Abstract
Context
Luteolin can affect multiple biological functions, such as anti-inflammatory, antioxidant and immune enhancement processes. Luteolin can inhibit inflammation of T2-high asthma, but its role in neutrophilic asthma has been insufficently studied.
Objective
This study determines the effect of luteolin on IL-36γ secretion-mediated MAPK pathway signalling in neutrophilic asthma.
Materials and methods
The asthma model was established by using ovalbumin/lipopolysaccharide (OVA/LPS). Female 6–8-week-old C57BL/6 mice were divided into control, asthma, luteolin (20 mg/kg) and asthma + luteolin (20 mg/kg) groups. To explore the mechanism of anti-inflammatory effects of luteolin in neutrophilic asthma, Beas-2B cells were treated with luteolin (20 µmol/L), LPS (100 ng/mL), recombinant human IL-36γ protein (rhIL-36γ; 100 ng/mL) or IL-36γ siRNA.
Results
IL-36γ secretion and MAPK/IL-1β signalling were significantly increased in the asthma mouse model compared with the control (p < 0.05). However, the levels of IL-36γ secretion and MAPK/IL-1β signalling were reduced by luteolin (p < 0.05). In addition, luteolin inhibited IL-36γ and MAPK/IL-1β levels after LPS (100 ng/mL) stimulation of Beas-2B cells (p < 0.05). We found that in Beas-2B cells, luteolin inhibited activation of the MAPK pathway and IL-1β secretion following stimulation with rhIL-36γ (100 ng/mL; p < 0.05). Finally, IL-1β and phosphorylated MAPK levels were found to be lower in the IL-36γ siRNA + LPS (100 ng/mL) group than in the nonspecific control (NC) siRNA + LPS group (p < 0.05).
Discussion and conclusions
Luteolin alleviated neutrophilic asthma by inhibiting IL-36γ secretion-mediated MAPK pathways. These findings provided a theoretical basis for the application of luteolin in the treatment of neutrophilic asthma.
KEYWORD:
Introduction
Bronchial asthma is a common, chronic, inflammatory disease of the airways characterized by a variety of respiratory symptoms and limited airflow. Many cells play an important role in the onset of asthma, including hypertrophic cells, macrophages, eosinophils, T lymphocytes, neutrophils and epithelial cells (Mims Citation2015). The internationally recognized system of classification of asthma divides it into two types: T2-high and T2-low. T2-high asthma is characterized by the presence of eosinophilic airway inflammation, while T2-low asthma is usually characterized by neutrophils or fewer granulocytes (Svenningsen and Nair Citation2017). T2-low asthma, especially neutrophilic asthma, is often associated with severe disease and a poor response to treatment (Samitas et al. Citation2017). Although the definition of neutrophilic asthma still lacks a consensus, neutrophilic asthma and eosinophil asthma are classified mainly according to the proportion of the cells in sputum (Simpson et al. Citation2006). Eosinophilic asthma is defined as an increase in eosinophils in the induced sputum of more than 2% or 3%, and neutrophilic asthma is defined as an increase in neutrophils in the induced sputum of more than 60% or 76% (Chung Citation2016).
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a typical flavonoid found in abundance in many vegetables, fruits and herbs, such as carrots, cabbage, artichokes, tea, celery and apples (Luo et al. Citation2017). Luteolin can affect multiple biological functions, such as anti-inflammatory, antioxidant and immune enhancement processes (Aziz et al. Citation2018). Luteolin can inhibit inflammation (Xie et al. Citation2021) and fibrosis (Chen et al. Citation2010) in the lungs and alleviate asthma, in which lung inflammation and airway remodelling are the main pathological characteristics. One previous study showed that luteolin could reduce bronchoconstriction and bronchial hyperreactivity in ovalbumin (OVA)-induced asthmatic mice (Das et al. Citation2003). Luteolin reduces the excessive production of airway mucus in asthmatic mice via inhibition of the γ-aminobutyric acid (GABA)ergic system (Shen et al. Citation2016). Luteolin has been shown to reduce airway inflammation in asthma by inducing CD4+CD25– transformation into CD4+CD25+ regulatory T cells (Kim SH et al. Citation2018). In addition, luteolin can improve asthma by activating the phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signalling pathway, inhibiting autophagy in allergic asthma via inhibition of Beclin-1-PI3K catalytic domain 3 (PI3KC3) complexes (Wang S et al. Citation2021). However, most studies of luteolin focused on T2-high asthma, and luteolin’s role in neutrophilic asthma has been poorly studied.
Interleukin (IL)-36 belongs to the IL-1 cytokine family; this molecule activates its target cells via the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways via the receptor IL-36R (Neurath Citation2020). IL-36 consists of three main agonists (IL-36α, IL-36β and IL-36γ) and two antagonists (IL-36Ra and IL-38). IL-36R has been found in human bronchial epithelial cells and pulmonary fibroblasts, and the activation of p38MAPK, extracellular signal-regulated kinase (ERK) and Akt signalling pathways via IL-36 signalling can regulate the expression of proteins and genes, including IL-6 and C-X-C motif chemokine ligand 8 (CXCL8) (Zhang et al. Citation2017). Studies have shown that IL-36 also plays a role in asthma. Compared with people with allergic rhinitis alone, the concentrations of IL-36α, IL-36β, IL-36γ, IL-36Ra and IL-38 and associated mRNA levels in the serum of people with allergic rhinitis combined with allergic asthma are significantly higher (Qin et al. Citation2019). A previous study found that IL-36 plays an important role in lung diseases, especially those characterized by the accumulation of neutrophils, including severe low T2 asthma (Koss et al. Citation2021).
The MAPK family is highly conserved and includes c-Jun NH2-end kinase (JNK), p38MAPK and extracellular signalling kinase (ERK) among its members, which regulate cellular activities, including proliferation, differentiation, apoptosis, survival, inflammation and innate immunity (Kim and Choi Citation2015). The MAPK pathway is a classic signal transduction pathway involved in the development of bronchial asthma (Khorasanizadeh et al. Citation2017).
Based on this previous research, the IL-36γ and MAPK pathways are known to participate in the development of asthmatic inflammation. Luteolin also has an anti-inflammatory effect on asthma. Therefore, this research aimed to determine the effect of luteolin on IL-36γ secretion-mediated MAPK pathway signalling in neutrophilic asthma. This study could provide a new approach to developing treatments of neutrophilic asthma.
Materials and methods
Materials
Female 6–8-week-old C57BL/6 mice were purchased from Jinan Pengyue Experimental Animals Co. (Jinan, China). OVA and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (St. Louis, MO). AL(OH)3 was purchased from Thermo Scientific (Waltham, MA). Luteolin was purchased from GlpBio (luteolin > 98%, Montclair, CA). The haematoxylin–eosin (HE) staining kit and glycogen periodic acid–Schiff (PAS/haematoxylin) stain kit were purchased from Solarbio Technology Co. (Beijing, China). The human IL-36γ enzyme-linked immunosorbent assay (ELISA) kit (IL-1F9) was purchased from Abcam (Waltham, MA). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and IL-1β polyclonal antibodies were purchased from Proteintech (Wuhan, China). The IL-1F9 antibody was purchased from Affinity Biosciences (Cincinnati, OH). The Ly-6G, phospho-p38 MAPK, p38 MAPK, phospho-p44/42 MAPK (Erk1/2), p44/42 MAPK (Erk1/2), phospho-SAPK/JNK and SAPK/JNK monoclonal antibodies were purchased from Cell Signaling Technology (Boston, MA). Horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-rabbit IgG antibody was purchased from Boster Biological Technology (Wuhan, China). Dulbecco’s modified Eagle’s medium (DMEM) and foetal bovine serum (FBS) were purchased from GIBCO (Carlsbad, CA). Recombinant human IL-36 γ/IL-1F9 protein (rhIL-36γ) was purchased from R&D Systems (Minneapolis, MN). Beas-2B cells were purchased from Cell Bank (Shanghai, China).
Animal grouping and model establishment
C57BL/6 mice were stratified into the following groups with six mice in each: control, asthma, luteolin and asthma + luteolin. Animal models of neutrophilic asthma were constructed according to previously described methods (Lu et al. Citation2016). OVA (100 µg) and 1 mg of AL(OH)3 were dissolved in 0.2 mL of physiological saline in the allergenic phase (asthma and asthma + luteolin group). On days 0, 7 and 14, mice in the asthma and asthma + luteolin groups received the resulting solution by intraperitoneal (IP) injection. In the excitation phase, the mice in the asthma and asthma + luteolin groups were intranasally administered 50 µg OVA and 1 µg LPS in 50 µL saline on days 15, 17 and 19, and atomization was carried out on the mice for 30 min with 3% OVA on days 21, 23 and 25. The mice were treated with IP luteolin (20 mg/kg body weight) (asthma + luteolin group and luteolin group) or an equivalent volume of physiological saline (control group and asthma group) 1 h after each nasal drop or atomization. Mice in the control and luteolin groups were given the same volume of physiological saline for sensitization and challenge. The mice were sacrificed within 24 h of the last stimulation, and lung tissue was isolated for HE, PAS, IHC and western blotting. Lung tissue was fixed in 4% polyformaldehyde for three days, dehydrated, and embedded in paraffin. Then, the embedded tissues were cut into 5 µm sections for HE staining, PAS staining and IHC.
HE staining
Prepared paraffin sections of mouse lung tissue were used for HE staining. Pathological changes around the mouse airways were assessed under a microscope at ×200 and ×400 magnification. The samples were scored based on the number of inflammatory cells around the airways in the HE-stained section as follows (Li et al. 2019): 0, no inflammatory cells; 1, a small number of inflammatory cells; 2, a circle of inflammatory cells (1–2 layers of cells); 3, a circle of inflammatory cells (3–5 layers of cells); 4, a circle of inflammatory cells (>5 layers of cells).
PAS staining
PAS staining was carried out on the pulmonary tissue paraffin sections; changes in airway mucus secretion were observed under a microscope at ×200 and ×400 magnification. The PAS staining results were analysed with ImageJ (Bethesda, MD).
Detection of IL-36γ expression by IHC
Fixed and paraffin-embedded sections of the dewaxed lung tissue of mice were placed in citric acid repair fluid for 15 min to carry out heat-induced epitope retrieval and allowed to cool naturally to room temperature (RT). Then, the sections were washed three times in PBS for 15 min. Endogenous peroxidase was removed from the sections with 3% hydrogen peroxide for 20 min at RT. After washing with PBS, the sections were incubated in goat serum for 90 min at RT. The sections were subsequently incubated in anti-IL-36γ antibody at 4 °C overnight. The next day, the sections were rewarmed to RT and washed with PBS. Then, the sections were incubated in goat anti-rabbit antibody for 60 min at RT and after washing, the 3,3-diaminobenzidine (DAB) solution was dropped on the sections for colouration. The sections were observed under a microscope as the colour developed and they were placed in PBS to terminate the reaction. Then, the tissue sections were stained with haematoxylin for 10 min, dehydrated, cleared and placed in neutral balsam for subsequent image acquisition.
Detection of Ly6G and IL-36γ expression and p38, ERK and JNK phosphorylation in lung tissue by Western blotting
The protein samples were extracted from the lung tissues and used for sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). The transfer time and current were determined according to the molecular weight of the target protein. After incubation for 90 min in 3% BSA, the PVDF membrane was incubated with the primary antibodies (against Ly6G, IL-1β, p-p38, p38, p-ERK, ERK, p-JNK, JNK and GAPDH) at 4 °C overnight. Then, the PVDF membrane was washed with Tris-buffered saline Tween 20 (TBST) and incubated for 60 min in a 1:5000 dilution of HRP-conjugated goat anti-rabbit IgG. After washing in TBST, the staining of the PVDF membrane was visualized. The images were subjected to grayscale analysis and quantification with ImageJ (Bethesda, MD).
Cell culture and treatment
Beas-2B cells were cultured in DMEM with 10% FBS at 37 °C and 5% CO2. The cells were pretreated with 20 µmol/L luteolin and PBS, 100 ng/mL LPS, or 100 ng/mL rhIL-36γ by adding these agents to the medium.
The cells were divided into the following groups: control (no stimulus), LPS (LPS alone), luteolin (luteolin treatment alone) and LPS + luteolin (luteolin pretreatment followed by LPS). After being cultured for 24 h, the cell supernatant was isolated and used for IL-36γ detection by ELISA after centrifugation for 20 min at 1000×g at 4 °C. Then, the cells were divided into the following groups: control group (no stimulus), rhIL-36γ (rhIL-36γ alone), luteolin (luteolin treatment alone) and rhIL-36γ + luteolin (luteolin pretreatment followed by rhIL-36γ). Finally, the cells were further divided as follows: control (no stimulus), LPS + NC siRNA (NC siRNA and LPS), NC siRNA (NC siRNA alone) and LPS + IL-36γ siRNA (IL-36γ siRNA and LPS). The cells were cultured for 24 h at 37 °C. Then, the protein samples were extracted and detected to determine the IL-1β expression by western blotting. The cells were cultured for 30 min to detect JNK, ERK and p38 phosphorylation by western blotting.
Statistical analysis
Statistical analysis was carried out using SPSS 18.0 software (SPSS, Chicago, IL). Unpaired t-tests were used for comparisons between the two groups. p < 0.05 was considered statistically significant.
Results
Pathological changes and the expression of Ly6G in a mouse model of asthma
In the neutrophilic asthma mouse model, the results of HE staining () showed no significant pathological difference between the luteolin and control groups. Compared with the control group, the asthma group showed oedema, a large number of inflammatory cells infiltrating the tissue around the small airways, and many inflammatory cells around the blood vessels. In the asthma + luteolin group, these inflammatory pathological changes were smaller than those in the asthma group. The results of PAS staining () indicated no significant lung change in the luteolin group compared with the control group, while the bronchial cavity of the asthma group showed a significant increase in mucus and inflammatory cells around the small airways. Inflammatory cells were significantly reduced in the asthma + luteolin group, with only a small amount of mucus visible in the bronchial cavity.
Figure 1. Luteolin reduces inflammation in a mouse model of severe asthma. (A) Animal experimental schedule. (B) Haematoxylin–eosin (HE) staining of airway lung tissue in mice. (C) Periodic acid–Schiff (PAS) staining of airway lung tissue in mice. (D) Analysis of HE staining of airway lung tissue in mice. (E) Analysis of PAS staining of airway lung tissue in mice. (F) Ly6G in airway lung tissue in mice as detected by western blotting. (G) Protein intensity analysis of Ly6G in airway lung tissue in mice. *p< 0.05 versus the control group. #p< 0.05 versus the asthma group.
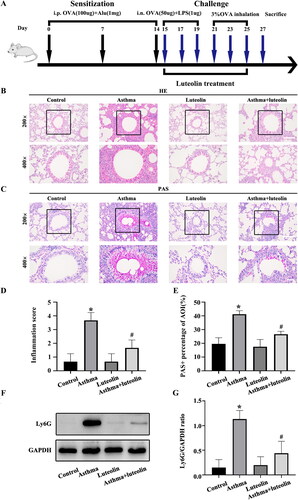
The expression of Ly6G, a neutral granulocyte marker, in the lung tissue of mice was detected by western blotting. The results () showed that the expression of Ly6G in the control and luteolin groups was similar. Compared with that in the control group, the expression of Ly6G in the asthma group was significantly increased. In the asthma + luteolin group, the expression of Ly6G was significantly reduced compared with that in the asthma group.
IL-36γ expression in vivo
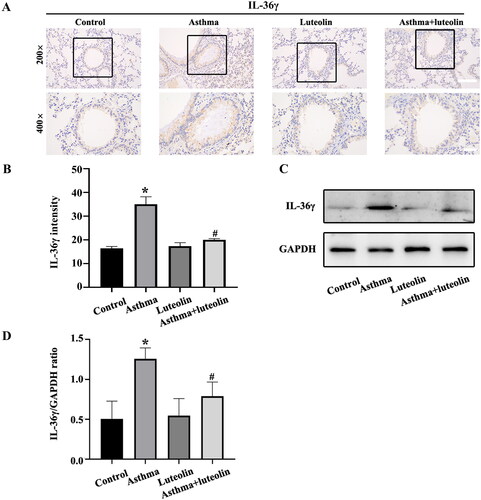
The IHC results () showed that IL-36γ in the asthma group was increased compared with that in the control and luteolin groups. There was no significant difference between the control and luteolin groups, while IL-36γ was significantly reduced in the asthma + luteolin group compared with the asthma group. The western blot results () also showed a significant increase in IL-36γ expression in the asthma group compared with the control group, while its expression was lower in the asthma + luteolin group compared with the asthma group.
Figure 2. Luteolin inhibits IL-36γ secretion in a mouse model of severe asthma. (A) Immunohistochemistry (IHC) of IL-36γ in mouse lung tissue. (B) Analysis of IL-36γ IHC in mouse lung tissue. (C) IL-36γ in mouse lung tissue as detected by western blotting. (D) Protein intensity analysis of IL-36γ in mouse lung tissue as detected by western blotting. *p< 0.05 versus the control group. #p< 0.05 versus the asthma group.
Effect of luteolin on the expression of IL-1β in animal experiments and its relationship with the MAPK pathway
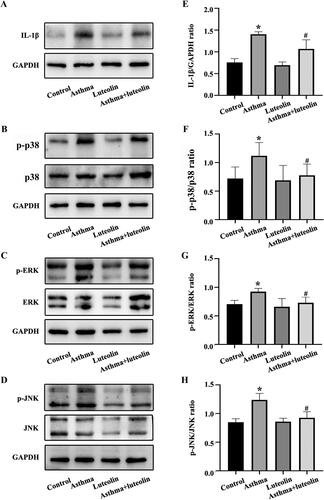
To explore possible signalling pathways mediating the improvements observed in response to luteolin in the mouse model of neutrophilic asthma, the protein phosphorylation levels of p38MAPK, ERK and JNK were detected by western blot. The phosphorylation levels of p38MAPK, ERK and JNK in mouse lung tissue () all increased significantly in the asthma group compared with the control group. The levels in the luteolin group were significantly lower than those in the asthma group. The expression of the typical inflammatory factor IL-1β, which is downstream of the MAPK pathway, was also detected; the results showed that the expression of IL-1β was significantly higher in the asthma group than in the control group (). The expression of IL-1β in the asthma + luteolin group was significantly lower than that in the asthma group ().
Figure 3. Luteolin relieves airway inflammation and inhibits the activation of mitogen-activated protein kinase (MAPK) pathways in asthmatic mice. (A–D) IL-1β, phosphorylated (p-)p38, p38, p-REK, ERK, p-JNK and JNK in mouse airway lung tissue as detected by western blotting. (E–H) Protein intensity analysis of IL-1β, p-p38, p38, p-REK, ERK, p-JNK and JNK in mouse airway lung tissue as detected by western blotting. *p< 0.05 versus the control group. #p< 0.05 versus the asthma group.
Luteolin inhibits the secretion of IL-36γ and IL-1β and the activation of the MAPK pathway
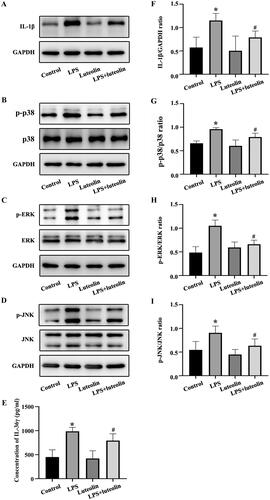
The effects of luteolin on asthmatic inflammation were further explored in vitro. The results indicated that the highest levels of IL-36γ and IL-1β secretion were observed in the LPS group (), while p38 MAPK, ERK and JNK phosphorylation also increased significantly. After pretreatment with luteolin, the secretion of IL-36γ and IL-1β and the phosphorylation of p38 MAPK, ERK and JNK in the LPS + luteolin group were reduced compared with those in the LPS group. Therefore, these results suggested that the improvement of inflammation after treatment of luteolin may be related to the secretion of IL-36γ and activation of the MAPK pathway.
Figure 4. Luteolin reduces the secretion of IL-36γ and IL-1β in Beas-2B cells and inhibits the activation of mitogen-activated protein kinase (MAPK) pathways under lipopolysaccharide (LPS) stimulation. (A–D) IL-1β, p-p38, p38, p-REK, ERK, p-JNK and JNK in Beas-2B cells under LPS stimulation as detected by western blot. (E) IL-36γ in Beas-2B cells under LPS stimulation as detected by enzyme-linked immunosorbent assay (ELISA). (F–I) Protein intensity analysis of IL-1β, p-p38, p38, p-REK, ERK, p-JNK and JNK in Beas-2B cells under LPS stimulation as detected by western blotting. *p< 0.05 versus the control group. #p< 0.05 versus the LPS group.
Luteolin inhibits IL-1β secretion induced by IL-36γ via the MAPK pathway
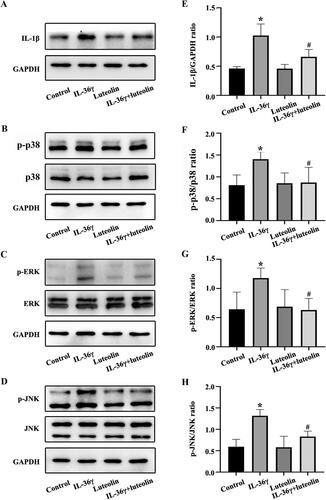
The changes in the level of IL-1β secretion and p38 MAPK, ERK and JNK phosphorylation in Beas-2B cells in response to stimulation with rhIL-36γ were detected by western blot. The secretion of IL-1β and the phosphorylation of p38MAPK, ERK and JNK were elevated in the rhIL-36γ group compared with the other groups (), while the levels in the rhIL-36γ + luteolin group were somewhat lower than those in the rhIL-36γ group. These results suggested that IL-36γ induced Beas-2B cell secretion of IL-1β via the MAPK pathway. Luteolin may also inhibit the production of IL-1β by inhibiting IL-36γ.
Figure 5. Luteolin reduces the secretion of IL-1β in Beas-2B cells and inhibits the activation of mitogen-activated protein kinase (MAPK) pathways under IL-36γ stimulation. (A–D) IL-1β, p-p38, p38, p-REK, ERK, p-JNK and JNK in Beas-2B cells under IL-36γ stimulation as detected by western blotting. (E–H) Protein intensity analysis of IL-1β, p-p38, p38, p-REK, ERK, p-JNK and JNK in Beas-2B cells under IL-36γ stimulation as detected by western blotting. *p< 0.05 versus the control group. #p< 0.05 versus the IL-36γ group.
IL-36γ affects IL-1β secretion
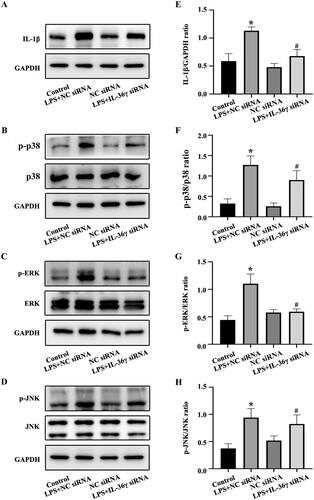
To further establish the relationship between IL-36γ and IL-1β, IL-36γ siRNA was used to reduce the synthesis and secretion of IL-36γ in cells. The levels of IL-1β and p38 MAPK, ERK and JNK phosphorylation were highest in the LPS + NC siRNA group, with reduced levels in the LPS + IL-36γ siRNA group compared with the LPS + NC siRNA group (). This suggests that luteolin may play a similar role to IL-36γ siRNA in inhibiting the secretion of IL-1β via inhibition of IL-36γ and that IL-36γ promotes the secretion of IL-1β.
Figure 6. IL-36γ siRNA reduces the secretion of IL-1β in Beas-2B cells and inhibits the activation of mitogen-activated protein kinase (MAPK) pathways under lipopolysaccharide (LPS) stimulation. (A–D) IL-1β, p-p38, p38, p-REK, ERK, p-JNK, JNK in Beas-2B cells under LPS stimulation as detected by western blotting. (E–H) Protein intensity analysis of IL-1β, p-p38, p38, p-REK, ERK, p-JNK, JNK in Beas-2B cells under LPS stimulation as detected by western blotting. *p< 0.05 versus the control group. #p< 0.05 versus the LPS + NC siRNA group.
Discussion
Bronchial asthma is a common chronic disease of the respiratory system. Asthma can be classified into T2-high asthma and T2-low asthma. T2-low asthma includes neutrophilic and less granulocytic types (Hudey et al. Citation2020).
IL-36 is naturally expressed in a variety of tissues, such as the skin, lungs and intestines, in which it induces inflammation (Bassoy et al. Citation2018). In lung tissue, viruses and bacteria may amplify neutrophil inflammation in the tissue via the production of IL-36γ (Chustz et al. Citation2011). Previous studies have found that IL-36 is a key upstream amplifier of neutrophilic pneumonia in mice (Koss et al. Citation2021). In a house dust mite (HDM) allergenic mouse asthma model, IL-36γ was increased (Ramadas et al. Citation2011). A study previously found that following stimulation with LPS, the expression of IL-36γ mRNA in monocytes was increased (Towne et al. Citation2004).
Luteolin is a typical flavonoid compound with significant anti-inflammatory, anti-allergic and immune-enhancing effects. Previous studies on luteolin-reducing asthma have focused on T2-high asthma, characterized by eosinophilic inflammation, while fewer studies have been conducted on T2-low neutrophilic asthma. T2-low asthma tends to respond less well to standard treatments, including glucocorticoids (Fitzpatrick et al. Citation2020). Therefore, research on the anti-inflammatory effect of luteolin in neutrophilic asthma is significant.
This research used a mouse model of severe neutrophilic asthma characterized by an increase in neutrophils stimulated by OVA and LPS to assess the ameliorative effects of luteolin. The pathological tissue staining results and the levels of Ly6G, a neutrophil marker, showed that luteolin improved inflammation and reduced the production of neutrophils. IL-36γ was found to be associated with neutrophilic inflammation in the lungs and was elevated in the neutrophilic asthma model; the expression of IL-36γ was found to be decreased after treatment with luteolin. Luteolin was also associated with improvements in neutrophilic asthma and IL-36γ inhibition.
Further research in lung tissues was conducted to determine the specific mechanisms underlying the inhibition of IL-36γ production and improvements in asthmatic inflammation mediated by luteolin. The results showed that p38MAPK, JNK and ERK phosphorylation increased to varying degrees in the model of asthma, while p38MAPK, JNK and ERK phosphorylation declined after treatment with luteolin. These results revealed that luteolin could improve neutrophilic asthma by inhibiting IL-36γ and the MAPK pathway.
To further explore the relationship between the improvement of neutrophilic asthma by treatment with luteolin and the inhibition of IL-36γ, in vitro experiments were conducted on Beas-2B cells. First, IL-36γ and IL-1β levels were increased after stimulation with LPS, while the secretion of inflammatory factors decreased with luteolin pretreatment. The phosphorylation levels of p38MAPK, JNK and ERK showed trends similar to those of IL-36γ in Beas-2B cells. This phenomenon suggested that luteolin may inhibit IL-36γ and IL-1β in bronchial epithelial cells via the MAPK pathway in neutrophilic asthma.
IL-36γ increased the secretion of IL-1β and activated the MAPK pathway in Beas-2B cells. Luteolin inhibited the upregulation of IL-1β stimulated by IL-36γ. Combined with the results of LPS stimulation in Beas-2B cells, it can be concluded that IL-36γ may induce an increase in IL-1β via the MAPK pathway. Luteolin may block inflammation by inhibiting this process. IL-36γ siRNA was used to downregulate IL-36γ in Beas-2B cells, and the secretion of IL-1β was reduced compared with that in cells treated with LPS + NC siRNA, indicating that IL-36γ promotes the secretion of IL-1β.
IL-1β is a cytokine that plays a role in inflammatory diseases such as asthma (Simpson et al. Citation2014; Fu et al. Citation2015; Peebles Citation2017); it has a significant role in severe steroid-resistant asthma, which is dominated by neutrophilic inflammation (Kim RY et al. Citation2017). IL-36γ promotes the secretion of the inflammatory factor IL-1β in keratinocyte (HaCaT) cells in psoriasis (Wang W et al. Citation2017). IL-1β can also promote the secretion of IL-36γ (Ahsan et al. Citation2016). Therefore, IL-36γ and IL-1β may be involved in a positive feedback system, which may be broken by luteolin, which blocks the induction of IL-36γ and IL-1β, thereby reducing the secretion of inflammatory factors, as in neutrophilic asthma. Our research showed that the MAPK pathway played an important role in this mechanism, further illustrating the role of IL-36γ and the efficacy of luteolin in the inhibition of asthmatic inflammation.
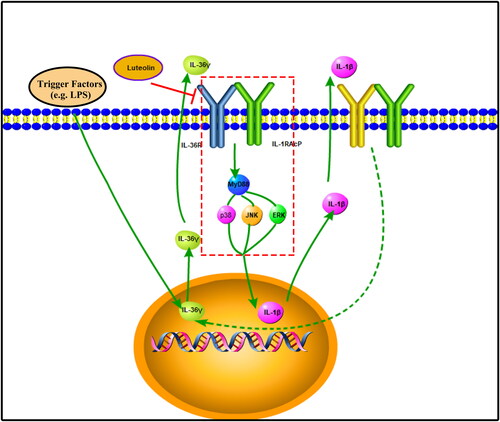
The research showed that the expression of IL-36γ in neutrophilic asthma was significantly increased in animal models, and luteolin played an important anti-inflammatory role in neutrophilic asthma. Combined with in vitro experiments, the specific anti-inflammatory mechanism of luteolin in neutrophil asthma inflammation was further demonstrated. We suspect that in the development and progression of neutrophilic asthma, its trigger factors (e.g., LPS) stimulate the airway and promote the secretion of IL-36γ in airway epithelial cells. IL-36γ up-regulates the expression of IL-1β through the MAPK pathway, and IL-1β promotes the secretion of IL-36γ, thus amplifying the inflammatory response. Luteolin can break this positive feedback by blocking the induction of IL-1β by IL-36γ in human bronchial epithelial cells, thus reducing the secretion of inflammatory factors and alleviating the inflammatory response (). Whether this conjecture is true remains to be further explored to prove that IL-1β can promote the secretion of IL-36γ in the Beas-2B cells.
Conclusions
Luteolin alleviated neutrophilic asthma by inhibiting IL-36γ secretion-mediated MAPK pathways. This study is the first to demonstrate the anti-inflammatory effects of luteolin in neutrophilic asthma and the inflammatory mechanism of neutrophilic asthma was further elucidated, establishing the important role of IL-36γ.
This study expanded our understanding of luteolin pharmacology and provided a theoretical basis for the application of luteolin in the treatment of neutrophilic asthma.
Acknowledgements
The authors would like to thank Shandong Institute of Respiratory Diseases.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Ahsan F, Moura-Alves P, Guhlich-Bornhof U, Klemm M, Kaufmann SH, Maertzdorf J. 2016. Role of interleukin 36γ in host defense against tuberculosis. J Infect Dis. 214(3):464–474.
- Aziz N, Kim MY, Cho JY. 2018. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 225:342–358.
- Bassoy EY, Towne JE, Gabay C. 2018. Regulation and function of interleukin-36 cytokines. Immunol Rev. 281(1):169–178.
- Chen CY, Peng WH, Wu LC, Wu CC, Hsu SL. 2010. Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: implications for therapy of lung fibrosis. J Agric Food Chem. 58(22):11653–11661.
- Chung KF. 2016. Asthma phenotyping: a necessity for improved therapeutic precision and new targeted therapies. J Intern Med. 279(2):192–204.
- Chustz RT, Nagarkar DR, Poposki JA, Favoreto S Jr, Avila PC, Schleimer RP, Kato A. 2011. Regulation and function of the IL-1 family cytokine IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 45(1):145–153.
- Das M, Ram A, Ghosh B. 2003. Luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin sensitized mice. Inflamm Res. 52(3):101–106.
- Fitzpatrick AM, Chipps BE, Holguin F, Woodruff PG. 2020. T2-"low" asthma: overview and management strategies. J Allergy Clin Immunol Pract. 8(2):452–463.
- Fu JJ, McDonald VM, Baines KJ, Gibson PG. 2015. Airway IL-1beta and systemic inflammation as predictors of future exacerbation risk in asthma and COPD. Chest. 148(3):618–629.
- Hudey SN, Ledford DK, Cardet JC. 2020. Mechanisms of non-type 2 asthma. Curr Opin Immunol. 66:123–128.
- Khorasanizadeh M, Eskian M, Gelfand EW, Rezaei N. 2017. Mitogen-activated protein kinases as therapeutic targets for asthma. Pharmacol Ther. 174:112–126.
- Kim EK, Choi EJ. 2015. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 89(6):867–882.
- Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR, Hansbro NG, et al. 2017. Role for NLRP3 inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 196(3):283–297.
- Kim SH, Saba E, Kim BK, Yang WK, Park YC, Shin HJ, Han CK, Lee YC, Rhee MH. 2018. Luteolin attenuates airway inflammation by inducing the transition of CD4+CD25– to CD4+CD25+ regulatory T cells. Eur J Pharmacol. 820:53–64.
- Koss CK, Wohnhaas CT, Baker JR, Tilp C, Przibilla M, Lerner C, Frey S, Keck M, Williams CMM, Peter D, et al. 2021. IL36 is a critical upstream amplifier of neutrophilic lung inflammation in mice. Commun Biol. 4(1):172.
- Li C, Dai J, Dong G, Ma Q, Li Z, Zhang H, Yan F, Zhang J, Wang B, Shi H, et al. 2019. Interleukin-16 aggravates ovalbumin-induced allergic inflammation by enhancing Th2 and Th17 cytokine production in a mouse model. Immunology. 157(3):257–267.
- Lu Y, Xing QQ, Xu JY, Ding D, Zhao X. 2016. Astragalus polysaccharide modulates ER stress response in an OVA-LPS induced murine model of severe asthma. Int J Biol Macromol. 93(Pt A):995–1006.
- Luo Y, Shang P, Li D. 2017. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front Pharmacol. 8:692.
- Mims JW. 2015. Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol. 5(Suppl. 1):S2–S6.
- Neurath MF. 2020. IL-36 in chronic inflammation and cancer. Cytokine Growth Factor Rev. 55:70–79.
- Peebles RS Jr. 2017. Is IL-1beta inhibition the next therapeutic target in asthma? J Allergy Clin Immunol. 139(6):1788–1789.
- Qin X, Liu M, Zhang S, Wang C, Zhang T. 2019. The role of IL-36γ and its regulation in eosinophilic inflammation in allergic rhinitis. Cytokine. 117:84–90.
- Ramadas RA, Ewart SL, Medoff BD, LeVine AM. 2011. Interleukin-1 family member 9 stimulates chemokine production and neutrophil influx in mouse lungs. Am J Respir Cell Mol Biol. 44(2):134–145.
- Samitas K, Zervas E, Gaga M. 2017. T2-low asthma: current approach to diagnosis and therapy. Curr Opin Pulm Med. 23(1):48–55.
- Shen ML, Wang CH, Lin CH, Zhou N, Kao ST, Wu DC. 2016. Luteolin attenuates airway mucus overproduction via inhibition of the GABAergic system. Sci Rep. 6:32756.
- Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. 2014. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 43(4):1067–1076.
- Simpson JL, Scott R, Boyle MJ, Gibson PG. 2006. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 11(1):54–61.
- Svenningsen S, Nair P. 2017. Asthma endotypes and an overview of targeted therapy for asthma. Front Med. 4:158.
- Towne JE, Garka KE, Renshaw BR, Virca GD, Sims JE. 2004. Interleukin (IL)-1F6, IL-1F8, and IL-1F9 signal through IL-1Rrp2 and IL-1RAcP to activate the pathway leading to NF-kappaB and MAPKs. J Biol Chem. 279(14):13677–13688.
- Wang S, Wuniqiemu T, Tang W, Teng F, Bian Q, Yi L, Qin J, Zhu X, Wei Y, Dong J. 2021. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int Immunopharmacol. 94:107460.
- Wang W, Yu X, Wu C, Jin H. 2017. IL-36gamma inhibits differentiation and induces inflammation of keratinocyte via Wnt signaling pathway in psoriasis. Int J Med Sci. 14(10):1002–1007.
- Xie K, Chai YS, Lin SH, Xu F, Wang CJ. 2021. Luteolin regulates the differentiation of regulatory T cells and activates IL-10-dependent macrophage polarization against acute lung injury. J Immunol Res. 2021:8883962.
- Zhang J, Yin Y, Lin X, Yan X, Xia Y, Zhang L, Cao J. 2017. IL-36 induces cytokine IL-6 and chemokine CXCL8 expression in human lung tissue cells: implications for pulmonary inflammatory responses. Cytokine. 99:114–123.