Abstract
Context
The relationship between resveratrol and histone acetylation in renal cell carcinoma (RCC) has not yet been reported.
Objective
To explore the functional role of resveratrol in RCC.
Materials and methods
Functional experiments were performed to determine proliferatio n of ACHN cells with treatment of resveratrol (0, 7.8125, 15.625, 31.25 and 62.5 μg/mL, for 12, 24 and 48 h of culture) or 0.1 μM SAHA. The enzyme activities of MMP-2/-9 were measured by gelatine zymography and histone acetylation by Western blot.
Results
When the cells were treated with 15.625, 31.25 and 62.5 μg/mL resveratrol, ACHN cells viability was 73.2 ± 3.5%, 61.4 ± 3.1%, 50.2 ± 4.7% for 12 h, 62.7 ± 4.5%, 52.4 ± 5.5%, 40.2 ± 3.8% for 24 h, and 60.8 ± 3.7%, 39.4 ± 5.1%, 37.6 ± 2.7% for 48 h, and the wound closure (%) of migration was increased from 0.6 to 0.7, 0.85, 0.9 for 12 h and from 0.23 to 0.3, 0.48, 0.59 for 24 h. The invasion rate was 8.5 ± 0.9%, 7.4 ± 0.3% and 5.8 ± 0.6%, and cell cycle was arrested at G1 from 42.5 ± 2.9% to 55.3 ± 5.7%, 59.8 ± 3.4%, 68.7 ± 4.6%. MMP-2/-9 expression (p < 0.05) was inhibited by resveratrol. The protein levels of histone acetylation (p < 0.01) was increased by resveratrol.
Discussion and conclusions
Our results suggest that these effects might be related to a high level of histone acetylation, and resveratrol can be considered as an alternative treatment for RCC.
Introduction
Renal cell carcinoma (RCC), a common malignant tumour of the kidney derived from renal tubular epithelial cells, accounts for almost 90% of adult renal malignant tumours and 3% of adult malignant tumours (Schrader et al. Citation2008; Rini et al. Citation2009). The incidence of renal cell carcinoma in newly diagnosed cases has increased steadily over the past two decades (Motzer et al. Citation1996). Therefore, a better understanding of molecular mechanisms underlying the occurrence and development of RCC is required to achieve the optimal therapeutic effect of RCC.
To the best of our knowledge, a cancer cell is characterized by invasion, easy transferring, reproduction, etc. (Ferraz da Costa et al. Citation2018; Konstantatou et al. Citation2019). Matrix metalloproteinases (MMPs) play an important role in tumour metastasis by degrading extracellular matrix (ECM) components, to be specific, they allow cells to pass through ECM to reach distant targets and their expressions and activations could lead to an increase in cancer cells in almost all people (Forget et al. Citation1999; Curran et al. Citation2004). Among them, MMP-2 and MMP-9 have been regarded as taking primarily responsibility for basement membrane and peri-cellular ECM rearrangement (Di Cara et al. Citation2018).
Studies have focussed on histone deacetylation, an important epigenetic modification involved in the development of many types of malignant tumours, including renal cell carcinoma, leukaemia, prostate cancer, lung cancer and colon cancer (Marti et al. Citation2012; Chiu et al. Citation2013). Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are enzymes that, respectively, control histone acetylation and deacetylation and play a pivotal role in the regulation of chromatin structure and gene expression. Breaking the balance between HAT and HDAC would directly cause the occurrence and development of cancer. In addition to this, immunohistochemical evaluation and microarray analysis provide evidence that histone acetylation is a common change in RCC (Kanao et al. Citation2008; Mosashvilli et al. Citation2010; De Vito et al. Citation2018). Histone acetylation may therefore be a potential therapeutic target for RCC.
Resveratrol (3, 5, 4′-trans-trihydroxystilbene), an antioxidant found in numerous plant species and in red wine, is a promising chemopreventive agent for cancer. Resveratrol has been reported to suppress the proliferation of a wide variety of tumour cells including RCC (Kim et al. Citation2016). Resveratrol regulates many cellular targets involved in cancer signalling pathways (Jang et al. Citation1997; Signorelli and Ghidoni Citation2005; Delmas et al. Citation2011; Shukla and Singh Citation2011; Scott et al. Citation2012). Studies have shown that resveratrol can result in many molecular processes such as apoptosis (Gwak et al. Citation2016), senescence (Kilic Eren et al. Citation2015), autophagy (Kumar et al. Citation2015), cellular metabolism, proliferation and migration (Li et al. Citation2015). The strong antitumor activity of resveratrol has attracted wide attention in the past few years (Aggarwal et al. Citation2004; Gescher et al. Citation2013). However, the functional roles of resveratrol in RCC regarding histone acetylation remain unexplored. Therefore, our study aims to determine whether resveratrol alleviated RCC through histone acetylation, and to demonstrate that resveratrol may be an effective therapeutic drug for RCC in clinics.
Materials and methods
Reagents
Resveratrol was purchased from Sigma-Aldrich (R5010; HPLC > 99%; St. Louis, MO). SAHA was obtained from Selleck (S1047; HPLC > 99.9%; Selleck, Houston, TX). CCK-8 assay kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Cell culture
Human renal carcinoma cell line (ACHN) was purchased from ATCC and cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA) containing 10% foetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA), 1% antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA) and 1% glutamine. The cells were incubated in an incubator at 37 °C under 5% CO2.
Cell viability assay
Resuspended ACHN cells were seeded in 96-well plates in DMEM containing 10% FBS and cultured overnight. Resveratrol with different doses (0, 7.8125, 15.625, 31.25 and 62.5 μg/mL) and 0.1 μM SAHA that cultured overnight were added to the well. After 12, 24 and 48 h of culture, 10 μL CCK-8 solution was added to the well and the cells were incubated at 37 °C for 2 h in an incubator under 5% CO2 in the dark. Subsequently, the optical density (OD) value of each well in different cell groups at an absorbance of 450 nm was determined by a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA). Cell viability was measured by CCK-8 assay kit according to the manufacturer’s protocol.
Cell scratch test
The suspended ACHN cells were seeded in six-well plates and cultured overnight in DMEM containing 10% FBS. When the cells reached 80% confluence, a straight gap was created by a 200 μL sterile tip in the middle of the well. The cells were washed twice with DMEM to smooth the edges of the scratch and then the floating cells were removed. DMEM containing different doses (0, 15.625, 31.25 and 62.5 μg/mL/control, low, medium and high) of resveratrol or deacetylase inhibitor SAHA was added into the well. After being incubated (37 °C, 5% CO2) for 12, 24 and 48 h, the migration images of the cells were observed under a microscope (Keyence, Osaka, Japan) and the distance of cell migration was measured by image-Pro Plus Analysis software (Media Cybernetics Company, Rockville, MD). The experiment was repeated for three times and the results were presented as mean value.
Transwell assay
A transwell chamber with 8 μm pores (3413, American Corning Company, New York, NY) was placed on a 24-well plate with a coating of 50 μL matrigel (BD Biosciences, Franklin Lakes, NJ). ACHN cells were cultured in serum-free medium for 12 h to eliminate the effects of the serum. Next, the cells were resuspended in DMEM containing bovine serum albumin (BSA; Sigma-Aldrich Chemical Company, St Louis, MO) with free FBS. The suspended cells (100 μL) were added to the transwell chamber, and 400 μL DMEM containing 20% FBS was added to the basolateral chamber, followed by treatment of the cells with different doses (0, 15.625, 31.25 and 62.5 μg/mL/control, low, medium, high) of resveratrol or SAHA. The cells were cultured for 24 h at 37 °C in an incubator under 5% CO2. The transwell chamber was then removed, the culture solution in the transwell chamber was discarded and the chamber was washed twice with calcium-free phosphate-buffered saline (PBS). After that, the chamber was fixed in methanol solution for 30 min and stained with 0.1% crystal violet for 20 min at room temperature. The chamber was washed several times with PBS, and the upper chamber liquid was aspirated. The unmigrated cells in the upper layer were gently wiped off using a cotton swab. The microporous membrane was removed carefully with small tweezers and dried with the bottom side up. Next, the membrane was transferred to a glass slide and sealed with a neutral gum. Images were observed and collected by an inverted optical microscope (Keyence, Osaka, Japan).
Cell cycle analysis
5 × 105 cells with 70% cold ethanol were cultured overnight under –20 °C. Then, the cells were centrifuged at 1200×g about 1 min and washed twice with PBS. Next, the cells were treated with 200 µL RNase A (1 mg/mL) for 10 min in suspension at 37 °C, and 300 µL PI (50 μg/mL, BioVision, Milpitas, CA) was then added into the cells in the dark. After 20 min of incubation at room temperature, cellular DNA content of the cells was analysed using FAC Scan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and the data were analysed by the Mod Fit LT software V2.0 (Becton Dickinson, Franklin Lakes, NJ).
Gelatine zymography
After treatment of ACHN cells with different concentrations (0, 15.625, 31.25 and 62.5 μg/mL/control, low, medium, high) of resveratrol or SAHA, the cells were cultured in serum-free medium for 24 h, and then lysed with protein lysis buffer (RIPA, Cell Signaling Technology, Inc., Danvers, MA) to collect the supernatant. Protein concentration was measured using a BCA protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA) and adjusted to 5 μg/μL using 1 × loading and diethyl pyrocarbonate (DEPC) water. Samples (6 μL) were loaded and fractioned on a 8% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gel supplemented with 0.1% gelatine (Bio-Rad Laboratories, Hercules, CA) under non-reducing conditions at 4 °C. The gels were washed twice in an eluent (2.5% Triton X-100, 50 mmol/L Tris–HCl, 5 mmol/L CaCl2, pH 7.6) for 30 min each time and then washed twice in a rinse solution (50 mmol/L Tris–HCl, 5 mmol/L CaCl2, pH 7.6) for 20 min each time. At 37 °C, the gels were incubated in an incubation solution (50 mmol/L Tris–HCl, 5 mmol/L CaCl2, 0.02% Brij-35, pH 7.6) overnight, stained with staining solution (0.5% Coomassie bright blue, 30% methanol, 10% acetic acid; Bio-Rad Laboratories, Hercules, CA) for 3 h and decoloured in a mixture of acetic acid and methanol at room temperature every 5 min until a colourless enzyme band was shown. Proteolytic activities of MMP-2 and MMP-9 were visualized as clear zone bands on a blue background and analysed using ImageJ software (version 1.50; National Institute of Mental Health, Bethesda, MD).
Western blot
After treatment of ACHN cells with different concentrations (0, 15.625, 31.25 and 62.5 μg/mL/control, low, medium, high) of resveratrol or SAHA, the cells were washed twice with PBS and added to protein lysis buffer (RIPA; Cell Signaling Technology, Inc., Danvers, MA) for 2 h on ice. Then, the cells were centrifuged at 12,000×g for 30 min at 4 °C. After that, the supernatant was collected. The protein concentration was tested using the BCA protein kit (Bio-Rad Laboratories, Inc., Hercules, CA) and adjusted to a concentration of 5 μg/μL using 1 × loading and DEPC water. Samples (6 μL) were electrophoresed (80 V for 30 min and then transferred to 120 V for 1.5 h) on 10% running gels. The gels were transferred onto polyvinylidene fluoride membrane (PVDF, Bio-Rad Laboratories, Inc., Hercules, CA) on ice for 110 min at 110 V. The membranes were blocked with 5% non-fat milk and eluted three times with TBS for 5 min each time. The bands were then incubated overnight with the corresponding primary antibody, and washed with TBS three times for 15 min; later, the bands were incubated with secondary antibody (1:2000; Santa Cruz Biotechnology, Inc., Dallas, TX) for 2 h at room temperature, washed with TBS three times for 15 min each time and then washed once with TBS/0.1% Tween-20 (TBST) for 15 min. The development was carried out with a developer (EZ-ECL kit; Biological Industries (BI), Beit HaEmek, Israel), and the grey values of the strips were analysed and counted by ImageJ (version 5.0; Bio-Rad, Hercules, CA). The antibodies used in the present study were as follows: anti-GAPDH (mouse; 1:1000; Santa Cruz Biotechnology, Inc., Dallas, TX), anti-acetyl-H3K14 (rabbit; 1:1000; ab52946; Abcam, Cambridge, UK), anti-acetyl-H3K9 (rabbit; 1:1000; ab4441; Abcam, Cambridge, UK), anti-acetyl-H4K12 (rabbit; 1:1000; ab46983; Abcam, Cambridge, UK), anti-acetyl-H4K5 (rabbit; 1:1000; ab51997; Abcam, Cambridge, UK), anti-acetyl-H4K16 (rabbit; 1:1000; ab109463; Abcam, Cambridge, UK), anti-H3 (rabbit; 1:1000; ab1791; Abcam, Cambridge, UK) and anti-H4 (rabbit; 1:1000; ab9051; Abcam, Cambridge, UK).
Statistical analyses
Data are shown as mean ± SEM and analysed by one-way ANOVA, p value <0.05 was considered as statistically significant.
Results
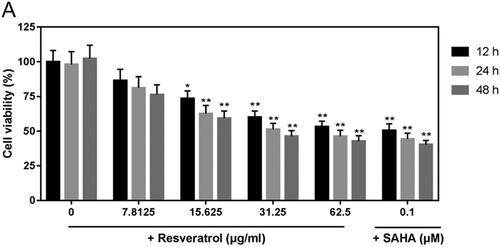
Resveratrol inhibits viability of ACHN cells
After 12, 24 and 48 h of post-treatment with different doses of resveratrol (0, 7.8125, 15.625, 31.25 and 62.5 μg/mL) and 0.1 μM SAHA, viability of ACHN cells was determined by CCK-8. The results showed that the viability was obviously lower in groups with 15.625, 31.25 and 62.5 μg/mL resveratrol than that in control group, and the inhibition of viability was also found in SAHA (). Thus, 15.625, 31.25 and 62.5 μg/mL resveratrol were selected and as assigned to low, medium and high groups for subsequent experiments.
Figure 1. Resveratrol inhibits viability of ACHN cells. ACHN cells were treated with 7.8125, 15.625, 31.25 and 62.5 μg/mL resveratrol and 0.1 μM SAHA for 12, 24 and 48 h. CCK-8 assay kit was used to detect cell viability, which was inhibited by resveratrol. All data were expressed by means ± SEM. *p < 0.05, **p < 0.01 vs. 0 dose.
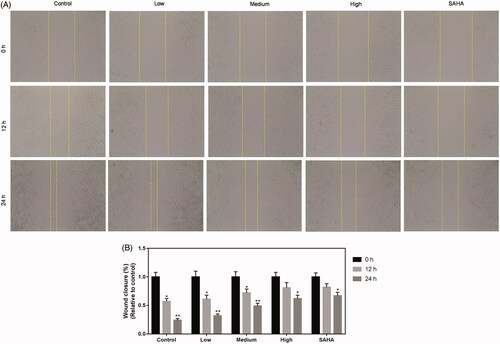
Resveratrol suppresses migration ability of ACHN cells
After treatment of ACHN cells with different doses of resveratrol (0, 7.8125, 15.625, 31.25 and 62.5 μg/mL) or SAHA (0.1 μM) for 0, 12 and 24 h, cell migration ability was determined by cell scratch test. The result revealed that wound distance was wider in high group than that in control group (). Thus, 24 h was selected for later experiments.
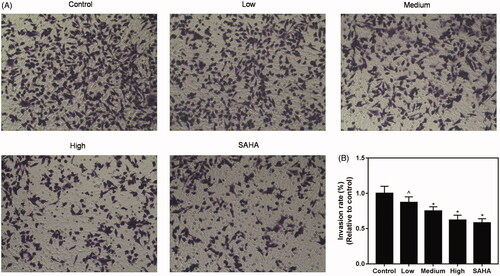
Resveratrol suppresses invasion ability of ACHN cells
Next, the effect of resveratrol on invasion ability of ACHN cell was detected. Resveratrol was found to significantly inhibit ACHN cells-induced invasion ability in medium and high groups, compared with control and SAHA groups ().
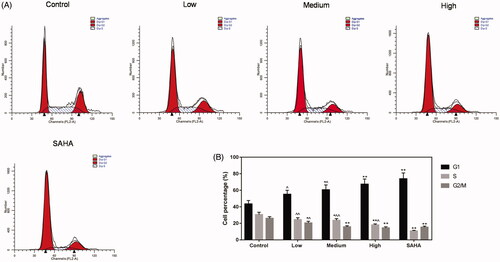
Resveratrol inhibits cycle of ACHN cells
Next, flow cytometry was performed to measure cycle of ACHN cells. The results indicated that resveratrol could significantly inhibit cell cycle in low, medium and high groups, compared with control and SAHA groups ().
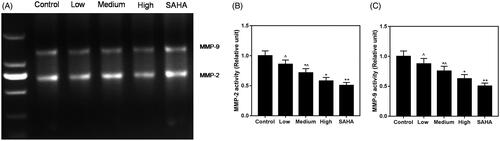
Treatment with resveratrol inhibits enzymatic activities of MMP-2 and -9
Resveratrol treatment induced inhibition of enzymatic activities of MMP-2 and -9 in ACHN cells (). Activities of MMP-2 (∼64 kDa) () and MMP-9 (∼84 kDa) () were decreased in resveratrol groups, compared with control group.
Figure 5. Effects of resveratrol on the gelatinolytic activities of MMP-9 and -2 in ACHN cells. (A) Band of clearance in gelatine zymogram. Activities of MMP-2 (B) and MMP-9 (C) were decreased in resveratrol group, respectively, compared to control and SAHA groups. All data were expressed by means ± SEM. ∧p < 0.05, vs. SAHA, *p < 0.05, **p < 0.01 vs. Control.
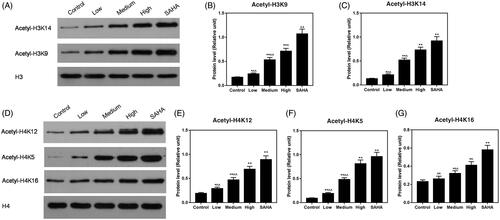
Resveratrol increases protein levels of histone acetylation
Protein levels of acH3K9, acH3K14, acH4K12, acH4K5 and acH4K16 were determined by Western blot. The results showed that the protein levels of acH3K9 (), acH3K14 (), acH4K12 (), acH4K5 () and acH4K16 () were higher in ACHN cells treated with resveratrol than those in the control group.
Figure 6. Effects of resveratrol on ACHN cells may be involved in histone acetylation. (A–C) Expression of acH3K9 and acH3K14 was assessed at the protein level by Western blot. (D–G) Expression of acH4K12, acH4K5 and acH4K16 was increased by resveratrol at the protein level using Western blot. All data were expressed by means ± SEM. ∧p < 0.05, ∧∧p < 0.01 vs. SAHA, *p < 0.05, **p < 0.01 vs. Control.
Discussion
In the present study, we assessed the effect of resveratrol on ACHN cells viability, migration, invasion and MMP-2/-9 enzymatic activities. We found that the cell viability was reduced by resveratrol in a dose-dependent manner rather than time-dependent manner. The wound healing and transwell assays showed that resveratrol impaired the migration and invasion abilities of ACHN cells. We showed that resveratrol decreased the activities of MMP-2/-9. We also found that the level of histone acetylation was higher in resveratrol groups than that in the control group.
RCC accounts for about 2–3% of adult malignant tumours and 80–90% of adult kidney malignancies. The ratio of male to female patients in morbidity is about 2:1 (Prasad et al. Citation2018; Qiao et al. Citation2018; Sun et al. Citation2018). Chemotherapy is a major treatment method for RCC, has serious adverse actions and causes a reduction of patients in treatment compliance due to its high cost (Safadi et al. Citation2018). Therefore, it is necessary to explore treatments featuring high effectiveness, low toxicity and reasonable price. In previous years, traditional Chinese medicine, including resveratrol, has high efficiency and low toxicity, its production and development attracted increasing attention in China (Olaku and White Citation2011). Resveratrol is a non-flavonoid polyphenol with wide pharmacological actions and has been reported to have anticancer effects (Michaille et al. Citation2018); however, its mechanism of action including histone acetylation has not been clearly elucidated.
Cell viability is an important hallmark of tumour cell survival (Shu et al. Citation2018). CCK-8 assay was used to detect the cell viability of ACHN cells. The results showed that the cell viability was decreased by resveratrol. Malignant tumours cells have typical characteristics such as invasiveness and metastatic ability (Sun et al. Citation2018). Tumour invasion is a key step leading to metastasis and poor prognosis (Kraljevic Pavelic et al. Citation2011). Metastasis arose from several consecutive mutation events in primary tumour subsets or disseminated cells, producing a small number of cells with full metastatic potential (Ramaswamy et al. Citation2003). Similar to previous research results (Zhao et al. Citation2018), we discovered that invasion, migration and cell cycle of ACHN cells were inhibited by resveratrol in a dose-dependent manner. Thus, we speculated that resveratrol had anticancer effect and then further studied its mechanism of action.
Matrix metalloproteinases are considered to be regulatory factors of tumour microenvironment and carcinogenesis (Strbac et al. Citation2018). The MMPs are involved in the key ECM degradation of proteolytic enzyme family (Curran et al. Citation2004; Chen KE et al. Citation2018; Qiao et al. Citation2018) and are used to disrupt the interaction of cells–cells and cells–ECM by tissue remodelling. Studies have shown that the MMPs, particularly MMP-2 and MMP-9, key members of the MMP family, play a vital role in angiogenesis, invasion and transferring of tumours, including RCC (Ramos et al. Citation2016; Chen et al. Citation2017; Liu et al. Citation2017; Beardo et al. Citation2019). Therefore, gelatine zymography was performed to determine the activity of MMP-2/-9. We found that resveratrol significantly decreased MMP-2 and MMP-9 expressions. However, one previous research reported that the expression level of MMP-2 in ACHN cells was not affected by resveratrol (Zhao et al. Citation2018). The difference may be explained by concentrations of resveratrol and action times applied in the two studies. These data indicated that resveratrol could inhibit activity of MMP-2/-9. Studies have shown that MMP-2/-9 activity is associated with histone acetylation (Yeh et al. Citation2016).
Epigenetics play an increasingly important role in the development of new treatments that can provide new prognostic biomarkers and early diagnosis (Baldewijns et al. Citation2008). Histone modification including deacetylase and acetylation is an epigenetic mechanism that plays a very important role in RCC. Acetylation is one of the important epigenetic markers associated with histones and leads to chromatin remodelling and gene expression regulation in the enhancers and promoters of genes (Pradeepa et al. Citation2016). Studies have shown that the degree of histone acetylation in renal cell carcinoma is low (Liu et al. Citation2017). The acetylation of histone H3 is diminished in the RCC 786-M-R cells (Wei et al. Citation2018). The histone H3 in BNIP3 (bcl-2/adenovirus E1B 19 kDa interacting protein 3) promoter of both 786-O and ACHN is deacetylated, while the histone H3 in BNIP3 promoter of A498 is acetylated (Liu et al. Citation2017). A novel HDAC inhibitor, N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA) has antitumor and pro-apoptotic effects on renal cell carcinoma xenografts (Park et al. Citation2015). Therefore, we hypothesized that resveratrol might increase the level of histone acetylation. To verify our hypothesis, Western blot was used directly to detect the degree of histone acetylation in this study due to our funding limitation. According to detection results of Western blot, we discovered that the levels of histone acetylation (histones 3 and 4) were elevated by resveratrol, and such a discovery has never been reported. These data suggest that the regulation of resveratrol on ACHN cells is probably related to the increase in degree of histone acetylation. It has been reported that epigenetic marks (acH4, acH3K9) were linked to transcription repression of tumour suppressor genes (Berger Citation2007). Kang et al. (Citation2019) found that lncRNA AY927503 enriched H3K4Me3 and acH3K9/14, promoted hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Acetylation of histone H3 activated FasL transcription and promoted apoptosis of cancer stem-like cells in colorectal cancer (Chen Z et al. Citation2018). Previous study has reported that the increase of H3K4me2 and AcH3K9 was associated with inhibition of the growth of breast cancer cells (Huang et al. Citation2012). Yu et al. (Citation2019) reported that suberoylanilide hydroxamic acid induced apoptosis of liver cancer cells by upregulating histone H4 acetylation and activating endoplasmic reticulum stress. In this study, resveratrol inhibited ACHN cell metastasis may be via increase of acH3K9 and acH3K14, resveratrol inhibited ACHN cell growth by increasing acH3K9, acH3K14, acH4K12, acH4K5 and acH4K16 expression levels.
Conclusions
We demonstrate the antitumor effects of resveratrol on RCC. The biological function of resveratrol on renal cancer cells may be related to the regulation of histone acetylation. These findings provide a promising treatment approach for RCC.
Disclosure statement
The authors declare no conflicts of interest.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. 2004. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 24:2783–2840.
- Baldewijns MM, van Vlodrop IJ, Schouten LJ, Soetekouw PM, de Bruine AP, van Engeland M. 2008. Genetics and epigenetics of renal cell cancer. Biochim Biophys Acta. 1785:133–155.
- Beardo P, Truan Cacho D, Izquierdo L, Alcover-Garcia JB, Alcaraz A, Extramiana J, Mallofre C. 2019. Cancer-specific survival stratification derived from tumor expression of tissue inhibitor of metalloproteinase-2 in non-metastatic renal cell carcinoma. Pathol Oncol Res. 25:289–299.
- Berger SL. 2007. The complex language of chromatin regulation during transcription. Nature. 447:407–412.
- Chen CM, Hsieh SC, Lin CL, Lin YS, Tsai JP, Hsieh YH. 2017. Alpha-mangostin suppresses the metastasis of human renal carcinoma cells by targeting MEK/ERK expression and mmp-9 transcription activity. Cell Physiol Biochem. 44:1460–1470.
- Chen KE, Chen C, Lopez T, Radecki KC, Bustamante K, Lorenson MY, Ge X, Walker AM. 2018. Use of a novel camelid-inspired human antibody demonstrates the importance of MMP-14 to cancer stem cell function in the metastatic process. Oncotarget. 9:29431–29444.
- Chen Z, Li W, Qiu F, Huang Q, Jiang Z, Ye J, Cheng P, Low C, Guo Y, Yi X, et al. 2018. Aspirin cooperates with p300 to activate the acetylation of H3K9 and promote FasL-mediated apoptosis of cancer stem-like cells in colorectal cancer. Theranostics. 8:4447–4461.
- Chiu HW, Yeh YL, Wang YC, Huang WJ, Chen YA, Chiou YS, Ho SY, Lin P, Wang YJ. 2013. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, enhances radiosensitivity and suppresses lung metastasis in breast cancer in vitro and in vivo. PLoS One. 8:e76340.
- Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. 2004. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res. 10:8229–8234.
- De Vito A, Lazzaro M, Palmisano I, Cittaro D, Riba M, Lazarevic D, Bannai M, Gabellini D, Schiaffino MV. 2018. Amino acid deprivation triggers a novel GCN2-independent response leading to the transcriptional reactivation of non-native DNA sequences. PLoS One. 13:e0200783.
- Delmas D, Aires V, Limagne E, Dutartre P, Mazue F, Ghiringhelli F, Latruffe N. 2011. Transport, stability, and biological activity of resveratrol. Ann N Y Acad Sci. 1215:48–59.
- Di Cara G, Marabeti MR, Musso R, Riili I, Cancemi P, Pucci Minafra I. 2018. New insights into the occurrence of matrix metalloproteases-2 and -9 in a cohort of breast cancer patients and proteomic correlations. Cells. 7:89.
- Ferraz da Costa DC, Campos NPC, Santos RA, Guedes-da-Silva FH, Martins-Dinis M, Zanphorlin L, Ramos C, Rangel LP, Silva JL. 2018. Resveratrol prevents p53 aggregation in vitro and in breast cancer cells. Oncotarget. 9:29112–29122.
- Forget MA, Desrosiers RR, Beliveau R. 1999. Physiological roles of matrix metalloproteinases: implications for tumor growth and metastasis. Can J Physiol Pharmacol. 77:465–480.
- Gescher A, Steward WP, Brown K. 2013. Resveratrol in the management of human cancer: how strong is the clinical evidence? Ann N Y Acad Sci. 1290:12–20.
- Gwak H, Kim S, Dhanasekaran DN, Song YS. 2016. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 371:347–353.
- Huang Y, Vasilatos SN, Boric L, Shaw PG, Davidson NE. 2012. Inhibitors of histone demethylation and histone deacetylation cooperate in regulating gene expression and inhibiting growth in human breast cancer cells. Breast Cancer Res Treat. 131:777–789.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. 1997. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science (New York, NY). 275:218–220.
- Kanao K, Mikami S, Mizuno R, Shinojima T, Murai M, Oya M. 2008. Decreased acetylation of histone H3 in renal cell carcinoma: a potential target of histone deacetylase inhibitors. J Urol. 180:1131–1136.
- Kang CL, Qi B, Cai QQ, Fu LS, Yang Y, Tang C, Zhu P, Chen QW, Pan J, Chen MH, et al. 2019. LncRNA AY promotes hepatocellular carcinoma metastasis by stimulating ITGAV transcription. Theranostics. 9:4421–4436.
- Kilic Eren M, Kilincli A, Eren O. 2015. Resveratrol induced premature senescence is associated with DNA damage mediated sIRT1 and sIRT2 down-Regulation. PLoS One. 10:e0124837.
- Kim C, Baek SH, Um JY, Shim BS, Ahn KS. 2016. Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPepsilon and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrol. 17:19.
- Konstantatou E, Fang C, Romanos O, Derchi LE, Bertolotto M, Valentino M, Kalogeropoulou C, Sidhu PS. 2019. Evaluation of intratesticular lesions with strain elastography using strain ratio and color map visualgrading: differentiation of neoplastic and nonneoplastic lesions. J Ultrasound Med. 38:223–232.
- Kraljevic Pavelic S, Sedic M, Bosnjak H, Spaventi S, Pavelic K. 2011. Metastasis: new perspectives on an old problem. Mol Cancer. 10:22.
- Kumar B, Iqbal MA, Singh RK, Bamezai RN. 2015. Resveratrol inhibits TIGAR to promote ROS induced apoptosis and autophagy. Biochimie. 118:26–35.
- Li L, Sun Q, Li Y, Yang Y, Yang Y, Chang T, Man M, Zheng L. 2015. Overexpression of SIRT1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. J Mol Neurosci. 56:858–867.
- Liu JB, Wang HZ, Liu ZH, Xu M, Li X. 2017. Histone deacetylation down-regulates the expression of BNIP3 in renal cell carcinoma. Sichuan da Xue Xue Bao Yi Xue Ban. 48:384–388.
- Liu QH, Wang Y, Yong HM, Hou PF, Pan J, Bai J, Zheng JN. 2017. XRCC1 serves as a potential prognostic indicator for clear cell renal cell carcinoma and inhibits its invasion and metastasis through suppressing MMP-2 and MMP-9. Oncotarget. 8:109382–109392.
- Marti RM, Sorolla A, Yeramian A. 2012. New therapeutic targets in melanoma. Actas Dermosifiliogr. 103:579–590.
- Michaille JJ, Piurowski V, Rigot B, Kelani H, Fortman EC, Tili E. 2018. MiR-663, a microRNA linked with inflammation and cancer that is under the influence of resveratrol. Medicines (Basel, Switzerland). 5:74.
- Mosashvilli D, Kahl P, Mertens C, Holzapfel S, Rogenhofer S, Hauser S, Buttner R, Von Ruecker A, Muller SC, Ellinger J. 2010. Global histone acetylation levels: prognostic relevance in patients with renal cell carcinoma. Cancer Sci. 101:2664–2669.
- Motzer RJ, Bander NH, Nanus DM. 1996. Renal-cell carcinoma. N Engl J Med. 335:865–875.
- Olaku O, White JD. 2011. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer (Oxford, England: 1990). 47:508–514.
- Park KC, Heo JH, Jeon JY, Choi HJ, Jo AR, Kim SW, Kwon HJ, Hong SJ, Han KS. 2015. The novel histone deacetylase inhibitor, N-hydroxy-7-(2-naphthylthio) hepatonomide, exhibits potent antitumor activity due to cytochrome-c-release-mediated apoptosis in renal cell carcinoma cells. BMC Cancer. 15:19.
- Pradeepa MM, Grimes GR, Kumar Y, Olley G, Taylor GC, Schneider R, Bickmore WA. 2016. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat Genet. 48:681–686.
- Prasad B, Tangedal K, Chibbar R, McIsaac M. 2018. Simultaneous clinical presentation of renal cell carcinoma and immunoglobulin light chain amyloidosis. Cureus. 10:e2585.
- Qiao S, Liu C, Xu W, AZhaTi W, Li C, Wang Z. 2018. Up-regulated expression of CD147 gene in malignant bone tumor and the possible induction mechanism during osteoclast formation. Braz J Med Biol Res. 51:e6948.
- Ramaswamy S, Ross KN, Lander ES, Golub TR. 2003. A molecular signature of metastasis in primary solid tumors. Nat Genet. 33:49–54.
- Ramos EA, Silva CT, Manica GC, Pereira IT, Klassen LM, Ribeiro EM, Cavalli IJ, Braun-Prado K, Lima RS, Urban CA, et al. 2016. Worse prognosis in breast cancer patients can be predicted by immunohistochemical analysis of positive MMP-2 and negative estrogen and progesterone receptors. Rev Assoc Med Bras. 62:774–781.
- Rini BI, Campbell SC, Escudier B. 2009. Renal cell carcinoma. Lancet. 373:1119–1132.
- Safadi A, Elias I, Visoki A, Zick A, Schwalb S, Katz R. 2018. Synchronous ipsilateral renal cell carcinoma and transitional cell carcinoma of the renal pelvic with complete remission of TCC after neoadjuvant chemotherapy. Urol Case Rep. 19:28–30.
- Schrader AJ, Sevinc S, Olbert PJ, Hegele A, Varga Z, Hofmann R. 2008. Gender-specific characteristics and survival of renal cell carcinoma. Der Urologe Ausg A. 47:1182, 1184–1186.
- Scott E, Steward WP, Gescher AJ, Brown K. 2012. Resveratrol in human cancer chemoprevention – choosing the ‘right’ dose. Mol Nutr Food Res. 56:7–13.
- Shu C, Yan D, Mo Y, Gu J, Shah N, He J. 2018. Long noncoding RNA lncARSR promotes epithelial ovarian cancer cell proliferation and invasion by association with HuR and miR-200 family. Am J Cancer Res. 8:981–992.
- Shukla Y, Singh R. 2011. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 1215:1–8.
- Signorelli P, Ghidoni R. 2005. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 16:449–466.
- Strbac D, Goricar K, Dolzan V, Kovac V. 2018. Matrix metalloproteinases polymorphisms as baseline risk predictors in malignant pleural mesothelioma. Radiol Oncol. 52:160–166.
- Sun L, Chao F, Luo B, Ye D, Zhao J, Zhang Q, Ma X, Zhang G. 2018. Impact of estrogen on the relationship between obesity and renal cell carcinoma risk in women. EBioMedicine. 34:108–112.
- Wei M, Mao S, Lu G, Li L, Lan X, Huang Z, Chen Y, Zhao M, Zhao Y, Xia Q. 2018. Valproic acid sensitizes metformin-resistant human renal cell carcinoma cells by upregulating H3 acetylation and EMT reversal. BMC Cancer. 18:434–448.
- Yeh CM, Lin CW, Yang JS, Yang WE, Su SC, Yang SF. 2016. Melatonin inhibits TPA-induced oral cancer cell migration by suppressing matrix metalloproteinase-9 activation through the histone acetylation. Oncotarget. 7:21952–21967.
- Yu L, Xie R, Tian T, Zheng L, Tang L, Cai S, Ma Z, Yang T, Han B, Yang Q. 2019. Suberoylanilide hydroxamic acid upregulates histone acetylation and activates endoplasmic reticulum stress to induce apoptosis in HepG2 liver cancer cells. Oncol Lett. 18:3537–3544.
- Zhao Y, Tang H, Zeng X, Ye D, Liu J. 2018. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed Pharmacother. 98:36–44.