Abstract
Context
Naoxintong Capsule (NXT), a Chinese medicine, has been widely used for the treatment of coronary heart disease (CHD) in clinics.
Objective
This study evaluated the cardioprotective effects of NXT alone and in combination with ticagrelor (TIC) and atorvastatin (ATO).
Materials and methods
Qi deficiency and blood stasis rats were established by 8 weeks high fat diet feeding and 16 days exhaustive swimming and randomly divided into seven groups, that is, NXT (250, 500 and 1000 mg/kg/d), TIC (20 mg/kg/d), ATO (8 mg/kg/d), NXT (500 mg/kg/d)+TIC (20 mg/kg/d) and NXT (500 mg/kg/d)+ATO (8 mg/kg/d) group, with oral administration for 12 weeks. The contents of TC, TG, LDL-C, HDL-C, IL-1β, IL-6, IL-8, TNF-α, AST, ALT, SOD, MDA, CK-MB, LDH, TXA2, PGI2, IgA, IgG, IgM and C3 in serum were measured.
Results
NXT + TIC group was significantly superior to the TIC group in decreasing the levels of TC (4.34 vs. 5.54), TG (3.37 vs. 4.66), LDL-C (1.21 vs. 1.35), LDH (4919.71vs. 5367.19) and elevating SOD level (248.54 vs. 192.04). NXT + ATO group was significantly superior to the ATO group in decreasing the levels of AST (195.931 vs. 241.63), ALT (71.26 vs. 83.16), LDH (4690.05 vs. 5285.82), TXA2 (133.73 vs. 158.67), IgG (8.08 vs. 9.80), C3 (2.03 vs. 2.35) and elevating the levels of HDL-C (1.19 vs. 0.91), SOD (241.91vs. 209.49).
Conclusions
The present findings demonstrate that the combined use of NXT with TIC and ATO had better integrated regulating effects than TIC and ATO, respectively. The mechanism of action requires further research.
Introduction
Coronary heart disease (CHD) is a primary cause of death in the world. Traditional Chinese medicine (TCM) is an indispensable part of alternative medicine having a unique theoretic system. A combination of differentiation syndrome and disease is the main therapeutic mode and feature of TCM (Chai et al. Citation2011). Qi deficiency and blood stasis syndrome (QDBS) is one of the common TCM syndromes and is closely related to CHD (Zhang et al. Citation2009). Wang et al. (Citation2008) reported that the most major heart syndrome element in 324 patients with CHD is Qi deficiency and blood stasis. Qi deficiency and blood stasis is a complex pathological system, accompanied by free radical accumulation, inflammation, vascular endothelial damage, liver and kidney injury, which in turn further promotes the occurrence of qi deficiency and blood stasis (Bi et al. Citation2019). Therefore, it is necessary to carry out treatment and mechanism research of CHD with QDBS at various conditions.
A high-fat diet is considered to be the indispensable factor to induce lipid metabolism disorder and result in CHD. Exhaustive swimming exercising is widely used to establish the animal model of CHD with QDBS worldwide (Zhang et al. Citation2010). Our previous study demonstrated that continual exhausting swimming followed by a high-fat diet could be a method to make a rat model of CHD with QDBS (Zhang et al. Citation2020). Thus, in the present study, we used high-fat diet feeding and exhaustive swimming exercising to establish the rat model of CHD with QDBS.
To date, antiplatelet drugs and statins are most widely used in reducing morbidity and mortality in patients with CHD. However, long-term use of these chemical drugs can bring some side effects, mainly including bleeding, liver damage and drug resistance. A combination of TCM and Western medicine may offer a new way to treat CHD due to it may increase the curative effect and reduce the recurrent rate and decrease the adverse reactions to Western medicine.
As a prescribed traditional Chinese medicine, the Naoxintong capsule (NXT) has been widely used to treat patients with CHD for more than 20 years due to its remarkable therapeutic effects and high safety. NXT contains the following 16 traditional Chinese medical herbs: Astragalus membranaceus (Fisch.) Bge. (Leguminosae), Salvia miltiorrhiza Bunge (Labiatae), Angelica sinensis (Oliv.) Diels (Umbelliferae), Paeonia lactiflora Pall (Ranunculaceae), Ligusticum chuanxiong Hort. (Umbelliferae), Prunus persica (L.) Batsch (Rosaceae), Carthamus tinctorius L. (Compositae), Boswellia carterii Birdw. (Burseraceae), Commiphora myrrha Engl. (Burseraceae), Spatholobus suberectus Dunn (Leguminosae), Achyranthes bidentata Bl. (Amaranthaceae), Cinnamomum cassia Presl (Lauraceae), Morus alba L. (Moraceae), Buthus martensii Karsch (Buthidae), Hirudo nipponica Whitman (Hirudinidae), and Pheretima aspergillum (E.Perrier) (Megascolecidae). The chemical fingerprints or the quantitative content of major active compounds in NXT and the in vivo biotransformation of the components have been investigated (Wang et al. Citation2018; He et al. Citation2019). In addition, numerous studies have demonstrated that NXT performed multiple protective effects on cardiovascular diseases (Zhao et al. Citation2013; Zhong et al. Citation2013; Han et al. Citation2019). In our previous study, NXT protected proatherogenic animals against atherosclerosis with the improvement of serum lipid profiles and recovery of intestinal microecology imbalance (Zhang et al. Citation2019). However, it is not clear what role NXT plays in multiple pathological conditions such as endothelial function and immune response, and how these effects are related to CHD. Little is known about its combination with antiplatelet drugs or statins on treating CHD.
This study determines the comprehensive pharmacodynamic effects on qi deficiency and blood stasis rats. Effects of NXT on oxidative stress, hepatic and renal function, lipid metabolism, inflammatory cytokines, immune response, myocardial enzyme and endothelial function were investigated via a rat model of CHD with QDBS and setting up three NXT dose groups. Meanwhile, to clarify what effect NXT can enhance when it is combined with antiplatelet drugs or statins, the therapeutic effects between NXT plus atorvastatin (N&A) and atorvastatin (ATO), NXT plus ticagrelor (N&T) and ticagrelor (TIC) were compared. This study will help to understand the pharmacodynamic effects of NXT in alone or in combination comprehensively.
Materials and methods
Drugs
NXT (Med-drug Permit no. Z20025001) was kindly provided by Xianyang Buchang Pharmaceutical Co. Ltd. (Shan’xi, China). NXT is composed of Astragalus membranaceus, Paeonia lactiflora, Salvia miltiorrhiza, Angelica sinensis, Ligusticum chuanxiong, Prunus persica, Achyranthes bidentata, Morus alba, Pheretima aspergillum, Hirudo nipponica, Spatholobus suberectus, Cinnamomum cassia, Carthamus tinctorius, Boswellia carterii, Commiphora myrrha, and Buthus martensii. All the above herbs, in the radios of 66:27:27:27:27:27:27:27:27:27:20:20:13:13:13:13 (dry weight), are crushed into a fine powder, screened through mesh size of 80 and mixed homogeneously, without any extraction (Chinese Pharmacopoeia Commission Citation2015). Ticagrelor tablets were purchased from AstraZeneca AB (Batch No. 1611060). Atorvastatin was purchased from Huirui Pharmacy Co., Ltd. (Batch No. R16877).
Experimental instruments and reagents
Electronic analytical balance (BS-3000A, Shanghai Yousheng Weighing Apparatus Co., Ltd.); Full-automatic biochemical analyzer (DS-261, Jiangsu Innova Medical Technology Co. Ltd.); Microplate reader (DG5033A, Nanjing Huadong Electronics Group Medical Equipment Co., Ltd.); Spectrophotometer (UNICO-UV2000, Unico (Shanghai) Instrument Co., Ltd.); Refrigerated centrifuge (KDC-2046, USTC ZONKIA); Ultra-low temperature freezer (DW-86L628, Haier); Hybrid triple quadrupole time-of-flight mass spectrometer (AB SCIEX Triple TOF™ 5600 plus).
MS grade formic acid was purchased from Sigma-Aldrich Co. (St. Louis, MO). MS grade methanol and acetonitrile were purchased from Fisher Scientific Inc. (Fair Lawn, NJ). Deionized water was purified by the Milli-Q system (Millipore Corporation, Billerica, MA) and filtered through 0.22 mm membrane filter prior to use.
UFLC-Q-TOF-MS/MS analysis of NXT
The capsule of NXT was completely removed, and 2.0 g NXT powder was accurately weighed. The powder was treated by an ultrasonic wave in 20 mL of 50% methanol for 30 min. The supernatant was filtrated by 0.22 μm filter membrane and then injected into an ultra-fast liquid chromatography/quadrupole-time-of-flight tandem mass spectrometry system (UFLC-Q-TOF-MS/MS) for analysis.
The analysis was performed with a connected system of UFLC XR (Shimadzu Corp., Japan)-hybrid triple quadruple time-of-flight mass spectrometer (Triple TOFTM 5600+, AB Sciex, Foster City, CA) equipped with electrospray ionization (ESI) source. Chromatographic separation was carried out on a Kinetex C18 column (Phenomenex, 3.0 × 100 mm, 2.6 μm, 100 Å). The mobile phase consisted of 0.1% formic acid (v/v) in both acetonitrile (A) and water (B) using a linear gradient from 5 to 95% A (0–30 min). Isocratic eluted at 95% for 5 min with a post-run of 10 min to equilibrate the system. The column temperature was set at 40 °C and the flow rate was kept at 0.3 mL/min. The conditions of MS/MS detector were as follows: ion source gas 1 55 psi; ion source gas 2 55 psi; curtain gas 35 psi; temperature 550 °C; ion spray voltage floating 5500 V in positive mode; ion spray voltage floating 4500 V in negative mode; collision energy 35 V; collision energy spread 15 V; declustering potential 80 V. Nitrogen was used as nebulizer and auxiliary gas. Samples were analyzed in both positive and negative ionization modes with scanning mas-to-charge (m/z) range from 50 to 1500. Data were collected in information-dependent acquisition (IDA) mode and analyzed by PeakView® 2.2 software (AB Sciex, Foster City, CA).
Ethical statement and animals
All experiment procedures were carried out according to the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Ethics Committee of Guangdong Medical Laboratory Animal Centre (Permission No. 2016022014). The harm to rats was minimized during the experimental process by taking appropriate measures.
Ninety male Sprague-Dawley rats, specific pathogen-free (SPF), weighing 220–260 g, aged 3 months, were obtained from Guangdong Medical Experimental Animal Centre (Certification No. SCXK-(Yue) 2013-0002, Quality Qualification Certificate No. 44007200028430) and raised in the SPF houses of Guangdong Medical Laboratory Animal Centre. The temperature of SPF houses was 20–26 °C and the relative humidity was 40-70%. Rats were fed by standard pellet feed and kept under a 12 h light/dark cycle. Experiments began after the rats adapted to the new environment for 1 week.
Experimental model and drug administration
The healthy Sprague-Dawley (SD) rats were initially divided into two groups, including the normal group (n = 10) and QDBS group (n = 80). The rats in the QDBS group were fed a high-fat diet (HFD: 1.2% cholesterol and 15% fat) for 8 weeks and then treated with exhaustive swimming exercising once a day for 16 days so that they were in a chronic state analogous to CHD with QDBS (Zhang et al. Citation2020). The swimming exercise protocol was arranged as follows: the rats were individually subjected to a swim to exhaustion (about 3–4 h) in a swimming pool (50 cm in height, 160 cm in diameter) filled with water 40 cm high. The water temperature was maintained at 19–21 °C. Exhaustion was defined by two criteria: the rats sank into the water and remained below the water surface for 10 s, and the rats showed a lack of a righting reflex when they were turned on their backs (Thomas and Marshall Citation1988). The swimming exercise was performed from 8:00 am to 12:00 pm daily for 16 days. These QDBS animals were then randomly divided into 8 groups (n = 10) for 12 weeks treatment, including model group, NXT-L group (250 mg/kg/d), NXT-M group (500 mg/kg/d, the human equivalent dose in clinic), NXT-H group (1000 mg/kg/d) (Chen et al. Citation2009), ATO group (8 mg/kg/d, the human equivalent dose in clinic) (Li et al. Citation2011), TIC group (20 mg/kg/d, the human equivalent dose in clinic) (Li et al. Citation2017), N&A group (NXT 500 mg/kg/d plus ATO 8 mg/kg/d), N&T group (NXT 500 mg/kg/d plus TIC 20 mg/kg/d). We treated rats with the above drugs which were dissolved with 0.5% CMC-Na orally once a day for 12 weeks, while animals in the normal group and model group received the same volume of 0.5% CMC-Na. During the period of drug administration, the model group and treatment groups were maintained a high-fat diet, while the normal group was fed a normal diet.
Collection of blood samples and detection of biochemical indexes
At the end of the experiment, all the rats were in narcotism by injecting 3% pentobarbital sodium into cavum abdominis (2.0 mL/kg) after an overnight fast, and blood samples were obtained from the abdominal aorta. Serum was separated from blood by centrifugation at 2000 g for 15 min. The serum was used to detect the biochemical parameters, including superoxide dismutase (SOD), methane dicarboxylic aldehyde (MDA), alanine transaminase (ALT), aspartate transaminase (AST), creatinine (Cr), blood urea nitrogen (BUN), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor-α (TNF-α), complement component 3 (C3), immunoglobulin A (IgA), immunoglobulin G (IgG), immunoglobulin M (IgM), creatine kinase-MB (CK-MB), lactic dehydrogenase (LDH), prostacyclin (PGI-2), and thromboxane A2 (TXA2). All the above serum biochemical indices were measured and quantified by kits (Nanjing Jiancheng Bio-engineering Institute, Nanjing, China) according to the manufacturer’s instructions.
Statistical analysis
Data were presented as means ± standard deviation (SD). One-way analysis of variance, Student’s t-test and Dunnett’s multiple comparisons were used to compare the results among groups. Statistical analysis was carried out with SPSS (Version: 21.0). p-Values less than 0.05 or 0.01 were considered statistically significant.
Results and discussion
Identification of major components of NXT
In this study, to ensure the quality of NXT, UFLC-Q-TOF-MS/MS was used to investigate the ingredients. As a result, a total of 114 components were presumed and identified in NXT based on high-accuracy protonated precursors and multi-stage mass spectrometry according to the reported literature and online databases such as ChemSpider (www.chemspider.com) and the Mass Bank (www.massbank.jp; ). Base peak chromatograms (BPC) were shown in . These compounds mainly included organic acids, flavones, phenanthraquinones, terpenoids, and saponins. Most of these constituents have been reported to show potentially important therapeutic activities for cardiovascular diseases (Ma et al. Citation2016).
Figure 1. Base peak chromatograms (BPCs) in negative ion mode (A) and in positive ion mode (B) of NXT by UFLC-Q-TOF-MS/MS.
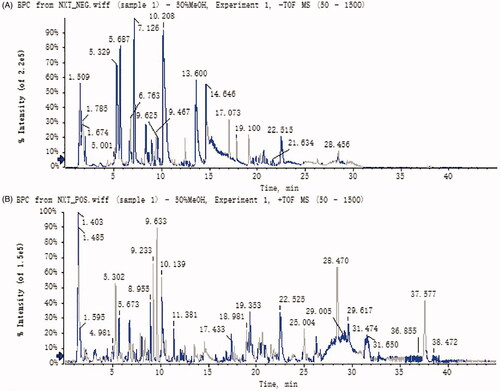
Table 1. Identification of the chemical constituents of NXT by UFLC-Q-TOF-MS/MS.
Effect of treatments on lipid metabolism
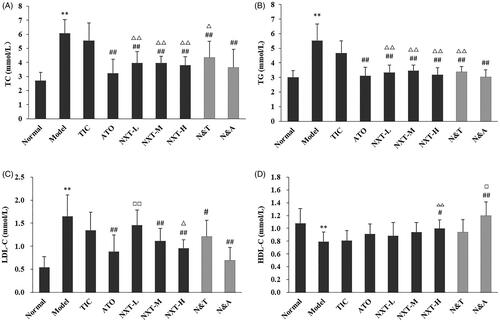
It has been demonstrated that disorders of lipid metabolism are related to the development of CHD. Higher TC and TG levels are closely associated with the risk of CHD (Gaw Citation2003; Cui et al. Citation2007). LDL contributes to the deposition of cholesterol in the blood vessel wall and the elevated concentration of LDL-C could lead to CHD (Jeppesen et al. Citation2006). HDL particles have functions with the potential to protect against arterial diseases, such as promoting cholesterol efflux from macrophages in the artery wall. The concentration of HDL-C is an independent, inverse predictor for CHD (Barter Citation2011). As shown in , compared with the normal group, higher TC, TG and LDL-C levels and lower HDL-C levels were observed in the model group (p < 0.01). Our results showed that the lipid metabolic abnormalities appeared in the model rats. And, three doses of NXT significantly decreased TC and TG levels (p < 0.01). NXT medium- and high-dose groups significantly LDL-C levels (p < 0.01), and the NXT high-dose group significantly increased the level of HDL-C (p < 0.05). The results indicated that NXT could improve hyperlipemia effectively. Moreover, NXT had a stronger effect on decreasing TC, TG and LDL-C levels than TIC (p < 0.05, p < 0.01). For drug combination, N&A and N&T significantly decreased the TC, TG and LDL-C levels (p < 0.05, p < 0.01). ATO alone had no effect on the HDL-C level, whereas N&A could increase the HDL-C level. TIC alone had no effects on affecting these four serum lipid profiles, whereas N&T had significant effects. These results indicated that the combined administration of NXT and ATO or TIC provided superior effects in lipid-lowering.
Figure 2. Effect of treatments on cholesterol (T-CHO) (A), triglyceride (TG) (B), low density lipoprotein (LDL-C) (C) and high-density lipoprotein (HDL-C) (D). Data are presented as means ± SD (n = 10). **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 and △△p < 0.01 compared with TIC group, □p < 0.05 and □□p < 0.01 compared with ATO group.
Effect of treatments on inflammatory response
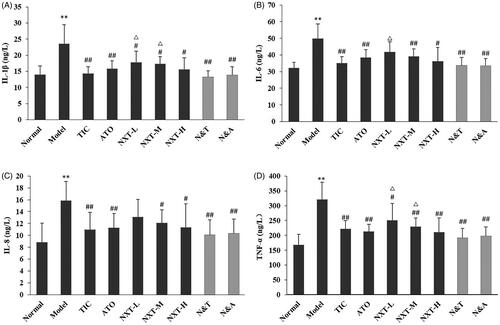
Evidence suggests that inflammation plays an important role in the pathogenesis of CHD (Jing et al. Citation2014). IL-1β, an inflammatory cytokine, is one of the central mediators in the cytokine network (Zhu et al. Citation2015). IL-1β can promote the expression of endothelial leukocyte adhesion molecules and stimulate the migration of inflammatory cells into the lesion site. IL-6 is an inflammatory factor that plays an important part in the development of CHD. IL-6 in the vessel wall can activate the autocrine and paracrine secretion of monocytes contributing to the deposition of fibrinogen, thus result in increased blood viscosity, platelet number, and activity (Yudkin et al. Citation2000). IL-8, a member of the α-chemokine subfamily, acts in concert with endothelium cell adhesion molecules to attract leukocytes to sites of inflammation (Romuk et al. Citation2002). The level of IL-8 is associated with the stability of atherosclerotic plaques (Frostegard et al. Citation1999). TNF-α could be involved in cardiovascular pathophysiology through its effects on lipid metabolism, coagulation and endothelial function (Herrmann et al. Citation1998). And, TNF-α is closely related to the occurrence and development of CHD (Leboeuf and Schreyer Citation1998). As shown in , the serum IL-1β, IL-6, IL-8 and TNF-α levels of rats in the model group significantly increased (p < 0.01), suggesting that the vascular endothelium of rats suffered from damage during high-fat diet feeding and exhaustive swimming, thus lead to the up-regulation of inflammatory factors. Three doses of NXT significantly inhibited the elevated levels of IL-1β and TNF-α (p < 0.05, p < 0.01), and NXT medium- and high-dose groups significantly decreased the levels of IL-6 and IL-8 (p < 0.05, p < 0.01), indicating that NXT had a good effect of anti-inflammation and this may be one of the mechanisms of anti-CHD of NXT. In terms of the drug combination, N&A and N&T significantly inhibited the up-regulation of the four inflammatory factors (p < 0.01). There was no significant difference between the combined group and Western drug group on decreasing IL-1β, IL-6, IL-8 and TNF-α levels, indicating that the enhanced effect of the drug combination in anti-inflammatory was not significant.
Figure 3. Effect of treatments on interleukin-1β (IL-1β) (A), interleukin-6 (IL-6) (B), interleukin-8 (IL-8) (C) and tumour necrosis factor-α (TNF-α) (D). Data are presented as means ± SD (n = 10). **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 and △△p < 0.01 compared with TIC group.
Effect of treatments on hepatic and renal function
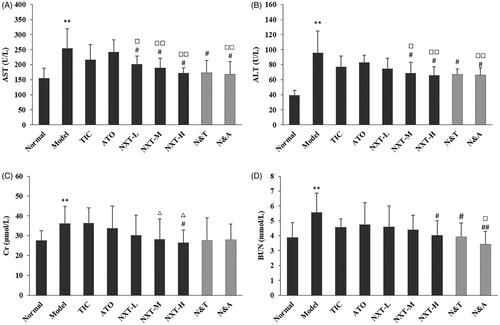
Liver function is closely related to CHD because lipid metabolism and kinds of clotting factor generation take place in the liver. When the liver cells are injured or necrotic, the activity of ALT and AST would sensitively increase. As shown in , compared with the normal group, serum ALT and AST levels were significantly increased in the model group (p < 0.01), indicating that the liver dysfunction appeared in model rats. After administration, NXT significantly lowered the levels of ALT and AST in serum (p < 0.05), indicating that NXT could perform a protective effect on the liver. And, our result showed that NXT had a stronger effect on decreasing ALT and AST level than ATO (p < 0.05, p < 0.01), indicating that NXT was superior to ATO on protecting the liver. In addition, the elevated levels of Cr and BUN in rats in the model group suggested the dysfunction of glomerular filtration and renal tubular secretion. A high dose of the NXT group significantly inhibited the abnormally increased levels of Cr and BUN (p < 0.05), which indicated that NXT had a certain protective effect on the kidney. In terms of the drug combination, N&A was superior to ATO on decreasing AST, ALT and BUN levels (p < 0.05, p < 0.01). N&T was superior to TIC on decreasing ALT and AST levels. These results indicated that these two combination therapies showed better protective effects on hepatic function.
Figure 4. Effect of treatments on glutamic-oxalacetic transaminease (AST) (A), glutamic-pyruvic transaminase (ALT) (B), creatinine (Cr) (C) and urea nitrogen (BUN) (D). Data are presented as means ± SD (n = 10). **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 compared with TIC group, □p < 0.05 and □□p < 0.01 compared with ATO group.
Effect of treatments on oxidative stress
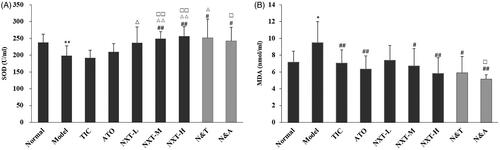
Evidence has revealed that the overproduction of reactive oxygen species (ROS) raises the risk for the development of CHD. SOD is a key enzyme in cellular defenses against oxidative damage (Vujaskovic et al. Citation2002). SOD can protect the human body from peroxidation with the consequent damage to the tissues by inactivating the oxygen-free radicals. MDA is a poisonous end-product of lipid peroxidation. The level of MDA represents the rate and extent of lipid peroxidation directly and shows the capability of eliminating free radicals indirectly (Su et al. Citation2005). A significant reduction in the antioxidant capacity of the rats with QDBS was determined as the increase of MDA content and the decrease of SOD activity. NXT medium- and high-dose groups significantly inhibited both the rise of MDA content and the decrease of SOD activity (p < 0.05, p < 0.01; ), suggesting that NXT could inhibit the aggravation of oxidative stress disorder. Moreover, the intermediate- and high-dose of NXT had a stronger effect on increasing the SOD level than TIC and ATO (p < 0.05, p < 0.01). For drug combination, N&A and N&T significantly increased the activity of SOD and decreased the level of MDA (p < 0.05, p < 0.01). N&A exhibited a significantly stronger effect on decreasing the MDA level and increasing the SOD activity than ATO (p < 0.05), while N&T exhibited a significantly stronger effect on increasing the SOD activity than TIC (p < 0.05). These results indicated that the addition of NXT to ATO or TIC could enhance the effect on the improvement of oxidative stress.
Figure 5. Effect of treatments on superoxide dismutase (SOD) (A) and methane dicarboxylic aldehyde (MDA) (B). Data are presented as means ± SD (n = 10). *p < 0.05 and **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 and △△p < 0.01 compared with TIC group, □p < 0.05 and □□p < 0.01 compared with ATO group.
Effect of treatments on myocardial enzyme
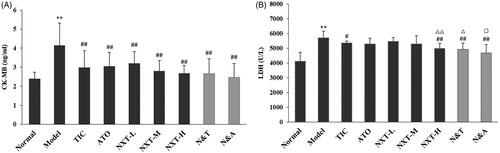
CK-MB is released into the bloodstream when myocardial cells are injured. An elevation in CK-MB is a significant predictor of myocardial damage and acute myocardial infarction (Adams et al. Citation1994). LDH is commonly used to diagnose the occurrence of myocarditis in the clinic (Snodgrass et al. Citation1959). As shown in , in the model rats, the levels of CK-MB and LDH were significantly increased (p < 0.01), which indicated the model rats exhibited myocardial damage. Three doses of NXT significantly decreased the level of CK-MB (p < 0.01) and a high dose of NXT significantly lowered the level of LDH (p < 0.01), suggesting that NXT showed good effects on myocardial protection. For drug combination, N&T and N&A both significantly decreased the CK-MB and LDH levels. N&A and N&T exhibited a significantly stronger effect on decreasing LDH levels than ATO and TIC (p < 0.05, p < 0.05), respectively, suggesting that the addition of NXT to ATO or TIC could enhance the effect on the improvement of myocardial injury.
Figure 6. Effect of treatments on creatine kinase-MB (CK-MB) (A) and lactic dehydrogenase (LDH) (B). Data are presented as means ± SD (n = 10). **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 and △△p < 0.01 compared with TIC group, □p < 0.05 compared with ATO group.
Effect of treatments on endothelial function
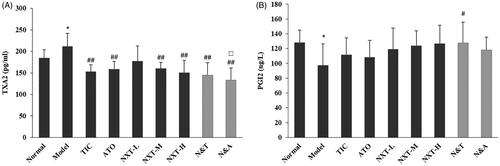
TXA2 and PGI2 are two arachidonic acid metabolites that were shown to be synthesized in the human body. TXA2 stimulates platelet aggregation and vasoconstriction, whereas PGI2 antagonizes its activities. The balance between TXA2 and PGI2 effects vascular homeostasis (Mitsuhashi et al. Citation1994; Niccoli et al. Citation2008). An elevation in TXA2 and a reduction in PGI2 could promote thrombogenesis and the imbalance between TXA2 and PGI2 is commonly seen in CHD. In the current study, the level of TXA2 significantly increased and the level of PGI2 significantly decreased in the model rats (p < 0.05), which suggested the endothelial dysfunction appeared. The medium- and high-dose of NXT significantly reduced the level of TXA2 (p < 0.01) and slightly increased the level of PGI2 (), suggesting NXT was effective in improving the vascular endothelial function. For drug combination, N&T and N&A significantly decreased the level of TXA2 (p < 0.01) and N&T significantly increased the level of PGI2(p < 0.05). N&A exhibited a significantly stronger effect on decreasing the TXA2 level than ATO (p < 0.05), indicating that the addition of NXT to ATO could enhance the effect on improving endothelial dysfunction.
Effect of treatments on immune response
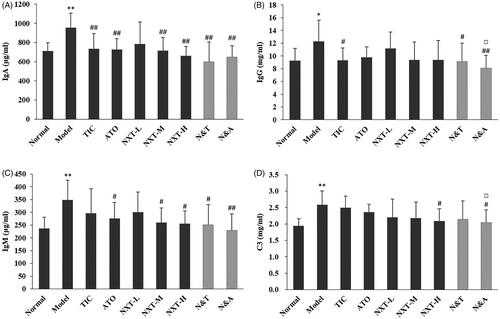
Evidence suggests that circulating immune complexes may play a pathogenetic role in various heart diseases, including CHD, myocarditis and myocardial infarction (Cristea et al. Citation1986; Dahlen et al. Citation1995). The incidence of CHD was correlated with the elevation of IgA, IgG, IgM and C3 (Yang et al. Citation2003). As shown in , compared with the control group, higher IgA, IgG, IgM and C3 levels were observed in the model group (p < 0.05, p < 0.01). After drug administration, the medium- and high-dose of NXT significantly decreased IgA and IgM levels (p < 0.05, p < 0.01) and high-dose of NXT significantly decreased the level of C3 (p < 0.05), suggesting NXT showed good performance in the improvement of the immune response. For drug combinations, no significant difference between N&T and TIC was observed. N&A had a greater effect on decreasing IgG and C3 than ATO (p < 0.05), indicating that the addition of NXT to ATO could enhance the effect on improving immune response.
Figure 8. Effect of treatments on immunoglobulin A (IgA) (A), immunoglobulin G (IgG) (B), immunoglobulin M (IgM) (C) and complement component 3 (C3) (D). Data are presented as means ± SD (n = 10). *p < 0.05 and **p < 0.01 compared with normal group, #p < 0.05 and ##p < 0.01 compared with model group, △p < 0.05 compared with TIC group, □p<0.05 compared with ATO group.
In this study, a high-fat diet and exhaustive swimming exercising were used to establish the rat model of CHD with qi deficiency and blood stasis. Our previous study showed that this animal model exhibited abnormalities in blood lipid metabolism, oxidative stress, immune response, liver and kidney function, etc. (Zhang et al. Citation2020). In the current study, the results of administration demonstrated that NXT, NXT plus TIC, NXT plus ATO effectively inhibited the development of CHD with QDBS through regulating blood lipid profiles, enhancing antioxidant capacity, inhibiting vascular inflammation and alleviating myocardial injury.
In the clinical application, the treatment with a single drug might not achieve hoped-for efficacy in the treatment of CHD for the complexity of occurrence and development of the disease. A combination of TCM and Western medicine provides a good treatment strategy as it takes full advantage of the quick therapeutic effect of Western medicine and multiple effects of TCM. To prevent the occurrence of severe cardiovascular events, long-term statin or antiplatelet drug therapy is the standard of care and recommended for patients with established cardiovascular diseases in the clinic. In the present study, in comparison with ATO, we demonstrated that co-treatment of NXT with ATO showed better efficacy on alleviating liver and kidney injury, improving oxidative stress, immune response and endothelial function, while the addition of NXT to TIC enhanced the effect on lipid-lowering, improving endothelial function and alleviating myocardial injury. Taken together, either alone or in combination, the efficacy of NXT has been demonstrated in the present study. We hope our study can offer fresh perspectives and help to provide better insight for the further application of NXT.
Conclusions
The present study demonstrated that NXT, NXT plus TIC and NXT plus ATO had an integrated regulating effect on the prevention and treatment in CHD with QDBS. The addition of NXT to ATO enhanced the effect on the improvement of oxidative stress, hepatic and renal function, immune response and endothelial function, while the addition of NXT to TIC enhanced the effect on the improvement of lipid metabolism, endothelial function and myocardial enzyme. The specific mechanisms require further research. The findings of this study provide further support for the clinical application of NXT.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams JE, 3rd, Schechtman KB, Landt Y, Ladenson JH, Jaffe AS. 1994. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin Chem. 40(7):1291–1295.
- Barter P. 2011. HDL-C: role as a risk modifier. Atheroscler Suppl. 12(3):267–270.
- Bi C, Li PL, Liao Y, Rao HY, Li PB, Yi J, Wang WY, Su WW. 2019. Pharmacodynamic effects of Dan-hong injection in rats with blood stasis syndrome. Biomed Pharmacother. 118:1–10.
- Chai C, Kou J, Zhu D, Yan Y, Yu B. 2011. Mice exposed to chronic intermittent hypoxia simulate clinical features of deficiency of both Qi and Yin syndrome in traditional Chinese medicine. Evid Based Complement Alternat Med. 2011:356252.
- Chen J, Lu YQ, Lv HH, Chen JJ, Wang Y, Yang K, Liu N. 2009. Protective effects of Naoxintong capsule on cerebral ischemia-reperfusion injury in rats. Chin J Rehabil Theory Pract. 15:138–140.
- Chinese Pharmacopoeia Commission. 2015. Pharmacopoeia of the People’s Republic of China Volume I. Beijing (China): China Medical Science Press.
- Cristea A, Rus H, Niculescu F, Bedeleanu D, Vlaicu R. 1986. Characterization of circulating immune complexes in heart disease. Immunol Lett. 13(1–2):45–49.
- Cui R, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, Kondo T, Watanabe Y, Koizumi A, Inaba Y, et al. 2007. Serum total cholesterol levels and risk of mortality from stroke and coronary heart disease in Japanese: the JACC study. Atherosclerosis. 194(2):415–420.
- Dahlen GH, Boman J, Birgander LS, Lindblom B. 1995. Lp(a) lipoprotein, IgG, IgA and IgM antibodies to Chlamydia pneumoniae and HLA class II genotype in early coronary artery disease. Atherosclerosis. 114(2):165–174.
- Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. 1999. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 145(1):33–43.
- Gaw A. 2003. HDL-C and triglyceride levels: relationship to coronary heart disease and treatment with statins. Cardiovasc Drugs Ther. 17(1):53–62.
- Han J, Tan H, Duan Y, Chen Y, Zhu Y, Zhao B, Wang Y, Yang X. 2019. The cardioprotective properties and the involved mechanisms of Naoxintong Capsule. Pharmacol Res. 141:409–417.
- He Y, Su WW, Chen TB, Zeng X, Yan ZH, Guo JM, Yang W, Wu H. 2019. Identification of prototype compounds and derived metabolites of Naoxintong capsule in beagle dog urine and feces by UFLC-Q-TOF-MS/MS. J Pharm Biomed Anal. 176:1–13.
- Herrmann SM, Ricard S, Nicaud V, Mallet C, Arveiler D, Evans A, Ruidavets JB, Luc G, Bara L, Parra HJ, et al. 1998. Polymorphisms of the tumour necrosis factor-alpha gene, coronary heart disease and obesity. Eur J Clin Invest. 28:59–66.
- Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C. 2006. Metabolic syndrome, low-density lipoprotein cholesterol, and risk of cardiovascular disease: a population-based study. Atherosclerosis. 189(2):369–374.
- Jing X, Chen SS, Jing W, Tan Q, Yu MX, Tu JC. 2014. Diagnostic potential of differentially expressed Homer1, IL-1β, and TNF-α in coronary artery disease. Int J Mol Sci. 16(1):535–546.
- Leboeuf RC, Schreyer SA. 1998. The role of tumor necrosis Factor-α Receptors in Atherosclerosis. Trends Cardiovasc Med. 8(3):131–138.
- Li JJ, Wu XY, Chen JL, Chen GR, Xu J, Gu Y, Song HP. 2017. Antiplatelet drug ticagrelor delays gastric ulcer healing in rats. Exp Ther Med. 14(4):3774–3779.
- Li SF, Fang JG, Sun YY. 2011. Effect of atorvastatin on the JAK/STAT signaling pathway and SOCS-1 in renal tissue of the diabetic nephropathy rat. Chin J Integr Trad and Western Nephrol. 9:765–768.
- Ma X, Lv B, Li P, Jiang X, Zhou Q, Wang X, Gao X. 2016. Identification of “multiple components-multiple targets-multiple pathways” associated with Naoxintong capsule in the treatment of heart diseases using UPLC/Q-TOF-MS and network pharmacology. Evid Based Complement Alternat Med. 2016:9468087.
- Mitsuhashi T, Ikata T, Morimoto K, Tonai T, Katoh S. 1994. Increased production of eicosanoids, TXA2, PGI2 and LTC4 in experimental spinal cord injuries. Paraplegia. 32(8):524–530.
- Niccoli G, Giubilato S, Russo E, Spaziani C, Leo A, Porto I, Leone AM, Burzotta F, Riondino S, Pulcinelli F, et al. 2008. Plasma levels of thromboxane A2 on admission are associated with no-reflow after primary percutaneous coronary intervention. Eur Heart J. 29(15):1843–1850.
- Romuk E, Skrzep-Poloczek B, Wojciechowska C, Tomasik A, Birkner E, Wodniecki J, Gabrylewicz B, Ochala A, Tendera M. 2002. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur J Clin Invest. 32(9):657–661.
- Snodgrass PJ, Wacker WE, Eppinger EC, Vallee BL. 1959. Metalloenzymes and myocardial infarction. III. Lactic dehydrogenase activity of serum-a determinate diagnostic measure. N Engl J Med. 261:1259–1266.
- Su X, Ma Y, Huang R, Wang X, Wang Y. 2005. Effects of shenmai injection on blood SOD activity and MDA level in senile patients with coronary heart disease. J Trad Chin Med. 25:50–53.
- Thomas DP, Marshall KI. 1988. Effects of repeated exhaustive exercise on myocardial subcellular membrane structures. Int J Sports Med. 9(4):257–260.
- Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. 2002. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 33(6):857–863.
- Wang HL, Jiang Y, Ding MY, Li J, Hao J, He J, Wang H, Gao XM, Chang YX. 2018. Simultaneous determination and qualitative analysis of six types of components in Naoxintong capsule by miniaturized matrix solid-phase dispersion extraction coupled with ultra high-performance liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J Sep Sci. 41(9):2064–2084.
- Wang J, Chu FY, Li J, Yao KW, Zhong JB, Zhou KH, He QY, Sun XW. 2008. Study on syndrome element characteristics and its correlation with coronary angiography in 324 patients with coronary heart disease. Chin J Integr Med. 14(4):274–280.
- Yang ZH, Ruan YJ, Huang ZJ. 2003. Changes of immunoreaction in patients with coronary heart disease. J Navy Med. 24:106–107.
- Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. 2000. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 148(2):209–214.
- Zhang H, Wang WR, Lin R, Zhang JY, Ji QL, Lin QQ, Yang LN. 2010. Buyang Huanwu decoction ameliorates coronary heart disease with Qi deficiency and blood stasis syndrome by reducing CRP and CD40 in rats. J Ethnopharmacol. 130(1):98–102.
- Zhang WJ, Liu H, Su WW, Zeng X, Wang YG, Li PB, Peng W, Yao HL. 2020. Establishment and evaluation on the animal model of Qi deficiency and blood stasis syndrome in hyperlipidemia rats with exhaustive swimming exercising. Acta Sci Natur Univ Sunyatseni. 59:131–136.
- Zhang WJ, Su WW, Li PB, Rao HY, Lin QW, Zeng X, Chen TB, Yan ZH, Liu H, Yao HL. 2019. Naoxintong Capsule inhibits the development of cardiovascular pathological changes in Bama Minipig through improving gut microbiota. Front Pharmacol. 10:1–19.
- Zhang YL, Zhang J, Hu XG. 2009. Establishment of rat model of ischemic stroke with qi deficiency and blood stasis syndrome and its evaluation system. Chinese J Integrated Trad and Western Med. 29:343–346.
- Zhao J, Zhu H, Wang S, Ma X, Liu X, Wang C, Zhao H, Fan S, Jin X, Zhao B, et al. 2013. Naoxintong protects against atherosclerosis through lipid-lowering and inhibiting maturation of dendritic cells in LDL receptor knockout mice fed a high-fat diet. CPD. 19(33):5891–5896.
- Zhong XN, Wang HH, Lu ZQ, Dai YQ, Huang JH, Qiu W, Shu YQ, Xu W, Cheng C, Hu XQ. 2013. Effects of Naoxintong on atherosclerosis and inducible nitric oxide synthase expression in atherosclerotic rabbit. Chin Med J. 126:1166–1170.
- Zhu H, Lin X, Zheng P, Chen H. 2015. Inflammatory cytokine levels in patients with periodontitis and/or coronary heart disease. Int J Clin Exp Pathol. 8(2):2214–2220.