Abstract
Context
Panax japonicus is the dried rhizome of Panax japonicus C.A. Mey. (Araliaceae). Saponins from Panax japonicus (SPJ) exhibit anti-inflammatory and antioxidative effects.
Objective
To explore the neuroprotective effect of SPJ on natural ageing of rat.
Materials and methods
Sprague-Dawley (SD) rats 18-month-old were divided into ageing control, ageing treated with SPJ 10 or 30 mg/kg (n = 8). Five-month-old rats were taken as the adult control (n = 8). Rats were fed regular feed or feed containing SPJ for 4 months. Cognitive level was evaluated by Morris water maze (MWM) test. The mechanisms of SPJ’s neuroprotection were evaluated by transmission electron microscope, western blot analysis, and immunofluorescence in vivo and in vitro.
Results
SPJ attenuated ageing-induced cognitive impairment as indicated by elevated number of times crossing the target platform (from 1.63 to 3.5) and longer time spent in the target platform quadrant (from 1.33 to 1.98). Meanwhile, SPJ improved the morphology of microglia and synapse, and activated M2 microglia polarisation including increased hippocampus levels of CD206 (from 0.98 to 1.47) and YM-1 (from 0.67 to 1.1), and enhanced autophagy-related proteins LC3B (from 0.48 to 0.82), Beclin1 (from 0.32 to 0.51), Atg5 (from 0.22 to 0.89) whereas decreased p62 level (from 0.71 to 0.45) of ageing rats. In vitro study also showed that SPJ regulated the microglial polarisation and autophagy.
Discussion and conclusions
SPJ improved cognitive deficits of ageing rats through attenuating microglial inflammation and enhancing microglial autophagy, which could be used to treat neurodegenerative disorders.
Introduction
Neurodegenerative disorders (NDDs) are often the consequence of degenerative response to numerous systemic-level processes, but the exact cause has not been fully elucidated, however, ageing is widely considered to be the greatest risk factor (Zia et al. Citation2021). It is predicted that the number of elderly people in the world will increase by 21% in the next 50 years (Tricco et al. Citation2012). The World Health Organisation (WHO) predicts that by 2040, ageing-related neurodegenerative disease such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) will overtake cancer as the second leading cause of death after cardiovascular disease (Gammon Citation2014). The link between ageing and increased inflammation has been well established. Both men and women over 65 years old have elevated serum levels of IL-6, TNF-α, and IL-18 (Ferrucci et al. Citation2005; Nilsson et al. Citation2020). Substantial evidence suggests that systemic low-grade inflammation contributed to the development of brain degeneration, especially with ageing progresses (Goldberg and Dixit Citation2015; Więckowska-Gacek et al. Citation2021). In the brain, the theory of ‘hypothalamic microinflammation’ mainly emphasises the fundamental role of inflammation in ageing-related NDDs development (Zhang et al. Citation2013, Citation2017). In general, inflammation represents a key regulator in both ageing and degenerative disorders (Lutshumba et al. Citation2021). Therefore, manipulating age-related inflammatory mechanisms may delay the development of NDDs (Guo et al. Citation2015).
As mononuclear phagocytes residing in the central nervous system (CNS) parenchyma, microglia are a crucial part of the CNS immune system and play a primary role in tissue injury, repair and regeneration (Smith et al. Citation2012; Hu et al. Citation2015; Fan and Pang Citation2017; Wang et al. Citation2019). Once activated by external stimuli, such as pathogens or injuries, resting microglia become polarised. The two extremes of this polarisation are called M1-like and M2-like phenotypes (Hu et al. Citation2015; Saxena et al. Citation2021). M1 microglia are usually activated by pro-inflammatory cytokines such as interferon-γ (IFNγ) or endotoxins such as lipopolysaccharide (LPS). Once in M1 polarisation state, microglia produce pro-inflammatory cytokines, nitric oxide (NO), and reactive oxygen species (ROS), resulting in neuron damage and neurogenic disorders (Block et al. Citation2007; Prinz and Priller Citation2014). In contrast, M2 microglia are activated by IL-4 and IL-10, which induce neurogenesis and brain tissue regeneration by secreting anti-inflammatory neurotrophic factors such as IL-10, transforming growth factor-β (TGF-β), insulin-like growth factor 1 (IGF-1) and brain-derived neurotrophic factor (BDNF) (Belarbi and Rosi Citation2013; Prinz and Priller Citation2014; Colonna and Butovsky Citation2017). The transition from M2 microglia to M1 microglia is related to the increased injury progression in NDDs (Hu et al. Citation2015). Therefore, regulating microglial polarisation from M1 towards M2 is considered to be a potential therapeutic strategy for NDDs.
Meanwhile, autophagy is a basic cellular homeostatic mechanism that is essential for controlling inflammatory responses and neurotoxicity in the CNS (Bussi et al. Citation2017; He et al. Citation2018). In the autophagic process, damaged organelles and denatured proteins are transported to lysosomes, in which their functional blocks are degraded and recycled via anabolic reactions (Carneiro and Travassos Citation2013; Kim et al. Citation2015). Autophagy-related proteins can either induce or inhibit immune and inflammatory responses, as well as immune and inflammatory signals can also regulate autophagy (Saitoh and Akira Citation2010; Su et al. Citation2016). Emerging studies demonstrated that autophagy is associated with the phenotypic transformation of microglia (Xia et al. Citation2016). For instance, both primary and BV2 microglia with autophagy gene knockout showed excessive M1 pro-inflammatory state (Ye et al. Citation2017; Houtman et al. Citation2019). However, when treated with various autophagy inducers, the inflammatory response of microglia was significantly inhibited and the M2 phenotypic polarisation of microglia increased (Munz Citation2016; Ye et al. Citation2017; Cheng et al. Citation2020).
Panax japonicus, the dried rhizome of Panax japonicus C.A. Mey. (Araliaceae), is a common traditional herbal medicine and widely distributed in Japan and southwest China and some ethnic minorities, it is used as a substitute for Ginseng root (Deng et al. Citation2017). Saponins from Panax japonicus (SPJ) are the bioactive rhizome component of Panax japonicus (Yuan et al. Citation2018). Mounting pharmacological studies have shown that SPJ have anti-inflammatory and antioxidative effects, as well as reduce blood lipids and regulate immunity (Wu et al. Citation2016), among which our previous studies have shown that the anti-inflammatory activity of SPJ is particularly prominent. For example, SPJ reduced the inflammatory response of LPS-induced BV2 microglia and RAW264.7 macrophages (Dai et al. Citation2014; Tu et al. Citation2018). We also showed that SPJ improved the cognitive deficits in natural ageing rats and D-galactose induction induced rats (Wang et al. Citation2015; Deng et al. Citation2017; Ruan et al. Citation2019; Wan et al. Citation2020). However, whether the effect of SPJ on cognitive impairment is related to regulation of microglial inflammatory phenotype and the level of autophagy remains unclear.
Materials and methods
Animal treatment
The specific pathogen free male Sprague-Dawley rats (n = 32) were purchased from the Experimental Animal Centre of China Three Gorges University in Yichang, Hubei province. Rats were housed in pathogen-free facilities, in a 12 h light/dark cycle in ventilated cages, with access to chow and water ad libitum. This study was approved by the China Three Gorges University Council on Animal Care Committee. The handling, experimental procedures, and care of the animals were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were divided into four groups and treated with various regimens: (a) adult control group (n = 8, normal feed, 1-month-old rats were purchased and raised to 5-month old); (b) ageing control group (n = 8, normal feed, 18-month-old rats were purchased and raised to 22-month old); (c) SPJ 10 mg/kg treated group (n = 8, contains SPJ feed, 18-month old rats were purchased and food administration for consecutive 4 months until they were 22-month-old); (d) SPJ 30 mg/kg treated group (n = 8, contains SPJ feed, 18-month old rats were purchased and food administration for consecutive 4 months until they were 22-month-old). Briefly, we measured the daily food intake of each rat, and calculated the SPJ content per kilogram of feed based on the average weight and food intake. Rat feed containing SPJ is processed by Beijing Huafukang Biotechnology Co., Ltd. At a predetermined time, rats were anaesthetized with intraperitoneal injecting urethanes. The cortex and hippocampus were snap frozen for further experiments.
Cell culture and viability assay
BV2 murine microglia cells were obtained from the Cell Resource Centre of the Institute of Basic Medicine, Chinese Academy of Medical Sciences. Cells were maintained in complete DMEM media (10% foetal bovine serum, 100 units/mL penicillin-streptomycin) and cultivated at 37 °C with 5% CO2.
The model of LPS activation was established according to the following steps: cells were pre-protected with 1, 5, 25, 50 μg/mL SPJ for 12 h and followed with 1 μg/mL LPS for 12 h. Cell viability was evaluated by MTT. Briefly, cells were seeded in 96-well plates (1 × 104 cells/well) and then pre-incubated with different concentrations of SPJ. After incubation, MTT solution was added and incubated for 4 h, then MTT solution was removed and 200 μL DMSO was added for 15-min incubation. Finally, the absorbance was measured at 570 nm with a microplate reader. Survival ratio of control group was defined as 100% and that of other groups expressed as percentage of control group.
Drug sources
Panax japonicus was harvested from the planting base of Chunmuying in Xuanen County, Enshi City, Hubei Province in October 2016. It was identified by Dr. He Yumin in Key Laboratory of Natural Products Research and Utilisation of Three Gorges University. SPJ were extracted according to the method of our research team (He et al. Citation2017). Briefly, the root of dried Panax japonicus (1000 g) was cut into small pieces and refluxed with 3-fold volume 70% ethanol for 3 times, 2 h each time. The extract was filtered and concentrated to 5 L, purified by M-5 macroporous resin, eluted with double distilled water, and eluted with 70% ethanol when the Molish reaction was negative. The collected eluent was then decompressed to concentrate, followed by freeze-drying. The dried sample was added with water to make a 20 mg/mL solution, and the pH value was adjusted to 10. The extraction rate is 18.4% and the purity is 83.5%.
Morris water maze (MWM) test
The MWM test was evaluated one week before the rats were killed. Briefly, tests were performed in a tank (120 cm diameter, 50 cm deep) filled with opacified water kept at 22 ± 1 °C. The tank was equipped with a 10 cm diameter platform submerged 1 cm under the water surface. Training includes daily sessions about 2 h for 5 consecutive days. The start positions varied pseudo randomly among the 4 cardinal points. Each trial ended when the animal reached the platform. The mice were gently guided to the platform if they failed to reach the platform in 1 min. After the last training, retention trial was evaluated during probe trial in which the platform was removed (day 6). Animals’ video track and test parameters including latency, swim speed, travelled distance, time spent in quadrant, platform crossings) were automatically calculated.
Transmission electron microscope
The tissue was cut into 1 mm3, fixed with stationary liquid for 24 h at 4 °C and then fixed in 1% osmic acid for another 2 h. After uranyl acetate staining under room temperature and dark condition for 3 h, samples were washed with redistilled water and dehydrated by ethanol. The dehydrated tissue was placed in a mixture of ethanol and epoxypropane (1:2) for 10 min, epoxypropane twice for 10 min, mixture of epoxypropane and embedding medium (1:1) for 40 min, mixture of epoxypropane and embedding medium (1:4) for 3 h, and then embedded with embedding medium at 4 °C overnight and subsequently polymerised at 60 °C for 48 h. After performing histological section, tissue slide was stained with uranyl acetate and lead citrate, and microglia were observed and photographed under transmission electron microscope.
Western blotting
All tissues or cells were lysed in RIPA buffer, and total protein concentrations were determined with BCA Protein Assay Kit. Total protein was loaded into precast 8%-12% SDS-PAGE gels and then transferred onto PVDF membranes. After that, the membranes were incubated with the following primary antibodies at 4 °C overnight: anti-β-actin (Servicebio, GB11001, 1:1000), anti-YM-1 (Abcam, ab192029, 1:2000), anti-CD206 (Abcam, ab64693, 1:1000), anti-LC3A/B (Cell signalling, #12741, 1:1000), anti-Beclin1 (Abcam, ab207612, 1:1000), anti-ATG5 (Proteintech, 10181-2-AP, 1:1000), anti-p62 (Abcam, ab56416, 1:2000), anti-IL-1β (Abcam, ab9722, 1:1000). The primary antibodies were recycled and membranes were washed 3 times in Tris-buffered saline with Tween-20 (TBST) for 6 min each time. Goat anti-Rabbit IgG and Goat anti-Mouse IgG secondary antibody (Jackson ImmunoResearch, 103069, 1:10,000) were incubated for 1 h at room temperature. After the removal of the secondary antibody, the membranes were washed 3 times with TBST for 6 min each time. Finally, the relative proteins levels were detected by enhanced chemiluminescence reagent and ImageJ software.
Immunofluorescence
Cells were seeded on the circular cover glass in 24-well plates and were pre-incubated with 25 and 50 μg/mL SPJ for 12 h and followed with 1 μg/mL LPS for another 12 h. After ending the incubation, cells were washed twice with PBS, followed by fixation with 4% paraform and then perforation with 0.1% Triton X-100 for 20 min. After blocking with 1% BSA, cells were incubated with the following primary antibodies at 4 °C overnight: anti-IL-1β (Abcam, ab9722, 1:2000), anti-TNF-α (Santa Cruz, SC-5746, 1:1000), anti-ARG1 (Proteintech, 16001-1-AP, 1:1000), TGF-β (Abcam, ab92486, 1:1000). Following incubation with primary antibody, cells were incubated with FITC-labeled secondary antibodies (Jackson ImmunoResearch) for 1.5 h. After washing, cells were mounted with 40, 60-diamidino-2-phenylindole (DAPI) and Prolong Antifade Reagent (G1401, Servicebio, China). Staining patterns were examined using a scanning fluorescence microscope (Leica Microsystems).
Statistical analysis
The obtained data are presented as mean ± SEM of at least three independent experiments. Groups were compared using one-way ANOVA with post hoc Tukey’s multiple comparisons test. All of the data were analysed with GraphPad Prism 6.0 software. Statistical significance was set at p < 0.05 for all the analyses.
Results
SPJ reduces cognitive impairment in ageing rats
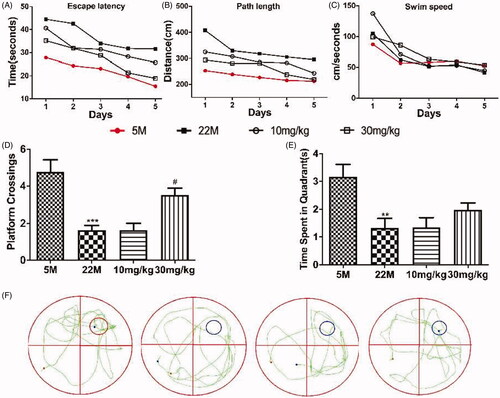
To evaluate the protective action of SPJ on cognitive impairment, rats were tested in the MWM (). All rats learned platform position across time during learning session, as demonstrated by decreased latency () or path length () to reach the platform over the 5 days of training. Although impaired learning ability was observed in the ageing rats as compared to the young, SPJ treatment with 10 and 30 mg/kg reduced impaired learning ability relative to the ageing group. It should be noted that the swim speed was comparable in all groups regardless of the treatment with SPJ, indicating no neuromotor differences among the groups (). After the last training trial, SPJ-treated rats showed a clear preference for the target quadrant by contrast to the ageing group (SPJ-treated vs. ageing rats: platform crossing, p < 0.05 (), time spent in target quadrant, p > 0.05 (). These results showed that SPJ alleviated cognitive impairments in memory retention of ageing rats.
Figure 1. Effect of SPJ on cognitive impairment in ageing rats. (A) Escape latency (B) path length and (C) swim speed of SD rats during learning session. (D) Comparison of numbers of crossing over platform site on day 6. (E) Comparison of time spent in target quadrant on day 6. (F) Representative route on day 6. **p < 0.01 vs. 5 M group; #p < 0.05 vs. 22 M group.
SPJ improves the morphology of microglia and synapses in ageing rats
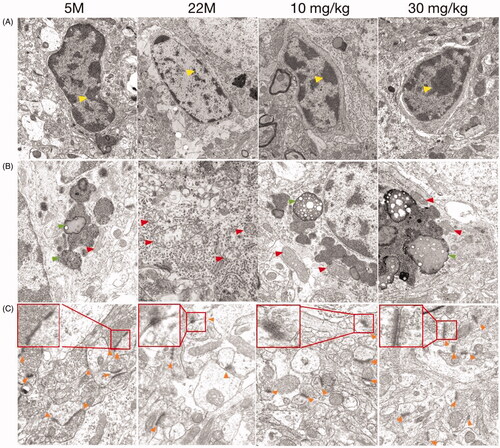
Compared to the 5 M group, cortical microglia in 22 M showed a variable degree of ultrastructural alterations as follows: vacuolisation, chromatin decreased, the nuclear membrane was unclear and the cell organelle (lysosomes, mitochondria) in the cytoplasm were blurred or even disappeared under the transmission electron microscope (). Beyond that, mitochondria with variable size (elongated, fragmental size or swelling) and with reduction or distorted/disrupted cristae were found in the ageing microglia (). After treatment with SPJ, the pathological phenomena of microglia in the ageing group were significantly improved. The electron density of microglia was high and the organelles were rich in the cytoplasm, as well as the mitochondria were well-structured with clear crista and inner boundary membrane. On the other hand, contrary to 5 M group, the aged group exhibits fewer synapses, blurred structure of the membrane, and significantly fewer synaptic vesicles, all of which have been reversed with intervention of SPJ ().
Figure 2. Effect of SPJ on the morphology of microglia and synapses in ageing rats through transmission electron microscope. The morphology of microglial nucleus (A), cytoplasm (B) and neuronal synapses (C) demonstrated that treatment with SPJ at 10 and 30 mg/kg exerted neuroprotective effect by improving damaged microglia and synapses in cortex. Yellow arrows indicate chromatin; Red arrows indicate mitochondria; Orange arrows indicate synapses.
SPJ promotes M2 polarisation in ageing rats
To explore the effect of SPJ on microglia polarisation in hippocampus of ageing rats, western blot analysis was used to determine the levels of CD206 and YM-1. The data demonstrated that the protein levels of CD206 and YM-1 were significantly reduced in the ageing rats when compared to the young. However, the above protein expression changes were obviously reversed by SPJ intervention (p < 0.05, ).
SPJ reverses autophagy impairment in ageing rats
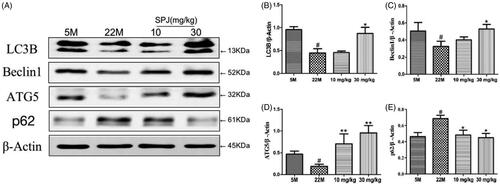
We further examined whether SPJ regulates autophagy progress in ageing rats. Western blotting showed that the levels of LC3B, Beclin1 and ATG5 in the hippocampus of ageing rats were significantly decreased in comparison with the young group (p < 0.05, ), whereas the level of p62 was obviously increased (p < 0.05, ). After 30 mg/kg SPJ intervention, the above changes of protein levels were reversed (p < 0.05 or p < 0.01, ).
Figure 4. Effect of SPJ on autophagy-related protein levels in the hippocampus of ageing rats. (A) Representative immunoblot bands of LC3, Beclin1, ATG5 and p62 levels in hippocampus. (B–E) Quantification of LC3, Beclin1, ATG5 and p62 after normalisation to β-actin. #p < 0.05 vs. 5 M group; *p < 0.05 or **p < 0.01 vs. 22 M group.
SPJ reverses LPS-induced M2-to-M1 polarisation in BV2 cells
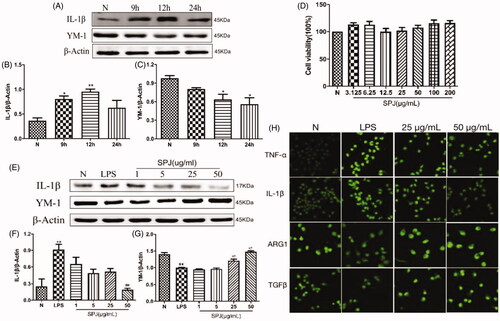
Upon stimulation by LPS for 9, 12 and 24 h, microglia were tested via western blotting to probe protein expressions related to inflammation. Our results found that the level of YM-1 (M2 marker) decreased gradually (, p < 0.05), whereas the level of M1 marker IL-1β peaked at 12 h (, p < 0.01). The above results showed that LPS enhanced microglial M2-to-M1 polarisation, so 1 μg/mL LPS was selected to stimulate BV2 for 12 h as the model time in the following experiment. showed that treatment with SPJ alone below the concentration of 200 μM did not induce toxicity in BV2 cells. Moreover, SPJ inhibited the protein levels of IL-1β in comparison to the LPS groups. Furthermore, the level of YM-1 increased after SPJ incubation (). Immunofluorescence further demonstrated an increase in the accumulation of M2 markers, including ARG1 and TGFβ, while SPJ suppressed LPS-induced increase of M1 markers, including TNF-α and IL-1β (). These results demonstrate that SPJ promotes microglial M1-to-M2 polarisation.
Figure 5. Effect of SPJ on microglial polarisation in LPS-induced BV2 cells. (A) Representative immunoblot bands of IL-1β and YM-1 in cells after stimulation by LPS. (B–C) Quantification of IL-1β and YM-1 after normalisation to β-actin (D) MTT showed that treatment with SPJ alone below the concentration of 200 μM did not induce toxicity in BV2 cells. (E) Representative immunoblot bands of IL-1β and YM-1 in cells upon a SPJ protection for 12 h followed by LPS stimulation. (F–G) Quantification of YM-1and IL-1β after normalisation to β-actin. (H) Confocal images show that the protein levels of TNF-α, IL-1β, ARG1 and TGFβ in BV2 after administrating SPJ in LPS groups. *p < 0.05, **p < 0.01 vs. control group; ##p < 0.01 vs. LPS group.
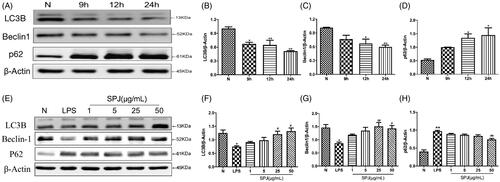
SPJ enhanced autophagy inhibited by LPS stimulation in BV2 cells
Emerging studies have shown the pivotal roles of autophagy involving in microglial inflammation and phenotype shift. In order to evaluate whether activation of inflammation would affect autophagy in BV2 cells, we also detected autophagy related proteins. Since LC3B is a classical marker of autophagy, we evaluated LC3B expression through western blotting and found that it was decreased in cells cotreated with LPS (p < 0.01, ). Similar to the LC3B, the level of Beclin1, an important participant in the formation of autophagosomes, significantly decreased after co-incubation with LPS (p < 0.01, ). Moreover, LPS treatment significantly increases p62, a protein substrate for autophagy used for monitoring autophagic turnover, in microglial cells (p < 0.01, ). The above results reflected impaired autophagy in BV2 with LPS stimulation. However, after treatment the cells with SPJ and LPS, the protein levels of LC3B and Beclin1 increased gradually, whereas the level of p62 gradually decreased (p < 0.05 or p < 0.01, ). The above findings indicate that SPJ enhance microglial autophagy.
Figure 6. Effect of SPJ on microglial autophagy in LPS-induced BV2 cells. (A) Representative immunoblot bands of LC3B, Beclin1 and p62 in cells after stimulation by LPS. (E) Representative immunoblot bands of LC3B, Beclin1 and p62 in cells upon a SPJ protection for 12 h followed by LPS stimulation. (B–D, F–H) Quantification of LC3B, Beclin1 and p62 after normalisation to β-actin. *p < 0.05, **p < 0.01 vs. control group; #p < 0.05, ##p < 0.01 vs. LPS group.
Discussion
The effects of SPJ on cognitive disorder are gradually illustrated by the behavioural experiment in the model of various CNS disorders (Wang et al. Citation2015; Deng et al. Citation2017; Ruan et al. Citation2019). But the underlying mechanism of SPJ mediated functional change in microglial phenotype remains unclear. This study reveals the consequences of SPJ in ageing-induced cognitive damage. Morris water maze showed that SPJ recovered the cognitive decline compared to the aged group. Transmission electron microscope showed the microglial and synapses’ morphological improvement by lowering the extent for (1) breakage membrane of microglia cell, (2) decreasing mass of chromatin in the nucleolus, (3) injury of organelles in the cytoplasm, and (4) decreasing number and damaging structure of synapses. These results suggested that SPJ may improve cognitive impairment through regulating microglia in ageing mice.
The balance between microglial inflammatory states affects the progression of a multitude of CNS disorders, however, the specific classification of M1 and M2 polarised microglia remains a topic for debate (Hu et al. Citation2015; Miron et al. Citation2013; Tian et al. Citation2016). M1/M2 polarisation of microglia is largely classified by the expression of M1 markers such as IL-1β, TNF-α, COX-2 and iNOS, and M2 markers such as ARG1, YM-1, TGF-β and CD206 (Hu et al. Citation2015). The polarised M1 or M2 subpopulation can reverse its phenotype and function in order to response to distinct microenvironmental cues. Thus, strategies focus on promoting M1-to-M2 phenotypic conversion of microglia may be regarded as a potential treatment of neuroinflammation-induced damage (Miron et al. Citation2013; Tian et al. Citation2016). Actually, emerging evidences indicated that SPJ can reduce the expression of pro-inflammatory factors and NOD-Like receptors-like receptor 3 (NLRP3) inflammatory bodies in hippocampus and cortex of natural ageing rats (Wang et al. Citation2015; Deng et al. Citation2017; Ruan et al. Citation2019). This study further showed that the SPJ induced microglial M2 polarisation in vivo, which indicated by the increase of M2 markers including YM-1 and CD206 in hippocampus. Western blotting and immunofluorescence analyses confirmed that SPJ could promote M1-to-M2 polarisation and exert anti-inflammatory effect in LPS-induced BV2 microglia in vitro.
Besides inflammatory response, autophagy is also affected by LPS as reported in a growing number of studies. For example, He et al. (Citation2018) demonstrated that LPS led to p38 MAPK-dependent phosphorylation of ULK1 in microglia, which inhibited ULK1 kinase activity and prevented ULK1 binding to the downstream effector ATG13, finally repressed autophagy in microglia. Lee et al. (Citation2019) found that LPS obviously inhibited autophagy via FOXO3 pathway and impaired the phagocytic capability of microglia. Furthermore, Ye et al. (Citation2020) found that LPS-induced neuroinflammation in microglia resulted from inhibition of autophagic flux through activation of the PI3KI/AKT/MTOR pathway, whereas elevated microglial autophagy alleviated LPS-induced neuroinflammation. Therefore, we evaluated the effect of LPS on autophagy-related proteins in BV2 microglial cell line. As is reported, the expression of LC3B and Beclin1 reduced and the level of p62 elevated in BV2 microglia after incubated with 1 μg/mL LPS for 12 h. The above phenomena can be reversed after the intervention of SPJ, indicating that SPJ can reverse LPS-induced autophagy inhibition in BV2 cells.
Recently, there is increasing evidence that autophagy regulates innate immunity by promoting the transformation of microglia from M1 to M2 (Saitoh and Akira Citation2010; Sumpter and Levine Citation2010). In various neuroinflammatory models (e.g., AD, PD, cerebral ischaemia and ALS), the inflammatory bodies in BV2 microglia are activated. Meanwhile, the expression of M1 pro-inflammatory factors is increased, but the M2 anti-inflammatory factors and autophagy are both suppressed (Jin et al. Citation2018). When autophagy was up-regulated by inhibiting serum or administrating various autophagy activators, microglia exhibited a decreased activation of inflammatory bodies and an increased polarisation to M2 (Cadwell Citation2016). These improvements were blocked with administration of the autophagy inhibitor 3-MA, which may be related to the regulation of mTOR or TLR2 signal pathway, inhibition of NLRP3 inflammatory bodies or other factors (Li et al. Citation2016; Xia et al. Citation2016; de Mattos Barbosa et al. Citation2018; Han et al. Citation2019). However, in other autophagy injury models of microglia (e.g., 3-MA treatment, siRNA-Beclin1 or siRNA-ATG5 transfection), NLRP3 signal, MAPKs and nuclear transcription factor (NF-κB) are all activated, and M1 pro-inflammatory cytokines are highly expressed. Such changes are however reversed, accompanied with an increased polarisation of M2, upon treating with various autophagy inducers (e.g., rapamycin, chloroquine and GSK-3β inhibitors) (Zhou et al. Citation2011; Cheng et al. Citation2020). Therefore, the inhibition of autophagy may be involved in the over-activation of microglia, while the promotion of autophagy can reduce the microglial pro-inflammatory response. Consistent to this analysis, our results showed that 50 μg/mL SPJ enhanced the autophagy of microglia, while the level of M1 pro-inflammatory factor IL-1β was significantly decreased and the expression of M2 anti-inflammatory factor YM-1 was significantly increased. These suggest that SPJ may exert its neuroprotective effect on microglia by enhancing autophagy that induces the transformation of inflammatory phenotype from M1 to M2.
Conclusions
Our research proved that SPJ reduced cognitive decline in ageing rats through upregulation of microglial M1 to M2 polarisation resulting in decreasing microglial inflammation and enhancing microglial autophagy ability.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Belarbi K, Rosi S. 2013. Modulation of adult-born neurons in the inflamed hippocampus. Front Cell Neurosci. 7:145–153.
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 8(1):57–69.
- Bussi C, Peralta RJ, Arroyo DS, Gaviglio EA, Gallea JI, Wang JM, Celej MS, Iribarren P. 2017. Autophagy down regulates pro-inflammatory mediators in BV2 microglial cells and rescues both LPS and alpha-synuclein induced neuronal cell death. Sci Rep. 7:43153–43161.
- Cadwell K. 2016. Crosstalk between autophagy and inflammatory signalling pathways: balancing defence and homeostasis. Nat Rev Immunol. 16(11):661–675.
- Carneiro LA, Travassos LH. 2013. The interplay between NLRs and autophagy in immunity and inflammation. Front Immunol. 4:361–374.
- Cheng J, Liao Y, Dong Y, Hu H, Yang N, Kong X, Li S, Li X, Guo J, Qin L, et al. 2020. Microglial autophagy defect causes Parkinson disease-like symptoms by accelerating inflammasome activation in mice. Autophagy. 16(12):2193–2205.
- Colonna M, Butovsky O. 2017. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 35:441–468.
- Dai YW, Yuan D, Wan JZ, Zhang CC, Liu CQ, Wang T. 2014. Study on protective effect of total saponins of Panax japonicus on LPS-induced RAW264.7 cell inflammation through NF-κB pathway. Chin J Chin Mater Med. 39:2076–2080.
- de Mattos Barbosa MG, de Andrade Silva BJ, Assis TQ, da Silva Prata RB, Ferreira H, Andrade PR, da Paixão de Oliveira JA, Sperandio da Silva GM, da Costa Nery JA, Sarno EN, Pinheiro RO. 2018. Autophagy impairment is associated with increased inflammasome activation and reversal reaction development in multibacillary leprosy. Front Immunol. 9:1223–1234.
- Deng LL, Yuan D, Zhou ZY, Wan JZ, Zhang CC, Liu CQ, Dun YY, Zhao HX, Zhao B, Yang YJ, et al. 2017. Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogen-activated protein kinase and nuclear factor kappa B signaling pathways. Neural Regen Res. 12(11):1877–1884.
- Fan LW, Pang Y. 2017. Dysregulation of neurogenesis by neuroinflammation: key differences in neurodevelopmental and neurological disorders. Neural Regen Res. 12(3):366–371.
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. 2005. The origins of age-related proinflammatory state. Blood. 105(6):2294–2299.
- Gammon K. 2014. Neurodegenerative disease: brain windfall. Nature. 515(7526):299–300.
- Goldberg EL, Dixit VD. 2015. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev. 265(1):63–74.
- Guo H, Callaway JB, Ting JP. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 21(7):677–687.
- Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, Chen M, Sun T, Xia M, Ding J, et al. 2019. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy. 15(11):1860–1881.
- He C, Yu Z, He Y, Xu C, Chang C, Yuan D. 2017. Purification of macroporous adsorption resin and decolorization of ion exchange resin of total saponins of Panax japonicus. Chia Tradit Herbal Drugs. 48:1146–1152.
- He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, Zhao Y, Mao Z. 2018. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 217(1):315–328.
- Houtman J, Freitag K, Gimber N, Schmoranzer J, Heppner FL, Jendrach M, 2019. Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. Embo J. 38(4):38–49. 4.
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. 2015. Microglial and macrophage polarization—new prospects for brain repair . Nat Rev Neurol. 11(1):56–64.
- Jin MM, Wang F, Qi D, Liu WW, Gu C, Mao CJ, Yang YP, Zhao Z, Hu LF, Liu CF. 2018. A critical role of autophagy in regulating microglia polarization in neurodegeneration. Front Aging Neurosci. 10:378–389.
- Kim JY, Paton JC, Briles DE, Rhee DK, Pyo S. 2015. Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget. 6(42):44161–44178.
- Lee JW, Nam H, Kim LE, Jeon Y, Min H, Ha S, Lee Y, Kim SY, Lee SJ, Kim EK, et al. 2019. TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia. Autophagy. 15(5):753–770.
- Li D, Wang C, Yao Y, Chen L, Liu G, Zhang R, Liu Q, Shi FD, Hao J. 2016. mTORC1 pathway disruption ameliorates brain inflammation following stroke via a shift in microglia phenotype from M1 type to M2 type. Faseb J. 30(10):3388–3399.
- Lutshumba J, Nikolajczyk BS, Bachstetter AD. 2021. Dysregulation of systemic immunity in aging and dementia. Front Cell Neurosci. 15:652111–652158.
- Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, et al. 2013. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 16(9):1211–1218.
- Munz C. 2016. Autophagy beyond intracellular MHC Class II antigen presentation. Trends Immunol. 37:755–763.
- Nilsson CJ, Nørgaard S, Foverskov E, Bruunsgaard H, Andersen PK, Lund R. 2020. Positive and negative aspects of social relations and low-grade inflammation in Copenhagen Aging and Midlife Biobank. Eur J Ageing. 17(4):531–546.
- Prinz M, Priller J. 2014. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 15(5):300–312.
- Ruan B, Wang R, Yang YJ, Wang DF, Wang JW, Zhang CC, Yuan D, Zhou ZY, Wang T. 2019. Improved effects of saponins from Panax japonicus on decline of cognitive function in natural aging rats via NLRP3 inflammasome pathway. Chin J Chin Mater Med. 44:344–349. Chinese
- Saitoh T, Akira S. 2010. Regulation of innate immune responses by autophagy-related proteins. J Cell Biol. 189(6):925–935.
- Saxena S, Kruys V, Vamecq J, Maze M. 2021. The role of microglia in perioperative neuroinflammation and neurocognitive disorders. Front Aging Neurosci. 13:671499–671534.
- Smith JA, Das A, Ray SK, Banik NL. 2012. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 87(1):10–20.
- Su P, Zhang J, Wang D, Zhao F, Cao Z, Aschner M, Luo W. 2016. The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience. 319:155–167.
- Sumpter RJ, Levine B. 2010. Autophagy and innate immunity: triggering, targeting and tuning. Semin Cell Dev Biol. 21(7):699–711.
- Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL. 2016. Deficiency in the voltage-gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke. J Neurochem. 139(1):96–105.
- Tricco AC, Soobiah C, Lillie E, Perrier L, Chen MH, Hemmelgarn B, Majumdar SR, Straus SE. 2012. Use of cognitive enhancers for mild cognitive impairment: protocol for a systematic review and network meta-analysis. Syst Rev. 1:25–34.
- Tu H, Zhou Q, Zhang X, Wang T, Yuan CF, Zhang CC, Yuan D, Liu ZQ. 2018. Protective effects of total saponins of Panax japonicas on HepG2 cell apoptosis induced with palmitic acid. Chin J Chin Mater Med. 43:390–395.
- Wan JZ, Wang R, Zhou ZZ, Deng LL, Zhang CC, Liu CQ, Zhao HX, Yuan CF, He YM, Dun YY, Yuan D, et al. 2020. Saponins of Panax japonicus confer neuroprotection against brain aging through mitochondria related oxidative stress and autophagy in rats. Curr Pharm Biotechnol. 21:1–14.
- Wang H, Shen Y, Chuang H, Chiu C, Ye Y, Zhao L. 2019. Neuroinflammation in Alzheimer’s disease: microglia, molecular participants and therapeutic choices. Curr Alzheimer Res. 16(7):659–674.
- Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, Peng B, Liu C, Xiong G, Zhang C, et al. 2015. Saponins from Panax japonicus attenuate d-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 67(9):1284–1296.
- Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U. 2021. Western diet as a trigger of Alzheimer’s disease: from metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 21:1568–1637.
- Wu QS, Chen P, Zhang QW. 2016. Research progress on chemical constituents, pharmacological activities and analytical methods of Panax japonicus. Asia-Pacific Trad Med. 12:46–54.
- Xia CY, Zhang S, Chu SF, Wang ZZ, Song XY, Zuo W, Gao Y, Yang PF, Chen NH. 2016. Autophagic flux regulates microglial phenotype according to the time of oxygen-glucose deprivation/reperfusion. Int Immunopharmacol. 39:140–148.
- Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N. 2017. The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro. J Neurochem. 142(2):215–230.
- Ye X, Zhu M, Che X, Wang H, Liang XJ, Wu C, Xue X, Yang J. 2020. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J Neuroinflammation. 17(1):18.
- Yuan D, Xiang T, Huo Y, Liu C, Wang T, Zhou Z, Dun Y, Zhao H, Zhang C. 2018. Preventive effects of total saponins of Panax japonicus on fatty liver fibrosis in mice. Arch Med Sci. 14(2):396–406.
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. 2013. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH . Nature. 497(7448):211–216.
- Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. 2017. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature. 548(7665):52–57.
- Zhou X, Zhou J, Li X, Guo C, Fang T, Chen Z. 2011. GSK-3β inhibitors suppressed neuroinflammation in rat cortex by activating autophagy in ischemic brain injury. Biochem Biophys Res Commun. 411:271–275.
- Zia A, Pourbagher-Shahri AM, Farkhondeh T, Samarghandian S. 2021. Molecular and cellular pathways contributing to brain aging. Behav Brain Funct. 17(1):6–36.

