Abstract
Context: Eclipta prostrata L. (Asteraceae) (EP) has been widely used for the treatment of skin disease in Asian traditional medicine.
Objective: This study investigates the potency of EP in promoting hair growth in vivo and in vitro.
Materials and methods: C57BL/6N mice were divided into four groups (n = 4) as follows: control (topical treatment of normal saline), topical 3% minoxidil to the dorsal skin of mice for 14 days, and low (1 mg/day) and high (10 mg/day) doses of EP orally administered once a day for 14 days. Dorsal hairs of C57BL/6N mice were depilated to synchronize anagen induction. Hair growth activity was evaluated by gross and microscopic observations. Sections of dorsal skin were stained with haematoxylin and eosin. We also treated the various concentrations of EP (5, 10 and 50 μg/mL) for 24 h on the human dermal papilla cells (HDPs) and examined the effects of EP on the expression of FGF-7 and mTOR signalling.
Results: EP enhanced the induction of anagen in the dorsal skin of mice, characterized by the appearance of inner root sheath along with hair shaft, the emergence of hair shaft through the epidermis. EP increased the expression of FGF-7, while decreased the level of FGF-5 in C57/BL6 mice. EP also increased the expression of FGF-7, activated the mTOR signalling in HDPs.
Discussion and conclusions: These results suggest that EP has a potency to enhance the growth of hair follicle, promoting hair growth through regulation of FGF-7 and FGF-5.
Introduction
Most people lose 50–100 of scalp hairs per day. New hairs grow again at the same time in the missing spot. Regeneration of hair shaft depends on the activity of underlying hair-follicle. The hair follicle constantly undergoes three distinct phase throughout the lifetime: anagen (growth phase), catagen (short transitional phase) and telogen (resting phase). Hair length and thickness varies among each part of the body which largely depends on the duration of hair follicular cycle. Hair loss occurs when hair growth cycles are disturbed, or when hair follicles are mechanically destroyed (Paus and Cotsarelis Citation1999; Stenn and Paus Citation2001).
Hair loss can occur throughout the scalp and whole body. Numerous studies on the physiology of hair growth have been carried out. Many growth factors, cytokines and hormones revealed to be implicated in the regulation of hair growth cycle; however, the clear mechanism of the hair loss has not been clarified. The factors associated with hair loss that have been known so far include genetic background, hormonal changes, pathological conditions, aging, and side effects of a drug. The leading cause of scalp hair loss is heredity and age. Although hair loss is not a life-threatening condition, it causes significant impairment of life quality. Currently, FDA-approved drugs for the treatment of androgenetic alopecia are a 5-α reductase type II inhibitor, oral finasteride (propecia) and minoxidil, which is an external agent for hair growth by increasing blood flow (Price Citation1999; Rogers and Avram Citation2008). Corticosteroids are also used to treat alopecia caused by a destruction of hair follicles by the immune system (Garg and Messenger Citation2009). There is a continuing need for drugs that prevent the progression of hair loss and facilitate hair regrowth.
The medicinal herb, Eclipta prostrata L. (Asteraceae) (EP) is an annual plant. In Asian traditional medicine, it has been used as an agent of tonifying the liver and kidney Yin, the essential fluid of the body (Lin et al. Citation2010). It has been applied for the treatment of skin disease in Thai traditional medicine (Tewtrakul et al. Citation2011). EP was reported to exhibit an anti-inflammatory (Ryu et al. Citation2013; Kim et al. Citation2017; Morel et al. Citation2017), antioxidative (Kim et al. Citation2008; Lee et al. Citation2009) and anti-hyperlipidaemic activities (Kumari et al. Citation2006; Dhandapani Citation2007).
In this study, we provide evidence that EP has hair growth facilitating activity in C57BL/6 mice. EP stimulates hair regrowth by modulating the expression of FGF-7.
Materials and methods
Cell culture and chemicals
Human dermal papilla cell lines (HDPs) are originally from Scien Cell (Carlsbad, CA). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% foetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, MA), supplemented with 50 units/mL of penicillin and 50 µg/mL of streptomycin. The cells were grown at 37 °C in humidified incubator containing a 5% CO2 atmosphere.
Preparation of EP extraction
EP was purchased from Hwalim Natural Drug Co. (Busan, Korea) and the characteristics of the sample were confirmed by the Division of Pharmacology, Pusan National University School of Korean Medicine. Voucher specimen (PNUKH-54) is deposited in the School of Korean Medicine, Pusan National University (Yangsan, Korea). EP (100 g) was soaked in 2 L of methanol and sonicated for 30 min; then kept for 24 h at room temperature to extract the active ingredients. The extract was filtered with Whatman filter paper (No. 20) and concentrated under reduced pressure through a vacuum decompression concentrator (Eyela, Tokyo, Japan). The concentrate was lyophilized with a freeze dryer (Labconco, Kansas City, MO) to give 6.38 g of final powder (yield 6.38%).
Fingerprinting analysis of EP
An Agilent 1200 series liquid chromatographic system comprising of binary solvent delivery pump, autosampler, vacuum degasser, thermostated column compartment and diode array detector were used, with data acquisition by Chemstation software (Agilent Corporation, Waldbronn, Germany). The chromatographic separation was carried out on a Capcell PAK MGII C18 column (4.6 × 150 mm, 3 μm; Shiseido, Tokyo, Japan) maintained at 35 °C. The mobile phase consisted of water containing 0.1% formic acid (A) and acetonitrile (B). The composition of the mobile phase was 0 min-10% (B), 13 min-10% (B), 20 min-25% (B), 24 min-30% (B), 28 min-35% (B), 32 min-45% (B), 35 min-45% (B), 40 min-50% (B), 43 min-55% (B), 47 min-60% (B) and 50 min-60% (B). The flow rate was 0.5 mL/min, and the injection volume was 15 μL. shows the typical chromatograms of the MeOH extracts of Eclipta prostrata.
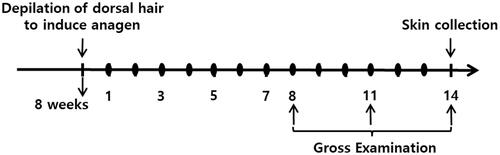
Figure 1. Scheme of the experiment. Dorsal hair of 7-week-old female C57BL/6 mice in the telogen stage of the hair growth cycle was depilated to induce initiation of the anagen stage. We assigned the mice into the following four groups (eight mice per group): group 1, control (vehicle treated); group 2, positive control (3% minoxidil-treated); group 3, experimental group 1 (low-dose Ep (EP-L) treated); group 4, experimental group 2 (high-dose Ep (EP-H) treated). One day after depilation of dorsal hair, mice were topically treated with 5% minoxidil or received with EP via the oral route. Dorsal skin was collected at 14 days after treatment and subjected to haematoxylin and eosin staining: ● Topical treatment with 5% minoxidil or oral administration of EP.
Reagents and antibodies
Minoxidil was purchased from Hyundai Pharm. Co. (Seoul, Korea) and Cayman Chemical (Ann Arbor, MI). Primary antibodies against fibroblast growth factor-7 (FGF-7), fibroblast growth factor-5 (FGF-5), β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and the antibodies against Akt, pAkt, S6K, pS6K, GAPDH were purchased from Cell Signaling Technology, Inc. (Danvers, MA). Biotinylated goat anti-mouse immunoglobulin G (IgG) and avidin–biotin peroxidase complex were purchased from Vector Laboratories, Inc. (Burlingame, CA). HRP-linked anti-mouse IgG antibody was purchased from GE Healthcare Life Science (Pittsburgh, PA). All chemicals and solvents used were purchased from E. Merck (Kenilworth, NJ), and Sigma-Aldrich Co. (St. Louis, MO), unless stated otherwise.
Hair growth activity in mice
Seven-week-old female C57BL/6 mice were purchased from Oriental Bio Co. (Seoul, Korea). After seven-days adaptation of breeding environment, the animals were divided into four randomized groups (n = 4); normal saline-, 3% minoxidil-, and low and high doses of EP-treated groups, to investigate the hair growth promoting the activity of EP. The hair cycle of the 8-week-old female C57BL/6 mice was synchronized by depilation as described (Seo et al. Citation2016). Hair growth activity was measured by gross and microscopic examination. The amount of EP was determined considering the dosage of human beings, the yield, and the metabolic rate of mice. Mice were sacrificed after 14 days, and tissues were processed as described below. The animal study protocol included in this study was approved by PNU-IACUC (PNU-2014-051) as approved by PNU-IACUC for the ethical and scientific evaluation of animal experiments.
Tissue preparation and histological examination
On the 14th day after treatment, the mice were sacrificed, and the dorsal skin tissues were collected and fixed in 4% formalin solution. Subsequently, dehydration, drying and penetration were carried out, followed by paraffin embedding. After cutting to a thickness of 7 μm, haematoxylin and eosin (H&E) staining was performed, and the change of hair follicles was observed through an optical microscope.
Immunohistochemistry
Dorsal skin tissues were fixed with 4% formaldehyde. After deparaffinization, tissues were autoclaved in 10 mM sodium citrate buffer for antigen retrieval. Skin tissues were blocked in PBS containing 1% goat serum for 30 min. Then, the skin tissues were incubated with the following primary antibodies for overnight at 4 °C in a moisture chamber: anti-FGF-7, FGF-5 (Santa Cruz Biotechnology, Santa Cruz, CA, 1:200). The next day, skin tissues were incubated with biotinylated goat anti-mouse IgG for 1 h. After washing, the tissues were incubated with the HRP-conjugated avidin–biotin complex. After washing, the tissues were incubated with 30 mg of 3,3′-diaminobenzidine dissolved in 150 mL of 0.1 M PBS solution for 5 min. Then, 0.005% hydrogen peroxide was added for 15 min to develop the colour. Immunohistochemical signals were visualized on light microscopy.
Western blot
Cells or tissues were lysed in RIPA buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% sodium dodecyl sulphate (SDS), 1.0% Triton X-100, 1% sodium deoxycholate, 5 mM EDTA). Samples were electrophoretically separated on 12% SDS-polyacrylamide gels and transferred onto PVDF membranes. After blocking in 5% nonfat skim milk in TBST buffer (Tris-buffered saline containing 0.1% Tween 20 (pH 7.8)) at room temperature for 1 h, the blots were incubated with the following primary antibodies overnight at 4 °C: anti-FGF-7, FGF-5 (Santa Cruz Biotechnology, Santa Cruz, CA, 1:500), p-Akt, p-S6K (Cell Signaling Technology, Danvers, MA, 1:1000), GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, 1:3000). After three times washing with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody, diluted 1:5000 in 5% (w/v) nonfat dry milk in TBST buffers for 1 h, and the antibody labelling was visualized with a Super signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) according to the manufacturer’s instructions and detected using Amersham Imager 600 (GE Healthcare Ltd., Little Chalfont, UK).
Immunofluorescence staining
HDPs were seeded on coverslips to the 70% of density. After EP and minoxidil treatment, cells were fixed with 4% paraformaldehyde-phosphate buffered saline (PBS) for 15 min and permeabilized with 0.1 T Triton X-100 in PBS. After blocking in 3% BSA in PBS for 30 min, cells were incubated with anti-FGF-7 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, 1:200) diluted in blocking buffer for overnight at 4 °C. After three times washing with PBS, cells were incubated with Alexa Fluor 488 secondary antibodies (Invitrogen, Carlsbad, CA, 1:1,000 dilution) for 1 h at room temperature. Then, slides were washed with PBS three times and mounted with ProLong Gold anti-fade mountant with DAPI. The stained cells were detected using Zeiss LSM 700 confocal microscopy (Oberkochen, Germany). The final images were obtained and analysed by using confocal microscopy with viewer software. Each image is a single Z section at the same cellular level.
Quantitative polymerase chain reaction (q-PCR)
Total RNA was isolated from the tissue samples of dorsal skin or HDPs with Trizol™ (GeneAll Biotechnology, Seoul, South Korea). The cDNA was generated from 1 µg of total RNA using Topscript™ Reverse Transcription System (Enzynomics, Inc., Daejeon, South Korea) following the manufacturer’s protocol. PCR was performed with TOPreal™ qPCR 2X Pre Mix (SYBR Green with low ROX, Enzynomics, Inc., Daejeon, South Korea) using Rotor Gene Q instrument (Corbett Research, Mortlake, Australia) at 95 °C for 5 min, followed by 25–30 cycles of 95 °C for 30 s, 60 °C for 1 min, and 75 °C for 1 min. The following primer sets were used: mFGF-7, forward 5rwCCCTTTGATTGCCACAATTC-3CTand reverse 5veTTGACAAACGAGGCAAAGTG-3G; mFGF-5, forward 5′-CGCGGACGCATAGGTATTAT-3CGand reverse 5veACGAGGAGTTTTCAGCAACAA-3G; mGAPDH, forward 5rwGGAGCCAAAAGGGTCATCAT-3AGand reverse 5veGTGATG GCATGGACTGTGGT-3A; hFGF-7, forward 5′-GACATGGATCCTGCCAACTTGGGCTGGAACAGTTCACATT-3CAand reverse 5veGGGCTGGAACAGTTCACATT-3G; hFGF-5, forward 5′-CTTGGAGCAGAGCAGTTTCC-3TGand reverse 5veCTTCGTGGGATCCATTGACT-3T; hGAPDH, forward 5rwAGGGCTGCTTTTAACTCTGGT-3GGand reverse 5veCCCCACTTGATTTTGGAGGGA-3C.
Statistical analysis
Values are expressed as mean ± standard deviation (SD) of three samples obtained from three independent experiments. Statistical comparisons between groups were performed using one-way analysis of variance (ANOVA) and considered at *p< 0.05.
Results
EP promotes hair-regrowth and induces FGF-7 in C57BL/6 mice
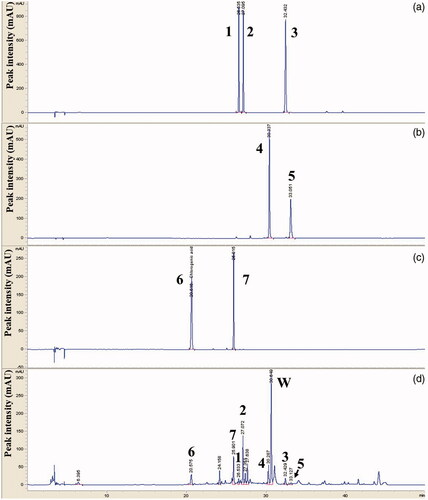
We fingerprinted the methanol extract of EP by HPLC to ensure the quality of EP used in this study and the reproducibility of the results. We identified apiin, apigenin 7-glucoside, apigenin, luteolin, kaempferol, chlorogenic acid and luteolin 7-glucoside as index compounds in EP ().
Figure 2. HPLC chromatogram of a standard mixture. Comparative chromatograms of phenolic components in the standard solution (a–c) and Eclipta extracts (d) by HPLC-MWD. Peak number indicated 1: apiin, 2: apigenin 7-glucoside, 3: apigenin, 4: luteolin, 5: kaempferol, 6: chlorogenic acid and 7: luteolin 7-glucoside. Phenolic compounds were eluted by CapcellPAK MGII C18 analytical column (4.6 × 150 mm, 3 µm) at 254–320 nm.
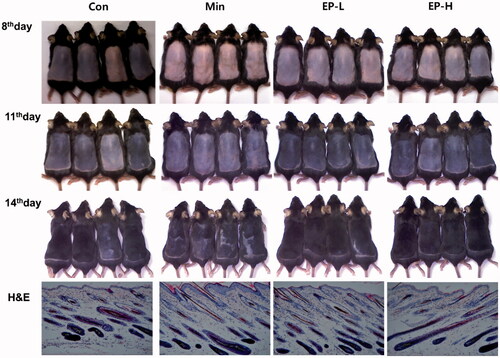
We observed the hair growth activity of EP. EP was orally administrated to C57BL/6 mice at two different doses (1, 10 mg/day). Min was separately applied daily onto the backs of the mice as a positive control. As shown in , from 8th day, the colour of dorsal skin was more rapidly changing to black in EP treated group, suggesting active transition to the anagen phase. The hair growth on the 11th and 14th days was more prominent in EP group than those of Con and Min group. Histologic examination reveals hair follicles reached the subcutaneous muscle layer, and hair shaft surrounded by hair canal emerges through epidermis in the Min or EP treated group (, H&E).
Figure 3. Gross and microscopic observation of the dorsal skin. The dorsal skin of mice in each group was photographed at 8, 11 and 14 days after treatment. Hair growth and skin darkness was more prominent in EP groups than Con and Min groups. Con: control, Min: minoxidil, EP-L: 1 mg/day of Eclipta prostrata, EP-H: 10 mg/day of Eclipta prostrata. After 14 days of treatment, skin tissues were collected, and stained with haematoxylin and eosin. Dermal papilla was enclosed in the control mice, while hair shaft was appeared through the hair canal in Min, EP treated mice. Original magnification: ×100 (H&E).
EP increased the expression of FGF-7, while decreased the FGF-5 in C57BL/6 mice
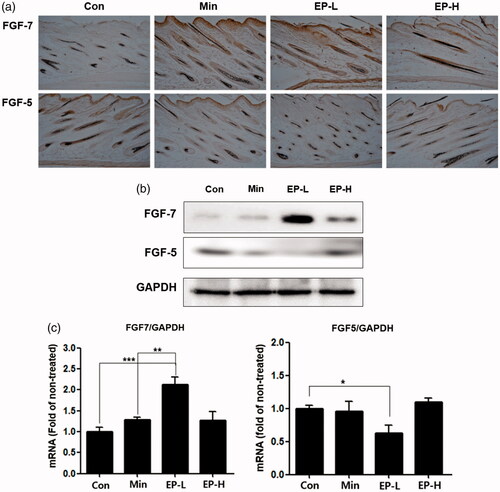
It was reported that FGF-7 is the key signalling protein that is required for the transition from telogen to anagen (Greco et al. Citation2009). FGF-5 was known to act a critical role in hair cycle transiting from the anagen to catagen (Hebert et al. Citation1994; Higgins et al. Citation2014). EP treatment increased the expression of FGF-7, while decreased the expression of FGF-5 in both protein and mRNA level (). Interestingly, extent of increased expression of FGF-7 was more prominent in low dose EP treated group (EP-L), the degree of decreased expression of FGF-5 was also more evident in EP-L group. This tendency, that the EP-L treatment is more efficient than EP-H, was also confirmed in the measurement of mRNA expression by real-time PCR ().
Figure 4. EP’s effects on the expression of FGF-7, FGF-5 in dorsal skin of mice. EP treatment increased the expression of FGF-7 which is responsible for the anagen induction, while decreased the expression of FGF-5 that induces regression of the hair follicle. (a) Immunohistochemical analysis of the expression of FGF-7 or FGF-5 in the EP-treated mice. Original magnification: ×100. (b) Western blotting analysis of the expression of FGF-7 and FGF-5. Proteins were extracted from the tissue of dorsal skin of mice. (c) mRNA expressions were measured by real-time polymerase chain reactions. Total RNA was also extracted from the tissue. Con: control; Min: minoxidil; EP-L: 1 mg/day of Eclipta prostrata; EP-H: 10 mg/day of Eclipta prostrata.
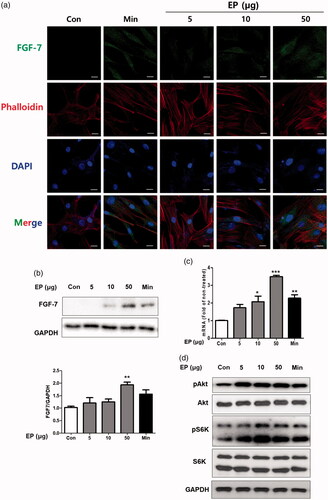
EP increased the expression of FGF-7, activated the mTOR signalling in human dermal papilla cells
We examined the EP’s effects on the HDPs. First, we measured the cytotoxic effects of EP. Although cell viability was slightly decreased from the 100 μg/mL concentration, no statistical difference was examined up to 500 μg/mL in HDPs (Supplementary Figure 1). We treated several concentration of EP (5, 10 and 50 μg/mL) to HDPs. Minoxidil (10 μM) was treated as a positive control. EP increased the cytosolic expression of FGF-7 measured by immunofluorescence assay (). We also measure the protein and mRNA level of FGF-7 in HDPs by western blot, and real-time PCR assay. EP also increased the expression of FGF-7 in both protein, mRNA level (). EP also activated the mTOR signalling in HDPs, confirmed by the measurement of the expression of pS6K, the major substrate of mTORC1 ().
Figure 5. EP's effects on the expression of FGF-7 in human dermal papilla cells (HDPs). (a) EP increased cytoplasmic expression of FGF-7. HDPs were treated with EP (5, 10 and 50 μg/mL) or minoxidil (10 μM) for 24h. Subcellular localization of FGF-7 was determined by immunofluorescence staining for endogenous FGF-7. Phalloidin and DAPI were respectively used to staining cytoskeleton and cell nuclei. Bar scale represents 10 μm. EP treatment increased the expression of FGF-7 in HDPs both protein (b) and mRNA (c) level. EP treatment also activated the Akt, mTOR signaling in HDPs (d).
Discussion
A subcutaneous hair follicle is the main structure to produce a hair shaft. Hair follicle perpetually goes through three distinct phases; anagen – growth phase, catagen – resting phase, and telogen – regression phase. Hair follicular cycling occurs over the lifetime. Although the driving force of hair cycling is not clear, cyclic regeneration requires many cellular signalling molecules.
At the beginning of anagen, new hair follicles are formed in the deep portion of the dermis along with the invagination of secondary hair germ cells downward into the dermis. During anagen, matrix cells located in the hair bulb rapidly proliferate to produce hair shaft. In this phase, hair continues to grow. During formation of the hair shaft in anagen phase, follicular melanocytes present in the matrix, produce melanin granules and transfer it into the medulla keratinocyte. Follicular melanogenesis only occurs in the anagen phase.
Duration of anagen in the hair growth cycle is different depending on each part of the body. Scalp hair has longest anagen phase, lasting 1–8 years. About 90–95% of scalp hairs are in the anagen phase, and 5–10% are in the telogen phase. Each of scalp hair is in a different state in the hair growth cycle, which means that hair growth and loss are continuous. The hair in the anagen phase is particularly sensitive; the duration of anagen can be shortened by exposure to external stresses or nutritional deficiencies.
Progressive miniaturization of scalp hair and subsequent hair loss, the main characteristic feature of androgenic alopecia is the result of follicular miniaturization. The main cause of follicular miniaturization is a progressive shortening of successive anagen phase. Therefore, prevention of shortening of the anagen phase can be the main strategy to prevent hair miniaturization or hair loss.
To evaluate the hair growth promoting effects of EP on the C57BL/6 mice, we synchronized the hair growth cycle by depilation of dorsal skin hair, one day before the administration of EP. We first examine the colour of dorsal skin, because the deposition of melanin pigment occurs only in the anagen phase. The dorsal skin pigmentation at 8th days after treatment was more prominent in EP group which means that EP induces robust cellular proliferation in the hair follicles of C57BL/6 mice. Then, we examined the hair regeneration by gross observation (, 8th day). After 14 days of treatment, hair regrowth was more prominent in low dose EP treated group than control or Min group (, 14th day). This result is consistent with the microscopic observation. In the EP group, we can observe the hair shaft contained in the hair canal of which the tip emerges through the epidermis (, H&E). This feature represents the EP efficiently induced anagen in C57BL/6 mice.
The underlying molecular mechanisms by which hair follicles are formed, differentiated, degenerated and regenerated, are not completely understood. However, complex interactions of molecules between the hair follicular cells such as dermal papilla and follicular epithelial cell are critical for the cycling of hair follicles. Among them, FGF-7 is an important mediator in the growth of hair follicle (Danilenko et al. Citation1995; Guo et al. Citation1996). FGF-7 has been shown to increase the survival of hair follicle as well as its regeneration (Booth and Potten Citation2000). FGF-7 is produced by dermal papilla and its receptors are highly expressed in the overlying matrix cells during anagen (Danilenko et al. Citation1996). In this study, we examined whether EP increases the expression of FGF-7 in vivo or in vitro. In C57BL/6 mice, EP enhanced the expression of FGF-7 both of the protein and mRNA level. Interestingly, low dose of EP was more efficient to enhance the growth of hair follicles (). EP also increased the expression of FGF-7 in cultured HDPs. The amount of cytosolic FGF-7 granules was increased by treatment of EP in HDPs (). EP also increased the expression of FGF-7 in cell lysates, at both protein and mRNA level ().
Conversely, EP treatment decreased the expression of FGF-5 in the dorsal skin of C57BL/6 mice. Protein and mRNA expression of FGF-5 was more prominently decreased in the EP-L group, indicating that low dose of EP is more efficient on the hair regrowth in C57BL/6 mice (). FGF-5 was known as an inhibitor of hair elongation (Hebert et al. Citation1994). FGF-5 in hair follicle begins to be expressed at the end of anagen stage, it has a critical role in the transition of the hair follicle from anagen to catagen. FGF-5 promotes the cessation of anagen by binding to FGFR1 on the dermal papilla (Ota et al. Citation2002). Therefore, it was postulated that inhibition of FGF-5 can provide the therapeutic benefit for the short anagen syndrome (Higgins et al. Citation2014). The inhibitory effects of EP on the expression of FGF-5, suggest that it can be helpful for activating the hair follicle by suppressing the termination of anagen phase.mTOR signalling was reported to be involved in tologen to anagen transition (Castilho et al. Citation2009). mTOR signalling also reported to promote stem cell activation during hair regeneration (Deng et al. Citation2015). Here, we further examined whether EP effects on the mTOR signalling in HDPs. Interestingly, EP activates the canonical signalling of mTOR in HDPs, confirmed by the expression of phospho-Akt and phospho-p70 S6 kinase, the major substrate of mTORC1 ().
Hair shaft producing cells were proliferated only in the anagen. Many mediators were involved in starting, sustaining and terminating anagen. Among them, we focused on the growth factor FGF-7 and FGF-5. Our results reveal the hair growth enhancing effects of EP were related with the anagen regulating growth factors. mTOR activating the potential of EP in HDPs was signifying that it exerts the positive role in the proliferation of follicular cells during anagen.
Supplementary_Figure.docx
Download MS Word (32.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Booth C, Potten CS. 2000. Keratinocyte growth factor increases hair follicle survival following cytotoxic insult. J Invest Dermatol. 114:667–673.
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. 2009. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 5:279–289.
- Danilenko DM, Ring BD, Pierce GF. 1996. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol Med Today. 2:460–467.
- Danilenko DM, Ring BD, Yanagihara D, Benson W, Wiemann B, Starnes CO, Pierce GF. 1995. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am J Pathol. 147:145–154.
- Deng Z, Lei X, Zhang X, Zhang H, Liu S, Chen Q, Hu H, Wang X, Ning L, Cao Y, et al. 2015. mTOR signaling promotes stem cell activation via counterbalancing BMP-mediated suppression during hair regeneration. J Mol Cell Biol. 7:62–72.
- Dhandapani R. 2007. Hypolipidemic activity of Eclipta prostrata (L.) L. leaf extract in atherogenic diet induced hyperlipidemic rats. Indian J Exp Biol. 45:617–619.
- Garg S, Messenger AG. 2009. Alopecia areata: evidence-based treatments. Semin Cutan Med Surg. 28:15–18.
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. 2009. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 4:155–169.
- Guo LF, Degenstein L, Fuchs E. 1996. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 10:165–175.
- Hebert JM, Rosenquist T, Gotz J, Martin GR. 1994. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 78:1017–1025.
- Higgins CA, Petukhova L, Harel S, Ho YY, Drill E, Shapiro L, Wajid M, Christiano AM. 2014. FGF5 is a crucial regulator of hair length in humans. Proc Natl Acad Sci USA. 111:10648–10653.
- Kim DI, Lee SH, Choi JH, Lillehoj HS, Yu MH, Lee GS. 2008. The butanol fraction of Eclipta prostrata (Linn) effectively reduces serum lipid levels and improves antioxidant activities in CD rats. Nutr Res (New York, NY). 28:550–554.
- Kim DS, Kim SH, Kee JY, Han YH, Park J, Mun JG, Joo MJ, Jeon YD, Kim SJ, Park SH, et al. 2017. Eclipta prostrata improves DSS-induced colitis through regulation of inflammatory response in intestinal epithelial cells. Am J Chin Med. 45:1047–1060.
- Kumari CS, Govindasamy S, Sukumar E. 2006. Lipid lowering activity of Eclipta prostrata in experimental hyperlipidemia. J Ethnopharmacol. 105:332–335.
- Lee MK, Ha NR, Yang H, Sung SH, Kim YC. 2009. Stimulatory constituents of Eclipta prostrata on mouse osteoblast differentiation. Phytother Res. 23:129–131.
- Lin XH, Wu YB, Lin S, Zeng JW, Zeng PY, Wu JZ. 2010. Effects of volatile components and ethanolic extract from Eclipta prostrata on proliferation and differentiation of primary osteoblasts. Molecules (Basel, Switzerland). 15:241–250.
- Morel LJF, Azevedo BC, Carmona F, Contini SHT, Teles AM, Ramalho FS, Bertoni BW, Franca SC, Borges MC, Pereira AMS. 2017. A standardized methanol extract of Eclipta prostrata (L.) L. (Asteraceae) reduces bronchial hyperresponsiveness and production of Th2 cytokines in a murine model of asthma. J Ethnopharmacol. 198:226–234.
- Ota Y, Saitoh Y, Suzuki S, Ozawa K, Kawano M, Imamura T. 2002. Fibroblast growth factor 5 inhibits hair growth by blocking dermal papilla cell activation. Biochem Biophys Res Commun. 290:169–176.
- Paus R, Cotsarelis G. 1999. The biology of hair follicles. N Engl J Med. 341:491–497.
- Price VH. 1999. Treatment of hair loss. N Engl J Med. 341:964–973.
- Rogers NE, Avram MR. 2008. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol. 59:547–566. Quiz 567–568.
- Ryu S, Shin JS, Jung JY, Cho YW, Kim SJ, Jang DS, Lee KT. 2013. Echinocystic acid isolated from Eclipta prostrata suppresses lipopolysaccharide-induced iNOS, TNF-α, and IL-6 expressions via NF-κB inactivation in RAW 264.7 macrophages. Planta Med. 79:1031–1037.
- Seo HS, Lee DJ, Chung JH, Lee CH, Kim HR, Kim JE, Kim BJ, Jung MH, Ha KT, Jeong HS. 2016. Hominis placenta facilitates hair re-growth by upregulating cellular proliferation and expression of fibroblast growth factor-7. BMC Complement Altern Med. 16:187.
- Stenn KS, Paus R. 2001. Controls of hair follicle cycling. Physiol Rev. 81:449–494.
- Tewtrakul S, Subhadhirasakul S, Tansakul P, Cheenpracha S, Karalai C. 2011. Antiinflammatory constituents from Eclipta prostrata using RAW264.7 macrophage cells. Phytother Res. 25:1313–1316.
