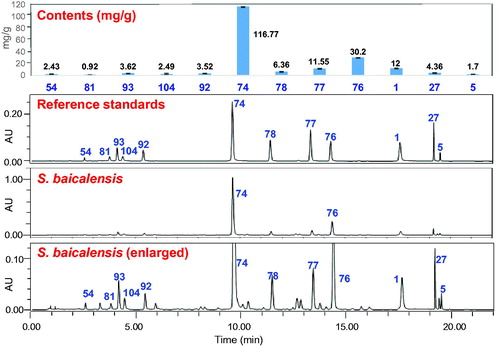
The contents of bioactive compounds are critically important for quality control of herbal medicines. Chinese Pharmacopoeia requires the content of baicalin in Huang-Qin should be no less than 9% (Chinese Pharmacopoeia Commission Citation2015). A number of HPLC methods have been developed to determine the contents of baicalin and other bioactive compounds in Huang-Qin (Xie et al. Citation2002; Zgórka and Hajnos Citation2003; Horvath et al. Citation2005; Islam et al. Citation2012). We developed a simple and rapid UPLC/UV method, and simultaneously determined the contents of 12 compounds in S. baicalensis within 20 min (Ji et al. Citation2015). Contents of these 12 compounds in 27 batches of Huang-Qin accounted for around 19.6% of dry weight of the herbal materials ().
Figure 3. UPLC/UV chromatograms (275 nm) for quantitative analysis of 12 major compounds in Scutellaria baicalensis (Huang-Qin crude drugs). 1, baicalein; 5, oroxylin A; 27, wogonin; 54, (2R,3R)-3,5,7,2′,6′-pentahydroxyflavanone; 74, baicalin; 76, wogonoside; 77, oroxylinA 7-O-β-D-glucuronoside; 78, norwogonin 7-O-β-D-glucuronoside; 81, scutellarin; 92, chrysin 6-C-β-D-glucoside-8-C-α-L-arabinopyranoside; 93, chrysin 6-C-α-L-arabinopyranoside-8-C-β-D-glucoside; 104, acteoside (Adapted from Ji et al. Citation2015).
Pharmacological activities of extracts and major compounds
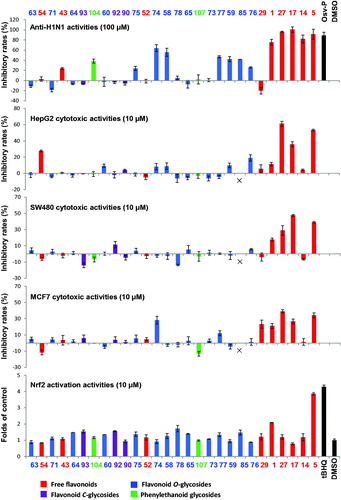
In China, Huang-Qin is widely used for the treatment of influenza, pneumonia, dysentery, and cancer. A large number of investigations have been reported on the pharmacological activities of different extracts of S. baicalensis (including water extract, methanol extract, and ethanol extract) and its major compounds such as baicalin, baicalein, and wogonin. These results were reported by different research groups, the investigations were conducted using different experimental models, and thus the results were difficult to be compared or summarized. Recently, our group isolated 28 compounds from this herb, and evaluated their anti-H1N1 viral, cytotoxic, and Nrf2 activation activities (Ji et al. Citation2015). The results indicated that free flavones were more potent than the other types as anti-influenza, cytotoxic, and antioxidative compounds of S. baicalensis (). They may be key players in the clinical therapeutic effects of Huang-Qin.
Figure 4. Screening of 28 compounds from Scutellaria baicalensis for their anti-H1N1 viral, cytotoxic, and Nrf2 activation activities. For compounds identification, see (Adapted from Ji et al. Citation2015).
In this section, we summarize literature reports on the anti-tumor, anti-viral, anti-microbial, anti-inflammatory, antioxidative, neuroprotective, and hepatoprotective activities of extracts and compounds of S. baicalensis, as well as their effects on cardiovascular and cerebrovascular diseases, and bones.
Anti-tumor activities
Scutellaria baicalensis extracts and compounds have been reported to show a wide spectrum of anti-tumor activities, both in vitro and in vivo (). These activities involve liver cancer, gastric cancer, lung cancer, breast cancer, prostate cancer, bladder cancer, brain cancer, squamous cell carcinoma, mucoepidermoid carcinoma, colorectal cancer, gallbladder carcinoma, oral cancer, leukemia, lymphoma, and myeloma.
Table 2. The anti-tumor activities of Scutellaria baicalensis and its compounds.
The extracts of S. baicalensis could inhibit the proliferation of human myeloma, lung cancer, liver cancer, and prostate cancer cells in vitro, and suppress tumor growth in bladder, prostate, lung and head/neck squamous xenograft tumor models. In the head/neck squamous cell carcinoma (HNSCC) murine model, oral administration of a water extract (75 mg/kg, 5 times/week for 7 weeks) led to 66% reduction of xenograft tumor (Zhang et al. Citation2003). The anti-cancer activities of S. baicalensis could be related with its inhibitory effects on PGE2 (prostaglandin E2) production via suppression of COX-2 (cyclooxygenase-2) expression and arachidonic acid release from cell membranes. The total free flavonoid extract (100 mg/kg for 30 d, p.o.) could also significantly reduce tumor size by 25.5% in A549 human lung cancer xenografted mice, via induction of growth arrest in S phase and inhibition of DNA synthesis (Wang Y et al. Citation2016).
The main flavones baicalin (74), baicalein (1), wogonin (27), and wogonoside (76) are the major bioactive constituents responsible for the anti-tumor activities, with IC50 values of 10–50 μM against most tested cancer cell lines in vitro (Chan et al. Citation2000; Chen et al. Citation2008; Wu et al. Citation2013). These flavones could scavenge oxidative radicals, attenuate NF-κB (nuclear factor-κB) activity, suppress COX-2 gene expression, and regulate cell cycle (Li-Weber Citation2009). Baicalin (200 mg/kg, 5 times/week for 2 weeks, i.p.) could inhibit mucoepidermoid carcinoma Mc3 cell growth by 50% in the xenograft murine model (Xu XF et al. Citation2011). It could suppress cell cycle progression and induce cell apoptosis through decreasing the mitochondrial membrane potential.
Baicalein (20 mg/kg, 5 d/week for 21 d, i.p.) could inhibit MDA468 breast cancer xenografts by 40%, the effect of which was comparable to that of the positive drug cisplatin (5 mg/kg). It could upregulate DDIT4 (DNA-damage-inducible transcript 4) expression, which mediated the inhibition of mTOR (mammalian target of rapamycin) (Wang YJ et al. Citation2015). In another prostate cancer xenograft murine model, baicalein (20 mg/kg for 14 d, p.o.) reduced tumor size by 55%, via reduction of expression of the androgen receptor and androgen-regulated genes (Bonham et al. Citation2005).
Wogonin (10 mg/kg for 4 weeks, p.o.) could inhibit tumor growth of T47D and MDAMB-231 breast cancer xenografts by up to 88% without significant toxicity in athymic nude mice (Chung et al. Citation2008). The mechanism could be downregulation of the Akt-dependent canonical Wnt signaling pathway and p27kip pathway. Wogonin could also act as CDK (cyclin-dependent kinase) inhibitors to potentiate the activities of anti-tumor drugs, such as the Bcl-2 (B-cell lymphoma 2) family inhibitor ABT-263. The combination of wogonin (50 mg/kg for 10 d, i.p.) and ABT-263 remarkably promoted tumor regression in human T-cell leukemia xenografted mice, but wogonin did not exhibit significant effects when used alone (Polier et al. Citation2015).
Wogonoside exerted anti-proliferative properties, suppressing tumor growth by 41% and prolonging survival durations up to 2.3-fold, in a U937 leucocythemia xenograft murine model (80 mg/kg/2 d for 14 d, i.p.) (Chen et al. Citation2013). The anti-tumor effect of wogonoside was related to cell cycle arrest and differentiation via inhibition of PLSCR1 (phospholipid scramblase 1) expression and regulation of subcellular localization in the nucleus.
PHY906 is an herbal preparation derived from the traditional Chinese medicine formula Huang-Qin Decoction, a four-herb formula with Huang-Qin as the key component (Ye M et al. Citation2007). PHY906 could enhance the anti-tumor activities of sorafenib against HepG2 tumor both in vivo and in vitro. Among the four component herbs, S. baicalensis played an important role in increasing tumor apoptosis by multiple mechanisms targeting on the inflammatory state of microenvironment of tumor tissue (Lam et al. Citation2015). PHY906 could also decrease gastrointestinal toxicity caused by the chemotherapeutic drug irinotecan. In a murine MCA-38 allograft model, PHY906 remarkably increased the anti-tumor activities of irinotecan and decreased weight loss (Lam et al. Citation2010).
Anti-viral activities
Scutellaria baicalensis extracts and compounds exerted broad-spectrum anti-viral activities against HIV, influenza virus, DENV, HBV, and HTLV-I.
The extracts of S. baicalensis could inhibit HIV on H9 cells (IC50, 0.6–4.74 μg/mL), DENV on Vero cells (86.59–95.19 μg/mL), and H1N1 and seasonal influenza A viruses on MDCK cells (14.16–41.49 μg/mL) (Zhang et al. Citation1991; Hour et al. Citation2013; Zandi et al. Citation2013). These investigations were conducted on cell models.
Baicalein (480 mg/kg for 4 d, p.o.) showed significant effects in preventing death, prolonging survival time, inhibiting lung consolidation, and reducing the viral titers in the lung in BALB/c mice infected with the influenza A/FM1/1/47 (H1N1) virus. The effects were comparable to lamivudine. The mechanism could be inhibition of neuraminidase activity and modulation of the immune system (Xu et al. Citation2010). The combination of baicalein (400 mg/kg for 5 d, p.o.) and ribavirin (50 mg/kg) provided a higher survival rate and lower body weight loss than either treatment alone in ICR mice infected with H1N1 virus (protection rates, 100% vs 20% and 50%) (Chen et al. Citation2011).
Wogonin could suppress HBV antigen secretion with an IC50 of 4 μg/mL for both HBsAg and HBeAg in the human HBV-transfected liver cell line HepG2.2.15, and was more potent than lamivudine. In vivo, wogonin (i.v. for 10 d) could reduce plasma duck hepatitis B virus (DHBV) DNA level in the liver of DHBV-infected ducks with an ED50 of 5 mg/kg, via inhibition of DHBV DNA polymerase and thus reducing the relaxed circular and linear forms of DHBV DNA (Guo et al. Citation2007).
5,7,4′-Trihydroxy-8-methoxyflavone (21, 50 μM) could remarkably inhibit influenza virus A/PR/8/34 (APR8) by reducing the replication of APR8 in MDCK cells, through inhibition of the fusion of the virus with endosome/lysosome membrane at early stage and inhibition of the budding of the progeny virus from the cell surface (Nagai et al. Citation1995).
Furthermore, baicalein, baicalin, and wogonin could also inhibit other types of viruses, including HIV, herpes simplex virus-1 (HSV-1), Moloney murine leukemia virus, and Rous-associated virus type 2 (Baylor et al. Citation1992; Li et al. Citation1993, Citation2000a; Kitamura et al. Citation1998; Huang et al. Citation2000; Wang et al. Citation2004; Guo et al. Citation2007; Błach-Olszewska et al. Citation2008; Nayak et al. Citation2014). Recently, Lin et al. (Citation2016) reported that S. baicalensis could be used to treat severe HFMD (Hand, Foot, and Mouth Disease) in patients aged >1 year, rapidly relieving fever, attenuating oral lesions and rashes, and improving nervous system involvement. This result was derived from a multi-center and retrospective analysis (Lin et al. Citation2016). It is reasonable to assume that S. baicalensis and its compounds possess a common, non-specific anti-viral mechanism, based on its inhibitory effects on different types of viruses.
Anti-microbial activities
Scutellaria baicalensis and its major compounds possess remarkable anti-microbial activities. The water extract of S. baicalensis could inhibit a wide spectrum of oral bacteria (MIC, 15.7–62.5 mg/mL; MBC, 20–125 mg/mL), including Streptococcus sanguis II, S. salivarius, Actinomyces viscosus, A. naeslundii, A. odontolyticus, two strains of Capnocytophaga, Bacteroides melaninogenicus ss intermedius, B. gingivalis, Fusobacterium nucleatum, and Actinobacillus actinomycetemcomitans (Tsao et al. Citation1982). It could also inhibit the growth of Candida albicans by 90% at 2.5 mg/mL (Wong and Tsang Citation2009).
Baicalin (100 mg/kg, p.o.) could protect mice from staphylococcal pneumonia caused by Staphylococcus aureus, reducing mortality from 80% to 28% and protecting the lung from accumulation of cellular infiltrates (Qiu et al. Citation2012). This activity is associated with inhibition of the cytolytic activity of α-hemolysin, which is a self-assembling and channel-forming toxin secreted by S. aureus. Baicalein also showed potent synergistic effect with penicillin G/amoxicillin against 20 clinical penicillinase-producing S. aureus strains. Baicalin at 32 μg/mL could enhance the bacteriostatic effects, and decrease the MIC50 values of penicillin and amoxicillin from 32–64 to 0.5–2 μg/mL (Qian et al. Citation2015).
Viscidulin (43, 5,7,2′,6′-tetrahydroxyflavanonol, 40 mg/kg for one time, i.v.) could protect mice against a lethal challenge with heat-killed Escherichia coli 35218, increasing the survival rate from 0% to 60% via neutralization of LPS (lipopolysaccharide) and reduction of proinflammatory cytokines (Fu et al. Citation2008).
Anti-inflammatory activities
An extract of S. baicalensis (750 mg/kg for 10 d, p.o.) showed potent anti-inflammatory activities in the zymosan-induced mice air-pouch, reducing NO production from 30 to 5 μM, through the down-regulation of IKKαβ (IκB kinase αβ) and NF-κB activation via suppression of c-Raf-1/MEK1/2 and MAPK phosphorylation (Kim et al. Citation2009). The flavonoids extract (100 μg/mL) also exhibited significant anti-inflammatory activities through inhibiting the NF-κB signaling pathway via the MAPK (mitogen-activated protein kinase) signaling pathway in RAW264.7 cells (Hong et al. Citation2013).
Baicalein (50–100 μM) showed anti-inflammatory effects in double-stranded RNA (dsRNA)-induced macrophages by inhibiting NO, cytokines, chemokines, and growth factors via the endoplasmic reticulum stress-CHOP/STAT pathway (Kim et al. Citation2018). Baicalin (100 mg/kg for 7 d, i.p.) could relieve ankle swelling, and protect the joint against inflammatory destruction in a murine adjuvant-induced arthritis model, by inhibiting splenic Th17 cell expansion and IL-17 (interleukin 17A)-mediated inflammation in synoviocytes (Yang X et al. Citation2013). Furthermore, baicalin (200 mg/kg for 7 d, p.o.) could alleviate LPS-induced liver inflammation in chicks, reducing the cloacal temperature from 41.5 to 40.3 °C and inhibiting NO production from 105 to 40 μM, via suppression of TLR4 (Toll-like receptor 4)-mediated NF-κB pathway (Cheng et al. Citation2017). Baicalin could also decrease inflammation by selective binding to chemokine ligands on CD4 and other leukocytes (Li et al. Citation2000b). Wogonoside (50 μM) could decrease the production of inflammatory mediators NO and PGE2, and inhibit the release of pro-inflammatory cytokines including TNF-α (tumor necrosis factor α) and IL-6 in LPS-induced RAW264.7 cells (Yang YZ et al. Citation2013). Wogonin treatment also regulated the production of inflammatory cytokines in mice with streptozotocin-induced vascular inflammation (Wang J et al. Citation2017).
Antioxidative activities
The extract of S. baicalensis (1 mg/mL) could protect cardiomyocytes in vitro from moderate hypoxia, ischemia/reperfusion, and antimycin A exposure, decreasing cell death from 47–49% to 23–26% by scavenging ROS (reactive oxygen species) (Shao et al. Citation1999).
Baicalein, baicalin, and wogonin showed potent antioxidative activities by scavenging ONOO- and inhibiting ONOO--mediated nitrotyrosine formation in endothelial cells with IC50 values of 0.71–6.70 μM, the activity of which was comparable to penicillamine (3.75 μM) (Kim et al. Citation2005). Baicalein and baicalin exhibited antioxidant activities against hydroxyl radical, DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, and alkyl radical, with IC50 values of 10–32 μM (Gao et al. Citation1999). Baicalein (50 μM) also exhibited antioxidative activity in ischemia/reperfusion cardiomyocyte model, decreasing subsequent cell death from 52.3% to 29.4% (Shao et al. Citation2002).
Neuroprotective activities
The extract of S. baicalensis (200 mg/kg for 40 or 32 d, p.o.) could improve rat act in the Morris water assay, reducing search error to about 50% in the chronic cerebral hypoperfusion and the LPS infusion models (Hwang et al. Citation2011). Treatment with the extract attenuated the neuroinflammatory responses and reduced the spatial memory impairments, via mitigating alterations of hippocampal MAPK signaling (). The extact could also protect animals from global cerebral ischemia and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced Parkinson's disease, and protect cortical and neuronal cells from glutamate, NMDA (N-methyl-D-aspartic acid), and H2O2 induced toxicity in vitro (Yang et al. Citation2014; Cao et al. Citation2016; Li et al. Citation2016).
Table 3. The neuroprotective activities of Scutellaria baicalensis and its compounds.
Baicalein (2 μM) could significantly promote mouse hippocampal HT22 cell survival by 50% after injury induced by iodoacetic acid (Lapchak et al. Citation2007). Baicalin (200 mg/kg for 7 d, i.p.) attenuated neurological impairment in gerbils after global ischemia, reducing neurological deficit scores from 2.88 to 1.63 (Dai et al. Citation2013). Baicalin could also protect against neuronal loss and apoptosis in gerbil hippocampus by activating GABAergic signaling, HSP70 (70 kilodalton heat shock proteins), and MAPKs cascades (Dai et al. Citation2013). The neuro-protective activities of S. baicalensis, baicalein, baicalin, and wogonin indicate they may be promising neuroprotective agents for the prevention of Alzheimer’s disease, Parkinson’s disease, ischemic strokes, and other neurologic diseases (Cho and Lee Citation2004; Li et al. Citation2005; Cheng et al. Citation2008; Tarragó et al. Citation2008; Mu et al. Citation2009; Choi et al. Citation2010).
Hepatoprotective activities
Extracts of S. baicalensis could inhibit liver injury and fibrosis in BDL (bile duct ligation), CCl4, and LPS-induced rat or mouse hepatotoxity, by inhibiting cytokine, COX-2, iNOS (nitric oxide synthases), and NF-κB (Nan et al. Citation2002; Thanh et al. Citation2015). Baicalin (5 mg/kg for 5 d, i.p.) could protect against t-BHP-induced rat liver injury, reducing the ALT (alanine transaminase) and AST (aspartate transaminase) levels from 226 to 110 U/l and from 607 to 197 U/l, respectively (Hwang et al. Citation2005). Baicalin also exerted hepatoprotective effects in alcohol-induced liver injury through inhibiting oxidative stress, inflammatory response, and regulation of the Shh pathway (Wang HF et al. Citation2016).
Effects on cardiovascular and cerebrovascular diseases
Baicalin (6 μM) could protect against the hyperglycemia-induced cardiovascular malformation during chick embryo development, decreasing the high incidence of cardiac bifida from 32% to 16%, by reducing ROS production and regulating SOD (superoxide dismutase), GSH-Px (glutathione peroxidase), and GABAA (γ-aminobutyric acid) levels (Wang et al. Citation2018). Baicalin also exerted angiogenesis and cardioprotective effects against chronic hypoxia-induced pulmonary hypertension and acute myocardial infarction in vivo, through mediation of MAPK cascades, the ERRα (estrogen-related receptor α) pathway, and the PI3K/AKT signaling (Zhang et al. Citation2011; Liu et al. Citation2013; Huang et al. Citation2017).
Baicalein could promote new blood vessel formation, attenuate cardiac remodeling and endothelium dysfunction against angiotensin II or myocardial ischemia reperfusion injury, via inhibition of AKT/mTOR, ERK1/2, NF-κB, and calcineurin sgnaling pathways in mice or chicks (Cho et al. Citation2008; Li et al. Citation2015; Wang AW et al. Citation2015).
Effects on bones
The extract of S. baicalensis (50 mg/kg for 42 d, p.o.) could significantly increase bone mineral density by 12–18%, and improve bone trabecula microstructure of weightlessness induced osteoporosis rats via the osteogenic differentiation enhancement effect (Zhang GW et al. Citation2017). A wogonin-rich fraction (50 μg/mL) exerted chondroprotective effects by inhibiting ROS production and suppressing catabolic markers (Khan et al. Citation2017). Baicalein and baicalin (10 μM) could significantly enhance the osteogenic differentiation of human periodontal ligament cells (hPDLCs) and rat bone marrow derived mesenchymal stem cells (rBMSC), reapectively, by increasing ALP (alkaline phosphatase) activities up to 1.5-2-fold and increasing the formation of mineralized nodules up to 2-fold (Chen et al. Citation2017; Zhang GW et al. Citation2017). Arjmandi et al. (Citation2014) reported that UP446 (a natural proprietary of S. baicalensis and Areca catechu L.) could reduce physical symptoms associated with knee osteoarthritis in patients after 500 mg/d treatment for 1 week.
Other activities
The extract of S. baicalensis and baicalein (1 mg/kg and 1 μg/kg for 2 d, respectively, p.o.) could reduce gastrointestinal dysfunction in ritonavir-treated rats (Mehendale et al. Citation2007). An ethanol extract exerted synergistic anti-diabetic effect with metformin in STZ-induced diabetic rats. Baicalin possessed anti-hyperglycemic activities by suppressing hepatic gluconeogenesis (Waisundara et al. Citation2008; Wang T et al. Citation2017). Baicalein could reduce endometriosis by suppressing the viability of human endometrial stromal cells in vitro (Jin et al. Citation2017). Furthremore, baicalin exhibited embryo-protection (Qi et al. Citation2016), weight losing (Yun and Jung Citation2014), sleep–wake regulation (Chang et al. Citation2011), anti-allergic (Kim et al. Citation2010), and anti-pyretic effects (Tsai et al. Citation2006). The polysaccharides from S. baicalensis showed antioxidative and immunostimulating activities (Olennikov et al. Citation2008a, Citation2011).
Biosynthesis of Scutellaria flavonoids
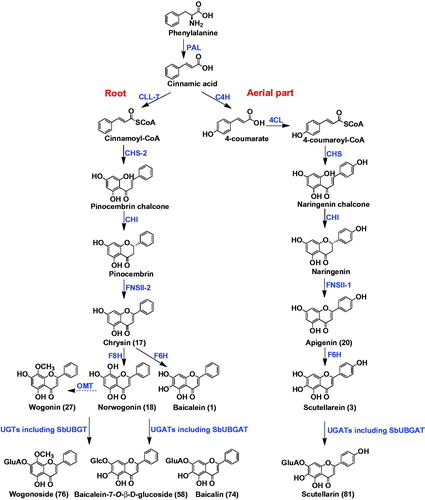
The flavonoids in S. baicalensis Georgi possess various pharmacological activities. Their biosynthesis in the living plant has gained increasing attention in recent years. Zhao et al. systematically investigated the biosynthetic pathways of free flavones. The Scutellaria flavones are originally derived from phenylalanine, which is catalyzed by phenylalanine ammonia lyase (PAL) to form cinnamic acid. Interestingly, the subsequent biosynthetic steps were different for flavones in the aerial parts and in the roots (). For the 4′-hydroxyl flavones, which are mainly distributed in the aerial parts, cinnamic acid is sequentially catalyzed by cinnamoyl 4 hydroxylase (C4H), p-coumaroyl CoA ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), and flavone synthase (FNSII-1) to form apigenin (Zhao et al. Citation2016a, Citation2016b). Then apigenin is hydroxylated by flavone 6-hydroxylase (F6H) to generate scutellarein, as shown in (Zhao Q et al. Citation2018). The flavones in the roots, however, usually lack a 4′-OH group on the B-ring. For their biosynthesis, cinnamic acid is catalyzed by cimmamoyl-CoA ligase (CLL-7), chalcone synthase (CHS-2), chalcone isomerase (CHI) to form pinocembrin. Pinocembrin is then converted by a specialized isoform of flavone synthase (FNSII-2) to form chrysin, which could be further hydroxylated by flavone 6-hydroxylase (F6H) and flavone 8-hydroxylase (F8H) to produce baicalein and wogonin, respectively (Zhao et al. Citation2016a, Citation2016b, Citation2018). O-methyltransferases (OMTs) may participate in the biosynthesis of wogonin, though no OMT has been reported yet. Among the biosynthetic enzymes, SbCLL-7, SbCHS-2, FNSII-2 and F8H are expressed preferentially in the roots. Functions of these genes have been validated by RNAi in hairy roots of S. baicalensis and overexpression in transgenic Arabidopsis.
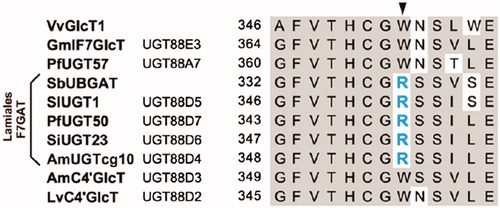
Glycosyltransferases are responsible for the formation of glycosidic bonds of flavonoid O-glucuronides and O-glucosides of S. baicalensis. SbUBGAT showed O-glucuronyltransferase activities for various flavones, and may take part in the biosynthesis of glucuronides like baicalin and wogonoside (Nagashima et al. Citation2000; Yang et al. Citation2016). SbUBGAT also showed O-glycosyltransferase activities. Together with SbUBGT discovered from the hairy root cultures of S. baicalensis, they may contribute to the production of flavonoid-O-glycosides (Hirotani et al. Citation2000). Furthermore, the Arg residue (R) in the PSPG (Plant Secondary Product Glycosyltransferase) box plays a critical role in the recognition of UDP-glucuronic acid sugar donor, while the corresponding Trp residue (W) has better selectivity for UDP-glucose donor (). This was validated by homology-modeling and site-directed mutagenesis analysis (Noguchi et al. Citation2009). Scutellaria baicalensis also contains abundant flavonoid-di-C-glycosides, and the responsible C-glycosyltransferases have not been reported yet.
Figure 6. Key amino acid residues for the catalytic selectivities of O-glucuronyltransferases and O-glycosyltransferases (Adapted from Noguchi et al. Citation2009).
Conclusions and future prospects
Scutellaria baicalensis contains at least 126 small molecules and 6 polysaccharides. It possesses anti-tumor, anti-viral, anti-microbial, anti-inflammatory, antioxidative, and neuroprotective activities. Chemical compounds responsible for many of these activities are still unknown, though the bioactivities of a few major compounds (baicalin, baicalein, wogonoside, and wogonin) have been extensively studied. Recently, our group reported the comprehensive correlations of chemicals and bioactivities of another popular herbal medicine Gan-Cao (licorice, Glycyrrhiza uralensis Fisch), and discovered a number of promising bioactive natural products (Ji et al. Citation2016). Similar research strategy could be applied to Huang-Qin to discover potential new drugs. In fact, the clinical trial of wogonin as an anti-cancer drug candidate has recently been approved by the State Drug Administration of China. On the other hand, the identified major bioactive compounds could be used as chemical markers to improve quality control of Huang-Qin crude drugs and related patent drugs. Furthermore, biosynthetic studies could help large-scale production of the bioactive compounds by metabolic engineering. Although enzymes involved in the biosynthesis of free flavones have been reported for S. baicalensis, many post-modification enzymes have yet to be characterized, including those responsible for the hydroxylation, methylation, and glycosylation reactions.
Disclosure statement
No potential conflict of interests was reported by the authors.
Additional information
Funding
References
- Arjmandi BH, Ormsbee LT, Elam ML, Campbell SC, Rahnama N, Payton ME, Brummel-Smith K, Daggy BP. 2014. A combination of Scutellaria baicalensis and Acacia catechu extracts for short-term symptomatic relief of joint discomfort associated with osteoarthritis of the knee. J Med Food. 17:707–713.
- Azimova SS, Vinogradova VI. 2013. Physicochemical and pharmacological properties of flavonoids. In: Natural compounds – flavonoids. New York (NY): Springer; p. 86–87.
- Baylor NW, Fu T, Yan YD, Ruscetti FW. 1992. Inhibition of human T cell leukemia virus by the plant flavonoid baicalin (7-glucuronic acid, 5,6-dihydroxyflavone). J Infect Dis. 165:433–437.
- Błach-Olszewska Z, Jatczak B, Rak A, Lorenc M, Gulanowski B, Drobna A, Lamer-Zarawska E. 2008. Production of cytokines and stimulation of resistance to viral infection in human leukocytes by Scutellaria baicalensis flavones. J Interf Cytok Res. 28:571–581.
- Bonham M, Posakony J, Coleman I, Montgomery B, Simon J, Nelson PS. 2005. Characterization of chemical constituents in Scutellaria baicalensis with antiandrogenic and growth-inhibitory activities toward prostate carcinoma. Clin Cancer Res. 11:3905–3914.
- Cantor M, Buta E, Zaharia A. 2009. Scutellaria genus - possibilities for use of species as floral and medicinal crop. J Plant Develop. 16:55–59.
- Cao YG, Mao XY, Sun CY, Zheng P, Gao JQ, Wang XR, Min DL, Sun HL, Xie N, Cai JQ. 2011. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 85:396–402.
- Cao YJ, Liang LZ, Xu J, Wu JL, Yan YX, Lin P, Chen Q, Zheng FM, Wang Q, Ren Q. 2016. The effect of Scutellaria baicalensis stem-leaf flavonoids on spatial learning and memory in chronic cerebral ischemia-induced vascular dementia of rats. Acta Biochim Biophys Sin. 48:437–446.
- Cha JH, Kim HW, Kim S, Jung SH, Whang WK. 2006. Antioxidant and antiallergic activity of compounds from the aerial parts of Scutellaria baicalensis Georgi. Yakhak Hoeji. 50:136–143.
- Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. 2000. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 160:219–228.
- Chang HH, Yi PL, Cheng CH, Lu CY, Hsiao YT, Tsai YF, Li CL, Chang FC. 2011. Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep-wake regulation. J Ethnopharmacol. 135:359–368.
- Chen LG, Hung LY, Tsai KW, Pan YS, Tsai YD, Li YZ, Liu YW. 2008. Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol Nutr Food Res. 52:1349–1357.
- Chen LJ, Hu BB, Shi XL, Ren MM, Yu WB, Cen SD, Hu RD, Deng H. 2017. Baicalein enhances the osteogenic differentiation of human periodontal ligament cells by activating the Wnt/β-catenin signaling pathway. Arch Oral Biol. 78:100–108.
- Chen LL, Dou J, Su ZZ, Zhou HM, Wang H, Zhou WD, Guo QL, Zhou CL. 2011. Synergistic activity of baicalein with ribavirin against influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 91:314–320.
- Chen Y, Hui H, Yang H, Zhao K, Qin YS, Gu C, Wang XT, Lu N, Guo QL. 2013. Wogonoside induces cell cycle arrest and differentiation by affecting expression and subcellular localization of PLSCR1 in AML cells. Blood. 121:3682–3691.
- Cheng CS, Chen J, Tan HY, Wang N, Chen Z, Feng Y. 2018. Scutellaria baicalensis and cancer treatment: recent progress and perspectives in biomedical and clinical studies. Am J Chinese Med. 46:25–54.
- Cheng OM, Li ZH, Han Y, Jiang QS, Yan Y, Cheng K. 2012. Baicalin improved the spatial learning ability of global ischemia/reperfusion rats by reducing hippocampal apoptosis. Brain Res. 1470:111–118.
- Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun XQ, Yang YQ, Li JR, Xiao TS, Zhang XY. 2017. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-κB pathway. Front Pharmacol. 8:547.
- Cheng YH, Li LA, Lin P, Cheng LC, Hung CH, Chang NW, Lin CJ. 2012. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharm. 263:360–367.
- Cheng YX, He GR, Mu X, Zhang TT, Li XX, Hu JJ, Xu B, Du GH. 2008. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 441:16–20.
- Chinese Pharmacopoeia Commission. 2015. Pharmacopoeia of the People’s Republic of China. Vol. 1. Beijing (China): Ministry of Health of the People’s Republic of China, p. 301–302.
- Cho H, Lee HY, Ahn DR, Kim SY, Kim S, Lee K, Lee YM, Park H, Yang EG. 2008. Baicalein induces functional hypoxia-inducible factor-1alpha and angiogenesis. Mol Pharmacol. 74:70–81.
- Cho J, Lee HK. 2004. Wogonin inhibits excitotoxic and oxidative neuronal damage in primary cultured rat cortical cells. Eur J Pharmacol. 485:105–110.
- Choi J, Conrad CC, Malakowsky CA, Talent JM, Yuan CS, Gracy RW. 2002. Flavones from Scutellaria baicalensis Georgi attenuate apoptosis and protein oxidation in neuronal cell lines. BBA-Gen Subject. 1571:201–210.
- Choi JH, Choi AY, Yoon H, Choe W, Yoon KS, Ha J, Yeo EJ, Kang I. 2010. Baicalein protects HT22 murine hippocampal neuronal cells against endoplasmic reticulum stress-induced apoptosis through inhibition of reactive oxygen species production and chop induction. Exp Mol Med. 42:811–822.
- Chung HY, Jung YM, Shin DH, Lee JY, Oh MY, Kim HJ, Jang KS, Jeon SJ, Son KH, Kong G. 2008. Anticancer effects of wogonin in both estrogen receptor-positive and -negative human breast cancer cell lines in vitro and in nude mice xenografts. Int J Cancer. 122:816–822.
- Dai J, Chen L, Qiu YM, Li SQ, Xiong WH, Yin YH, Jia F, Jiang JY. 2013. Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin's neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull. 90:1–9.
- Dong P, Zhang Y, Gu J, Wu WG, Li ML, Yang JH, Zhang L, Lu JH, Mu JS, Chen L. 2011. Wogonin, an active ingredient of Chinese herb medicine Scutellaria baicalensis, inhibits the mobility and invasion of human gallbladder carcinoma GBC-SD cells by inducing the expression of maspin. J Ethnopharmacol. 137:1373–1380.
- Fu JF, Cao HW, Wang N, Zheng XC, Lu YL, Liu X, Yang D, Li B, Zheng J, Zhou H. 2008. An anti-sepsis monomer, 2′,5,6′,7-tetrahydroxyflavanonol (THF), identified from Scutellaria baicalensis Georgi neutralizes lipopolysaccharide in vitro, and in vivo. Int Immunopharmacol. 8:1652–1657.
- Gao J, Morgan WA, Sanchez-Medina A, Corcoran O. 2011. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol. 254:221–228.
- Gao L, Li C, Yang RY, Lian WW, Fang JS, Pang XC, Qin XM, Liu AL, Du GH. 2015. Ameliorative effects of baicalein in MPTP-induced mouse model of Parkinson's disease: a microarray study. Pharmacol Biochem Behaviorbiochem. 133:155–163.
- Gao ZH, Huang KX, Yang XL, Xu HB. 1999. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. BBA-Gen Subjects. 1472:643–650.
- Gu J, Huang W, Zhang WS. 2013. [Resources distribution survey of wild and cultivated Scutellaria baicalensis Georgi]. Chin J Inf Trad Chin Med. 20:42–45. Chinese.
- Guo Q, Zhao L, You Q, Yang Y, Gu H, Song G, Lu N, Xin J. 2007. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 74:16–24.
- Han J, Ye M, Xu M, Sun JH, Wang BR, Guo DA. 2007. Characterization of flavonoids in the traditional Chinese herbal medicine-Huangqin by liquid chromatography coupled with electrospray ionization mass spectrometry. J Chromatogr B. 848:355–362.
- Hattori S. 1930. Spectrography of the flavone series. III. The constitution of wogonin. Acta Phytochim. 5:99–116.
- Hirotani M, Kuroda R, Suzuki H, Yoshikawa T. 2000. Cloning and expression of UDP-glucose: flavonoid 7-O-glucosyltransferase from hairy root cultures of Scutellaria baicalensis. Planta. 210:1006–1013.
- Hong GE, Kim JA, Nagappan A, Yumnam S, Lee HJ, Kim EH, Lee WS, Shin SC, Park HS, Kim GS. 2013. Flavonoids identified from Korean Scutellaria baicalensis Georgi inhibit inflammatory signaling by suppressing activation of NF- κB and MAPK in RAW 264.7 cells. Evid-Based Compl Alt. 2013:912031.
- Horvath CR, Martos PA, Saxena PK. 2005. Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. J Chromatogr A. 1062:199–207.
- Hour MJ, Huang SH, Chang CY, Lin YK, Wang CY, Chang YS, Lin CW. 2013. Baicalein, ethyl acetate, and chloroform extracts of Scutellaria baicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza A viruses. Evid-Based Compl Alt Med. 2013:1.
- Huang RL, Chen CC, Huang HL, Chang CG, Chen CF, Chang C, Hsieh MT. 2000. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Medica. 66:694–698.
- Huang ST, Wang CY, Yang RC, Chu CJ, Wu HT, Pang JHS. 2010. Wogonin, an active compound in Scutellaria baicalensis, induces apoptosis and reduces telomerase activity in the HL-60 leukemia cells. Phytomedicine. 17:47–54.
- Huang XY, Wu PL, Huang FF, Xu M, Chen MY, Huang KT, Li GP, Xu MH, Yao D, Wang LX. 2017. Baicalin attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2A receptor-induced SDF-1/CXCR4/PI3K/AKT signaling. J Biomed Sci. 24:52.
- Hussein AA, Torre MCDL, Jimeno ML, Rodríguez B, Bruno M, Piozzi F, Servettaz O. 1996. A neo-clerodane diterpenoid from Scutellaria baicalensis. Phytochemistry. 43:835–837.
- Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Hsu JD, Chu CY. 2005. Protective effect of baicalin on tert-butyl hydroperoxide-induced rat hepatotoxicity. Arch Toxicol. 79:102–109.
- Hwang YK, Jinhua M, Choi BR, Cui CA, Jeon WK, Kim H, Kim HY, Han SH, Han JS. 2011. Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion-induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments. J Ethnopharmacol. 137:681–689.
- Ishimaru K, Nishikawa K, Omoto T, Asai I, Yoshihira K, Shimomura K. 1995. Two flavone 2'-glucosides from Scutellaria baicalensis. Phytochemistry. 40:279–281.
- Ji S, Li R, Wang Q, Miao WJ, Li ZW, Si LL, Qiao X, Yu SW, Zhou DM, Ye M. 2015. Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J Ethnopharmacol. 176:475–484.
- Ji S, Li ZW, Song W, Wang YR, Liang WF, Li K, Tang SN, Wang Q, Qiao X, Zhou DM, et al. 2016. Bioactive constituents of Glycyrrhiza uralensis (licorice): Discovery of the effective components of a traditional herbal medicine. J Nat Product. 79:281–292.
- Jiang D, Zhao ZY, Zhang T, Zhong WH, Liu CS, Yuan QJ, Huang LQ. 2017. The chloroplast genome sequence of Scutellaria baicalensis provides insight into intraspecific and interspecific chloroplast genome diversity in Scutellaria. Gene. 8:227.
- Jin ZX, Huang JQ, Zhu ZL. 2017. Baicalein reduces endometriosis by suppressing the viability of human endometrial stromal cells through the nuclear factor-κB pathway in vitro. Exp Therapeut Med. 14:2992–2998.
- Karimov AM, Botirov EK. 2017. Structural diversity and state of knowledge of flavonoids of the Scutellaria L. genus. Russ J Bioorganic Chem. 43:691–711.
- Khan NM, Haseeb A, Ansari MY, Haqqi TM. 2017. A wogonin-rich-fraction of Scutellaria baicalensis root extract exerts chondroprotective effects by suppressing IL-1β-induced activation of AP-1 in human OA chondrocytes. Sci Rep. 7:43789
- Kim DH, Cho KH, Moon SK, Kim YS, Kim DH, Choi JS, Chung HY. 2005. Cytoprotective mechanism of baicalin against endothelial cell damage by peroxynitrite. J Pharm Pharmacol. 57:1581–1590.
- Kim D-S, Son E-J, Kim M, Heo Y-M, Nam J-B, Ro J, Woo S-S. 2010. Antiallergic herbal composition from Scutellaria baicalensis and phyllostachys edulis. Planta Medica. 76:678–682.
- Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. 2009. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and map kinase signaling molecules. J Ethnopharmacol. 126:320–331.
- Kim YO, Leem K, Park J, Lee P, Ahn DK, Lee BC, Park HK, Suk K, Kim SY, Kim H. 2001. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J Ethnopharmacol. 77:183–188.
- Kim YJ, Kim HJ, Lee JY, Kim DH, Kang MS, Park W. 2018. Anti-inflammatory effect of baicalein on polyinosinic–polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses. 10:224.
- Kitamura K, Honda M, Yoshizaki H, Yamamoto S, Nakane H, Fukushima M, Ono K, Tokunaga T. 1998. Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res. 37:131–140.
- Kubo M, Kimura Y, Odani T, Tani T, Namba K. 1981. Studies on Scutellariae Radix. Part II: the antibacterial substance. Planta Medica. 43:194–201.
- Kumagai T, Müller CI, Desmond JC, Imai Y, Heber D, Koeffler HP. 2007. Scutellaria baicalensis, a herbal medicine: anti-proliferative and apoptotic activity against acute lymphocytic leukemia, lymphoma and myeloma cell lines. Leuk Res. 31:523–530.
- Islam MN, Chung HJ, Kim DH, Yoo HH. 2012. A simple isocratic HPLC method for the simultaneous determination of bioactive components of Scutellariae Radix extract. Nat Product Res. 26:1957–1962.
- Lam W, Bussom S, Guan FL, Jiang ZL, Zhang W, Gullen EA, Liu SH, Cheng YC. 2010. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2:45ra59.
- Lam W, Jiang Z, Guan F, Huang X, Hu R, Wang J, Bussom S, Liu S-H, Zhao H, Yen Y, et al. 2015. PHY906 (KD018), an adjuvant based on a 1800-year-old Chinese medicine, enhanced the anti-tumor activity of sorafenib by changing the tumor microenvironment. Sci Rep. 5:9384.
- Lapchak PA, Maher P, Schubert D, Zivin JA. 2007. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 150:585–591.
- Li BQ, Fu T, Dongyan Y, Mikovits JA, Ruscetti FW, Wang JM. 2000a. Flavonoid baicalin inhibits HIV-1 infection at the level of viral entry. Biochem Biophys Res Commun. 276:534–538.
- Li BQ, Fu T, Gong WH, Dunlop N, Kung HF, Yan YD, Kang J, Wang JM. 2000b. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunopharmacol. 49:295–306.
- Li BQ, Fu T, Yan YD, Baylor NW, Ruscetti FW, Kung HF. 1993. Inhibition of HIV infection by baicalin-a flavonoid compound purified from Chinese herbal medicine. Cell Mol Biol Res. 39:119–124.
- Li FQ, Wang T, Pei Z, Liu B, Hong JS. 2005. Inhibition of microglial activation by the herbal flavonoid baicalein attenuates inflammation-mediated degeneration of dopaminergic neurons. J Neural Transm (Vienna, Austria: 1996). 112:331–347.
- Li L, Li L, Chen C, Yang J, Li JX, Hu N, Li Y, Zhang DM, Guo T, Liu X. 2015. Scutellarin's cardiovascular endothelium protective mechanism: important role of PKG-iα. Plos One. 10:e0139570.
- Li XL, Xu XF, Bu QX, Jin WR, Sun QR, Feng DP, Zhang QJ, Wang LX. 2016. Effect of total flavonoids from Scutellaria baicalensis on dopaminergic neurons in the substantia nigra. Biomed Report. 5:213–216.
- Li YS, Li Q. 2017. [The research of chemical constituents and biological activities in Scutellaria baicalensis]. Health Way. 16:220–221. Chinese.
- Lin W, Liu S, Wu B. 2013. Structural identification of chemical constituents from Scutellaria baicalensis by HPLC-ESI-MS/MS and NMR spectroscopy. Asian J Chem. 25:3799–3805.
- Lin HL, Zhou J, Lin KC, Wang HJ, Liang ZH, Ren XS, Huang LT, Xia C. 2016. Efficacy of Scutellaria baicalensis for the treatment of hand, foot, and mouth disease associated with encephalitis in patients infected with ev71: a multicenter, retrospective analysis. Biomed Res Int. 2016:1.
- Liu GZ, Ma JY, Chen YZ, Tian QQ, Shen Y, Wang XS, Chen B, Yao SZ. 2009. Investigation of flavonoid profile of Scutellaria bacalensis Georgi by high performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J Chromatogr A. 1216:4809–4814.
- Liu GZ, Rajesh N, Wang XS, Zhang MS, Wu Q, Li SJ, Chen B, Yao SZ. 2011. Identification of flavonoids in the stems and leaves of Scutellaria baicalensis Georgi. J Chromatogr. B, Anal Technol Biomed Life Sci. 879:1023–1028.
- Liu XB, Gu JM, Fan YQ, Shi HH, Jiang ME. 2013. Baicalin attenuates acute myocardial infarction of rats via mediating the mitogen-activated protein kinase pathway. Biol Pharm Bull. 36:988–994.
- Liu YX, Liu ZG, Su L, Yang RP, Hao DF, Pei YH. 2009. [Chemical constituents from Scutellaria baicalensis Georgi]. Chin J Med Chem. 19:59–62. Chinese.
- Liu YX. 2008. [Studies on chemical constituents of Scutellaria baicalensis Georgi] [master’s thesis]. Liaoning (Shenyang): Shenyang Pharmaceutical University. Chinese.
- Li-Weber M. 2009. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and Baicalin. Cancer Treat Rev. 35:57–68.
- Long HL, Xu GY, Deng AJ, Li ZH, Ma L, Lu Y, Zhang ZH, Wu F, Qin HL. 2015. Two new flavonoids from the roots of Scutellaria baicalensis. J Asian Nat Product Res. 17:756–760.
- Lu J-H, Ardah MT, Durairajan SSK, Liu L-F, Xie L-X, Fong W-FD, Hasan MY, Huang J-D, El-Agnaf OMA, Li M. 2011. Baicalein inhibits formation of α-synuclein oligomers within living cells and prevents Aβ peptide fibrillation and oligomerisation. Chembiochem. 12:615–624.
- Ma JL. 2013. [Study on chemical constituents from stems and leaves of Scutellaria baicalensis]. Chin J Exp Trad Med Formulae. 19:147–149. Chinese.
- Mehendale S, Aung H, Wang CZ, Tong R, Foo A, Xie JT, Yuan CS. 2007. Scutellaria baicalensis and a constituent flavonoid, baicalein, attenuate ritonavir-induced gastrointestinal side-effects. J Pharm Pharmacol. 59:1567–1572.
- Miyaichi Y, Tomimori T. 1994. Studies on the constituents of Scutellaria species XVI phenol glycosides of the root of Scutellaria baicaleinsis Georgi. J Nat Med. 48:215–218.
- Miyaichi Y, Tomimori T. 1995. Studies on the constituents of Scutellaria Species XVII: phenol glycosides of the root of Scutellaria baicalensis Georgi (2). J Nat Med. 49:350–353.
- Mu JS, Liu TR, Jiang L, Wu XS, Cao Y, Li ML, Dong Q, Liu YB, Xu HN. 2016. The traditional Chinese medicine baicalein potently inhibits gastric cancer cells. J Cancer. 7:453–461.
- Mu X, He GR, Cheng YX, Li XX, Xu B, Du GH. 2009. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 92:642–648.
- Nagai T, Miyaichi Y, Tomimori T, Yamada H. 1989. Inhibition of mouse liver sialidase by plant flavonoids. Biochem Biophys Res Commun. 163:25–31.
- Nagai T, Moriguchi R, Suzuki Y, Tomimori T, Yamada H. 1995. Mode of action of the anti-influenza virus activity of plant flavonoid, 5,7,4'-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis. Antiviral Res. 26:11–25.
- Nagashima S, Hirotani M, Yoshikawa T. 2000. Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. Cell suspension cultures. Phytochemistry. 53:533–538.
- Nan JX, Park EJ, Kim YC, Ko G, Sohn DH. 2002. Scutellaria baicalensis inhibits liver fibrosis induced by bile duct ligation or carbon tetrachloride in rats. J Pharm Pharmacol. 54:555–563.
- Nayak MK, Agrawal AS, Bose S, Naskar S, Bhowmick R, Chakrabarti S, Sarkar S, Chawla-Sarkar M. 2014. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of ns1-mediated cellular innate immune responses. J Antimicrob Chemoth. 69:1298–1310.
- Nishikawa K, Furukawa H, Fujioka T, Fujii H, Mihashi K, Shimomura K, Ishimaru K. 1999. Flavone production in transformed root cultures of Scutellaria baicalensis Georgi. Phytochemistry. 52:885–890.
- Noguchi A, Horikawa M, Fukui Y, Fukuchi-Mizutani M, Iuchi-Okada A, Ishiguro M, Kiso Y, Nakayama T, Ono E. 2009. Local differentiation of sugar donor specificity of flavonoid glycosyltransferase in Lamiales. Plant Cell. 21:1556–1572.
- Oszmiański J, B??kowska A, Piacente S. 2004. Thermodynamic characteristics of copigmentation reaction of acylated anthocyanin isolated from blue flowers of Scutellaria baicalensis Georgi with copigments. J Sci Food Agri. 84:1500–1506.
- Olennikov DN, Chirikova NK, Tankhaeva LM. 2008a. Lamiaceae carbohydrates. IV. Water-soluble polysaccharides from Scutellaria baicalensis. Chem Nat Compound. 44:556–559.
- Olennikov DN, Rokhin AV, Tankhaeva LM. 2008b. Lamiaceae carbohydrates. V. structure of glucoarabinogalactan from Scutellaria baicalensis. Chem Nat Compound. 44:560–563.
- Olennikov DN, Stolbikova AV, Rokhin AV, Khobrakova VB. 2011. Carbohydrates from Lamiaceae. VIII. α-glucan from Scutellaria baicalensis roots. Chem Nat Compound. 47:190–193.
- Park HS, Park KI, Hong GE, Nagappan A, Lee HJ, Kim EH, Lee WS, Shin SC, Seo ON, Won CK, et al. 2014. Korean Scutellaria baicalensis Georgi methanol extracts inhibits metastasis via the forkhead box M1 activity in hepatocellular carcinoma cells. J Ethnopharmacol. 155:847–851.
- Park KI, Park HS, Kang SR, Nagappan A, Lee DH, Kim JA, Han DY, Kim GS. 2011. Korean Scutellaria baicalensis water extract inhibits cell cycle G1/S transition by suppressing cyclin D1 expression and matrix-metalloproteinase-2 activity in human lung cancer cells. J Ethnopharmacol. 133:634–641.
- Polier G, Giaisi M, Kohler R, Muller WW, Lutz C, Buss EC, Krammer PH, Li-Weber M. 2015. Targeting CDK9 by wogonin and related natural flavones potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263. Int J Cancer. 136:688–698.
- Popova TP, Litvinenko VI, Kovalev IP. 1973. Flavones of the roots of Scutellaria baicalensis. Chem Nat Compound. 9:699–702.
- Paton A, Suddee S, Bongcheewin B. 2016. Two new species of Scutellaria, (Lamiaceae) from Thailand and Burma. Kew Bull. 71:1–6.
- Qi XN, Li HT, Cong X, Wang X, Jiang ZL, Cao RF, Tian WR. 2016. Baicalin increases developmental competence of mouse embryos in vitro by inhibiting cellular apoptosis and modulating HSP70 and DNMT expression. J Reprod Develop. 62:561–569.
- Qian MY, Tang SS, Wu CM, Wang Y, He T, Chen TT, Xiao XL. 2015. Synergy between baicalein and penicillins against penicillinase-producing Staphylococcus aureus. Int J Med Microbiol. 305:501–504.
- Qiao X, Li R, Song W, Miao WJ, Liu J, Chen HB, Guo DA, Ye M. 2016. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-highperformance liquid chromatography coupled with hybrid quadrupoleorbitrap mass spectrometry and key ion filtering. J Chromatogr A. 1441:83–95.
- Qiu JZ, Niu XD, Dong J, Wang DC, Wang JF, Li HE, Luo MJ, Li ST, Feng HH, Deng XM. 2012. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of α-hemolysin. J Infect Dis. 206:292–301.
- Scheck AC, Perry K, Hank NC, Clark WD. 2006. Anticancer activity of extracts derived from the mature roots of Scutellaria baicalensis on human malignant brain tumor cells. BMC Compl Alternat Med. 6:1–9.
- Shang XF, He XR, He XY, Li MX, Zhang RX, Fan PP, Zhang QL, Jia ZP. 2010. The genus Scutellaria an ethnopharmacological and phytochemical review. J Ethnopharmacol. 128:279–313.
- Shang YZ, Qin BW, Cheng JJ, Miao H. 2006. Prevention of oxidative injury by flavonoids from stems and leaves of Scutellaria baicalensis Georgi in PC12 cells. Phytotherapy Res. 20:53–57.
- Shao ZH, Hoek TLV, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, et al. 2002. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 282:H999–H1006.
- Shao ZH, Li CQ, Hoek TLV, Becker LB, Schumacker PT, Wu JA, Attele AS, Yuan CS. 1999. Extract from Scutellaria baicalensis Georgi attenuates oxidant stress in cardiomyocytes. J Mol Cell Cardiol. 31:1885–1895.
- Shibata K, Iwata S, Nakamura M. 1923. Baicalin, a new flavone-glucuronic acid compound from the roots of Scutellaria baicalensis. Acta Phytochim. 1:105–139.
- Shieh DE, Cheng HY, Yen MH, Chiang LC, Lin CC. 2006. Baicalin-induced apoptosis is mediated by Bcl-2-dependent, but not p53-dependent, pathway in human leukemia cell lines. Am J Chinese Med. 34:245–261.
- Takagi S, Yamaki M, Inoue K. 1981a. Flavone di-C-glycosides from Scutellaria baicalensis. Phytochemistry. 20:2443–2444.
- Takagi S, Yamaki M, Inoue K. 1981b. On the minor constituents of the roots of Scutellaria baicalensis Georgi (Wogon). Yakugaku Zasshi. 101:899–903.
- Takagi S, Yamaki M, Inoue K. 1980. Studies on the water-soluble constituents of the roots of Scutellaria baicalensis Georgi (wogon). Yakugaku Zasshi. 100:1220–1224.
- Takido M, Aimi M, Yamanouchi S, Yasukawa K, Torii H, Takahashi S. 1976. Studies on the constituents in the water extracts of crude drugs. II. on the leaves of Scutellaria baicalensis Georgi. (1). Yakugaku Zasshi. 96:381–383.
- Takido M, Yasukawa K, Matsuura S, Iinuma M. 1979. On the revised structure of skullcapflavone I, a flavone compound in the roots of Scutellaria baicalensis Georgi (wogon). Yakugaku Zasshi. 99:443–444.).
- Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E. 2008. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem. 16:7516–7524.
- Thanh HN, Minh HPT, Le TA, Ly HDT, Huu TN, Duc LV, Kim TD, Thanh TB. 2015. Ethanol extracts of Scutellaria baicalensis protect against lipopolysaccharide-induced acute liver injury in mice. Asian Pac J Trop Biomed. 5:761–767.
- Tomimori T, Miyaichi Y, Imoto Y, Kizu H, Suzuki C. 1984b. Studies on the constituents of Scutellaria species. IV. On the flavonoid constituents of the root of Scutellaria baicalensis Georgi (4). Yakugaku Zasshi. 104:529–534.
- Tomimori T, Miyaichi Y, Imoto Y, Kizu H, Tanabe Y. 1984a. Studies on the constituents of Scutellaria species. III. On the flavonoid constituents of the root of Scutellaria baicalensis Georgi. Yakugaku Zasshi. 104:524–528.
- Tomimori T, Miyaichi Y, Imoto Y, Kizu HA, Tanabe Y. 1983. Studies on the constituents of Scutellaria species II. On the flavonoid constituents of the roots of Scutellaria baicalensis Georgi (2). Yakugaku Zasshi. 103:607–611.
- Tomimori T, Miyaichi Y, Kizu H. 1982. [On the flavonoid constituents from the roots of Scutellaria baicalensis Georgi. I]. Yakugaku Zasshi. J Pharmaceut Soc Japan. 102:388–391.
- Tsai CC, Lin MT, Wang JJ, Liao JF, Huang WT. 2006. The antipyretic effects of baicalin in lipopolysaccharide-evoked fever in rabbits. Neuropharmacology. 51:709–711.
- Tsao TF, Newman MG, Kwok YY, Horikoshi AK. 1982. Effect of Chinese and western antimicrobial agents on selected oral bacteria. J Dental Res. 61:1103–1106.
- Waisundara VY, Hsu A, Huang DJ, Tan BKH. 2008. Scutellaria baicalensis enhances the anti-diabetic activity of metformin in streptozotocin-induced diabetic Wistar rats. Am J Chinese Med. 36:517–540.
- Wang AW, Song L, Miao J, Wang HX, Tian C, Jiang X, Han QY, Yu L, Liu Y, Du J, et al. 2015. Baicalein attenuates angiotensin II-induced cardiac remodeling via inhibition of AKT/mTOR, ERKL/2, NF-κB, and calcineurin signaling pathways in mice. Am J Hypertens. 28:518–526.
- Wang CZ, Li XL, Wang QF, Mehendale SR, Yuan CS. 2010. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomed: Int J Phytother Phytopharmacol. 17:63–68.
- Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. 2015. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 47:1749–1758.
- Wang G, Liang J, Gao L-r, Si Z-p, Zhang X-t, Liang G, Yan Y, Li K, Cheng X, Bao Y, et al. 2018. Baicalin administration attenuates hyperglycemia-induced malformation of cardiovascular system. Cell Death Dis. 9:234.
- Wang HF, Zhang YL, Bai RX, Wang M, Du SY. 2016. Baicalin attenuates alcoholic liver injury through modulation of hepatic oxidative stress, inflammation and sonic hedgehog pathway in rats. Cell Physiol Biochem. 39:1129–1140.
- Wang HL, Cao J, Xu SQ, Gu DZ, Wang YP, Xiao SY. 2013. Depletion of high-abundance flavonoids by metal complexation and identification of low-abundance flavonoids in Scutellaria baicalensis Georgi. J Chromatogr A. 1315:107–117.
- Wang HW, Yin ZF, Li HB, Yuan XX, Yang MM, Zhao GQ. 2016. [Chemical constituents from stems and leaves of Scutellaria baicalensis]. Chin J Exp Trad Med Formulae. 22:41–44. Chinese.
- Wang HY. 2002. [Studies on anxiolytic constituents of Scutellaria baicalensis Georgi] [dissertation]. Liaoning (Shenyang): Shenyang Pharmaceutical University. Chinese.
- Wang J, Li K, Li Y, Wang Y. 2017. Mediating macrophage immunity with wogonin in mice with vascular inflammation. Mol Med Report. 16:8434–8440.
- Wang Q, Wang YT, Pu SP, Zheng YT. 2004. Zinc coupling potentiates anti-HIV-1 activity of baicalin. Biochem Biophys Res Commun. 324:605–610.
- Wang T, Jiang H, Cao S, Chen Q, Cui M, Wang Z, Li D, Zhou J, Wang T, Qiu F, et al. 2017. Baicalin and its metabolites suppresses gluconeogenesis through activation of AMPK or AKT in insulin resistant HepG-2 cells. Eur J Med Chem. 141:92–100.
- Wang Y, Cao H-j, Sun S-j, Dai J-y, Fang J-w, Li Q-h, Yan C, Mao W-w, Zhang Y-y. 2016. Total flavonoid aglycones extract in Radix Scutellariae, inhibits lung carcinoma and lung metastasis by affecting cell cycle and DNA synthesis. J Ethnopharmacol. 194:269–279.
- Wang YJ, Han E, Xing Q, Yan J, Arrington A, Wang C, Tully D, Kowolik CM, Lu DM, Frankel PH, et al. 2015. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 358:170–179.
- Wong KS, Tsang WK. 2009. In vitro antifungal activity of the aqueous extract of Scutellaria baicalensis Georgi root against Candida albicans. Int J Antimicrob Agents. 34:284–285.
- Wong YK, Chou MK, Shen YC, Wang YH, Yen JC, Chen CF, Lin SK, Liao JF. 2014. Preventive effect of baicalein on methamphetamine-induced amnesia in the passive avoidance test in mice. Pharmacology. 93:278–285.
- Wu JY, Tsai KW, Li YZ, Chang YS, Lai YC, Laio YH, Wu JD, Liu YW. 2013. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid-Based Compl Alternat Med: Ecam. 2013:579751
- Xie LH, Wang X, Basnet P, Matsunaga N, Yamaji S, Yang DY, Cai SQ, Tani T. 2002. Evaluation of variation of acteoside and three major flavonoids in wild and cultivated Scutellaria baicalensis roots by micellar electrokinetic chromatography. Chem Pharmaceut Bull. 50:896–899.
- Xiong Z, Jiang B, Wu PF, Tian J, Shi LL, Gu J, Hu ZL, Fu H, Wang F, Chen JG. 2011. Antidepressant effects of a plant-derived flavonoid baicalein involving extracellular signal-regulated kinases cascade. Biol Pharmaceut Bull. 34:253–259.
- Xu DY, Chen PD, Zhang L, Cao YD, Ding AW. 2011. [Study on chemical constituents of Scutellaria baicalensis]. Chin J Exp Trad Med Formulae. 17:78–80. Chinese.
- Xu G, Dou J, Zhang L, Guo QL, Zhou CL. 2010. Inhibitory effects of baicalein on the influenza virus in vivo is determined by baicalin in the serum. Biol Pharmaceut Bull. 33:238–243.
- Xu JX, Ding LQ, Jiang MM, Qiu F. 2016. [Non-flavonoid constituents from the roots of Scutellaria baicalensis Georgi]. Chin J Med Chem. 26:480–483. Chinese.
- Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang Y, Liu B. 2011. Baicalin induces human mucoepidermoid carcinoma Mc3 cells apoptosis in vitro and in vivo. Investig New Drugs. 29:637–645.
- Yang J, Wu X, Yu H, Liao X, Teng L. 2014. NMDA receptor-mediated neuroprotective effect of the Scutellaria baicalensis Georgi extract on the excitotoxic neuronal cell death in primary rat cortical cell cultures. Sci World J. 2014:459549.
- Yang X, Yang J, Zou HJ. 2013. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin Dev Immunol. 2013:268065
- Yang Y, Wang HM, Tong YF, Liu MZ, Cheng KD, Wu S, Wang W. 2016. Systems metabolic engineering of Escherichia coli to enhance the production of flavonoid glucuronides. RSC Adv. 6:33622–33630.
- Yang YZ, Tang YZ, Liu YH. 2013. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J Ethnopharmacol. 148:271–276.
- Ye F, Che YF, Mcmillen E, Gorski J, Brodman D, Saw D, Jiang B, Zhang DY. 2009. The effect of Scutellaria baicalensis on the signaling network in hepatocellular carcinoma cells. Nutr Cancer. 61:530–537.
- Ye F, Jiang S, Volshonok H, Wu J, Zhang DY. 2007. Molecular mechanism of anti-prostate cancer activity of Scutellaria baicalensis extract. Nutr Cancer. 57:100–110.
- Ye M, Liu SW, Jiang ZL, Lee YS, Tilton R, Cheng YC. 2007. Liquid chromatography/mass spectrometry analysis of PHY906, a Chinese medicine formulation for cancer therapy. Rapid Commun Mass Spectrometr. 21:3593–3607.
- Yin F, Liu JH, Ji XH, Wang YW, Zidichouski J, Zhang JZ. 2011. Baicalin prevents the production of hydrogen peroxide and oxidative stress induced by Aβ aggregation in SH-SY5Y cells. Neurosci Lett. 492:76–79.
- Yun JY, Jung YS. 2014. A Scutellaria baicalensis Radix water extract inhibits morphine-induced conditioned place preference. Pharm Biol. 52:1382–1387.
- Zandi K, Lim TH, Rahim NA, Shu MH, Teoh BT, Sam SS, Danlami MB, Tan KK, Abubakar S. 2013. Extract of Scutellaria baicalensis, inhibits dengue virus replication. BMC Compl Alternat Med. 13:91.
- Zgórka G, Hajnos A. 2003. The application of solid-phase extraction and reversed phase high-performance liquid chromatography for simultaneous isolation and determination of plant flavonoids and phenolic acids. Chromatographia. 57:S77–S80.
- Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J, Zhang W, Wei H, Sung M, Wang W, et al. 2003. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutellaria baicalensis. Cancer Res. 63:4037–4043.
- Zhang GW, Li CR, Niu YB, Yu Q, Chen YL, Liu EQ. 2017. Osteoprotective effect of Radix Scutellariae in female hindlimb-suspended Sprague-Dawley rats and the osteogenic differentiation effect of its major constituent. Molecules. 22:1044.
- Zhang KQ, Lu JM, Mori T, Smith-Powell L, Synold TW, Chen S, Wen W. 2011. Baicalin increases VEGF expression and angiogenesis by activating the ERRα/PGC-1α pathway. Cardiovasc Res. 89:426–435.
- Zhang X, Du L, Zhang W, Yang Y, Zhou QM, Du GH. 2017a. Therapeutic effects of baicalein on rotenone-induced Parkinson's disease through protecting mitochondrial function and biogenesis. Sci Rep. 7:9968.
- Zhang X, Tang X, Chen H. 1991. Inhibition of HIV replication by baicalin and S. baicalensis extracts in H9 cell culture. Chinese Med Sci J [Chung-Kuo i Hsueh K'o Hsueh Tsa Chih]. 6:230–232.
- Zhang X, Yang YL, Du LD, Zhang W, Du GH. 2017b. Baicalein exerts anti-neuroinflammatory effects to protect against rotenone-induced brain injury in rats. Int Immunopharmacol. 50:38–47.
- Zhang YY, Guo YZ, Ageta H, Harigaya Y, Onda M, Hashimoto K, Ikeya Y, Okada M, Maruno M. 1997. [A new flavone C-glycoside from Scutellaria baicalensis]. J Chin Pharm Sci. 6:182–186. Chinese.
- Zhang YY, Guo YZ, Onda M, Hashimoto K, Ikeya Y, Okada M, Maruno M. 1994. Four flavonoids from Scutellaria baicalensis. Phytochemistry. 35:511–514.
- Zhang YJ, Wei DF, Kai XU, Zhang ZJ. 2014. [Progress in research of Scutellaria baicalensis improving learning and memory of brain and affecting alzheimer's desease]. Chinese Pharmacol Bull. 30:9–13. Chinese
- Zhao Q, Chen XY, Martin C. 2016a. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Science Bulletin (Beijing). 61:1391–1398.
- Zhao Q, Cui M-Y, Levsh O, Yang D, Liu J, Li J, Hill L, Yang L, Hu Y, Weng J-K, et al. 2018. Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4′-deoxyflavones in Scutellaria baicalensis. Mol Plant. 11:135–148.
- Zhao Q, Zhang Y, Wang G, Hill L, Weng J-K, Chen X-Y, Xue H, Martin C. 2016b. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci Adv. 2:e1501780.
- Zhao WZ, Wang HT, Huang HJ, Lo YL, Lin AMY. 2018. Neuroprotective effects of baicalein on acrolein-induced neurotoxicity in the nigrostriatal dopaminergic system of rat brain. Mol Neurobiol. 55:130–137.
- Zhou Y, Hirotani M, Yoshikawa T, Furuya T. 1997. Flavonoids and phenylethanoids from hairy root cultures of Scutellaria baicalensis. Phytochemistry. 44:83–87.