Abstract
Context
Cisplatin, as a first-line treatment for ovarian cancer, is associated with debilitating adverse effects, including nephrotoxic and haematotoxic effects.
Objective
This study determines whether nanocurcumin, combined with cisplatin, would give additional benefit to kidney function and haematological parameters in rats with ovarian cancer.
Materials and methods
Twenty-five Wistar rats were divided into five untreated rats and 20-dimethylbenz(a)anthracene (DMBA)-induced ovarian cancer rats. The 20 ovarian cancer rats were divided into four treatment groups: vehicle, cisplatin, cisplatin-curcumin, and cisplatin-nanocurcumin. Cisplatin was given at the dose of 4 mg/kg BW once weekly, while curcumin or nanocurcumin was administered at 100 mg/kg BW daily for four weeks. At the end of treatment, we analysed kidney function, haematological parameters, and inflammatory and oxidative stress markers from plasma.
Results
Nanocurcumin alleviates the increase in kidney function markers and abnormalities in haematological indices in rats treated with cisplatin. Compared to cisplatin-treated rats, plasma urea levels decreased from 66.4 to 47.7 mg/dL, creatinine levels lowered from 0.87 to 0.82 mg/dL, and neutrophil gelatinase-associated lipocalin (NGAL) levels declined from 8.51 to 3.59 mIU/mg protein. Furthermore, the therapy increased glutathione activities (from 2.02 to 3.23 U/µL), reduced lipid peroxidation (from 0.54 to 0.45 nmol/mL), and decreased plasma TNF-α (from 270.6 to 217.8 pg/mL).
Conclusions
Cisplatin with nanocurcumin in an ovarian cancer rat model may provide additional benefits as a preventive agent against renal impairment and cisplatin-induced haematological toxicity. However, further research is required to prove that using nanocurcumin for a more extended time would not affect its anticancer properties.
Introduction
Ovarian cancer is the most prevalent cancer in women worldwide and the second most common cause of death due to gynaecologic cancer. Data shows that five-year survival rates in ovarian cancer were less than 45% (Webb and Jordan Citation2017; Lheureux et al. Citation2019). With the emergence of targeted therapy in cancer management, platinum-based chemotherapy drugs such as cisplatin are still used as first-line treatment in ovarian cancer (Oronsky et al. Citation2017; Lheureux et al. Citation2019). However, about a third of ovarian cancer patients treated with cisplatin do not respond well. Cisplatin is associated with significant toxicities, including nephrotoxicity, hepatotoxicity, and haematotoxicity (Higuchi and Yanagawa Citation2019; Mikuła-Pietrasik et al. Citation2019; Ben Ayed et al. Citation2020). Some of the known mechanisms of organ toxicities of cisplatin are the activation of inflammatory and oxidative stress pathways (Mikuła-Pietrasik et al. Citation2019; Volarevic et al. Citation2019).
A vast number of studies have investigated the use of combination therapy of cisplatin with phytochemicals to increase the drug’s efficacy and reduce its toxicity (Fang et al. Citation2021; Rajendran et al. Citation2021). Curcumin is among those that have been studied in combination with cisplatin. Studies by Kumar et al. (Citation2017) and Soetikno et al. (Citation2019) reported that curcumin prevents and improves the severity of nephrotoxicity due to cisplatin by inhibiting various pro-inflammatory cytokines, namely NF-kB, TNF-α, and IL-6.
Nanoparticle technology has been used to improve the pharmacokinetic limitation of curcumin. Our research group has succeeded in formulating curcumin nanoparticles encapsulated in chitosan-tripolyphosphate. The curcumin nanoparticle formulation has resulted in a 16-fold increase in the area under the curve of plasma curcumin concentrations and a 10-fold increase in maximum plasma concentrations (Arozal et al. Citation2021; Made Dwi Sandhiutami et al. Citation2021). Further pharmacodynamics in the rat ovarian cancer model also provided data of increased efficacy when nanocurcumin was given in combination with cisplatin (Sandhiutami et al. Citation2020). However, we still did not know whether the combination of cisplatin and curcumin nanoparticles in the ovarian cancer rat model would also be beneficial in reducing nephrotoxicity and haematotoxicity induced by cisplatin treatment. To induce a tumour, we used an aromatic hydrocarbon carcinogen called DMBA (7,12-dimethylbenz[a]anthracene), which causes cancerous mutations by binding to DNA (Huang et al. Citation2012). Thus, this study investigates the effects of nanocurcumin in preserving kidney function and haematological parameters in DMBA-induced ovarian cancer rats treated with cisplatin.
Materials and methods
Nanocurcumin
Curcumin nanoparticles were previously prepared from conventional curcumin (Plamed, China) using the ionic gelation method. The particle size of nanocurcumin obtained was 11–30 nm. The report on the complete characterization of pharmacokinetic profiles of nanocurcumin was provided previously (Arozal et al. Citation2021; Made Dwi Sandhiutami et al. Citation2021).
Animals and treatments
In the current study, 25 Wistar female rats aged 8 weeks and weighing 150–200 g were employed. As previously explained, ovarian cancer was induced in 20 rats using DMBA (Dwi Sandhiutami et al. Citation2018). Five rats were used as healthy controls and given sham operations. The 20 DMBA-induced ovarian cancer rats were divided into four treatment groups (n = 5 per group). The number of rats in each group follows the ethics committee’s recommendation. The four groups were as follows: ovarian cancer rats treated with drug vehicle only (OvCa), cisplatin only (4 mg/kg BW weekly) (OvCa-CPT), cisplatin (4 mg/kg BW weekly) with curcumin (100 mg/kg BW daily) (OvCa-CPT-CUR), and cisplatin (4 mg/kg BW weekly) with nanocurcumin (100 mg/kg BW daily) (OvCa-CPT-NanoCUR). The curcumin and nanocurcumin doses followed those used to determine their efficacy in ovarian cancer (Sandhiutami et al. Citation2020). After four weeks of treatment, rats were sacrificed and analysed for kidney functions, haematological parameters, and plasma inflammatory and oxidative stress markers. All the parameters resulted from the analysis of five rats per group. All analysis from each rat was done in duplicate. The Faculty of Medicine Ethics Committee Universitas Indonesia approved the study protocol before the study commencement, approval number 0531/UN2.F1/ETIK/2018. All the animals had a regular diet and unlimited access to clean water during the experiment.
Biochemical analysis
Urea (Cat no. 1 3101 99 10 021) and creatinine (Cat no. 1 1711 99 10 021) were quantified from rat plasma using colorimetric kits from DiaSysTM (Germany). Using the method recommended by the manufacturer, measurement of malondialdehyde from rat plasma was done using colorimetric analysis at 534 nm (Elabscience, USA, Cat no. E-BC-K025-M).
Enzyme-linked immunoassay analysis
Enzyme-linked immunoassay methods were used to quantify tumour necrosis factor (TNF)-α (Invitrogen, USA, Cat no. KRC 3011), transforming growth factor (TGF)-β (Elabscience, USA, Cat no. E-EL-R0084), neutrophil gelatinase-associated lipocalin (NGAL) (Cusabio Technology LLC, China, Cat no. CSB-E09409r), and follicle-stimulating hormone (FSH) ELISA Kit (Cusabio Technology LLC, China, Cat no. CSB-E06869r), superoxide dismutase (SOD) (Randox, UK, Cat no. SD125), and glutathione (GSH) (Invitrogen, USA, Cat no. EIAGSHC) from rat plasma.
Haematological analysis
Haematology parameters were analysed using fresh whole blood in an animal haematology analyser (VetAutoTM).
Histopathology analysis
After fixation, the histopathological assessment of the ovarium and kidney sections was used using 10% neutral buffer formalin. The samples were then processed into paraffin blocks for staining using haematoxylin-eosin staining. Afterward, the slides were examined under a microscope to assess inflammation in the kidney tissue in the form of renal tubule epithelial cell necrosis. Histopathological assessments were assessed qualitatively (Shea et al. Citation2014).
Data analysis
Data are presented in mean ± standard error of the mean value. The data were tested by first conducting a normality test using Kolmogorov-Smirnov or Shapiro-Wilk and homogeneity tests. Normalized and homogeneous data were then analysed using the one-way ANOVA parametric test and the Tukey comparison test. Data that were not normally distributed and homogeneous was analysed using the Kruskal–Wallis and Mann–Whitney test. A p value of <0.05 is considered statistically significant between groups. The figures in the present manuscript were created using GraphPad Prism version 9 (Prism Academy, USA).
Results
The results of the experiments are arranged as follows: proof of the ovarian cancer model as shown with FSH concentrations and ovarian histopathology (), followed by kidney function and histopathology improvement by nanocurcumin ( and ). Afterward, we investigated the effects of nanocurcumin on oxidative stress markers () and inflammatory markers (). The effect of nanocurcumin on haematology parameters is given in .
Figure 1. Plasma follicle-stimulating hormone concentrations (A) and rat ovarium histopathological appearance (B) after treatment with vehicle, cisplatin and cisplatin in combination with curcumin/nanocurcumin. Normal ovarian histology shown in healthy rats (B1) DMBA-induced rats exhibited sarcomas with or without budding (black arrows) (B2), cisplatin-treated rats mostly presented with endometrioid neoplasms (yellow arrows) (B3), in cisplatin-curcumin (B4) and cisplatin-nanocurcumin (B5) treated rats, most tissues presented with abnormal hyperplasia (white arrows) (B4, B5). Magnification: 400×; N = 5 rats per group; *p < 0.05 vs. Sham; #p < 0.05 vs. OvCa.
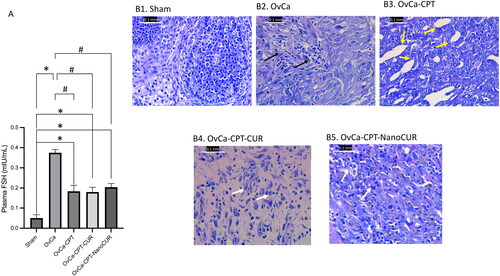
Figure 2. Markers of kidney functions in the rats treated with cisplatin or cisplatin-curcumin or cisplatin-nanocurcumin as shown by (A) urea, (B) creatinine and (C) neutrophil gelatinase-associated lipocalin (NGAL). N = 5 rats per group, *p < 0.05 vs. Sham; #p < 0.05 vs. OvCa; $p < 0.05 vs. OvCa-CPT-Cur.
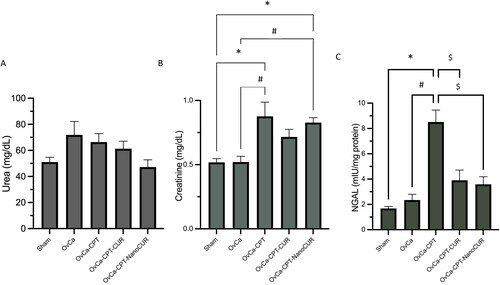
Figure 3. Histopathology of the kidney in ovarian-cancer rats treated with cisplatin or cisplatin- curcumin or cisplatin-nanocurcumin. Magnification: 400×. The red arrow indicates the presence of congestion; the black arrows indicate the presence of necrosis.
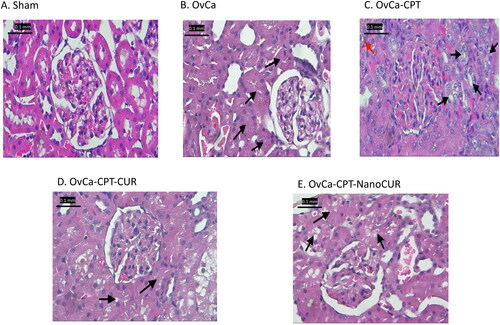
Figure 4. Markers of antioxidant enzyme activity and lipid peroxidation in the rat plasma treated with cisplatin or cisplatin-curcumin or cisplatin-nanocurcumin as shown by (A) GSH, (B) SOD and (C) MDA. N = 5 rats per group; *p < 0.05 vs. Sham; #p < 0.05 vs. OvCa.
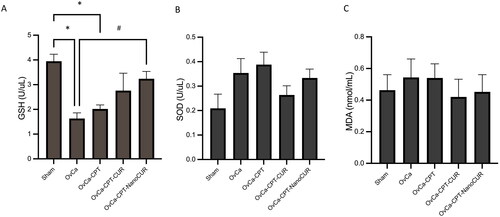
Figure 5. Markers of inflammation in the rat plasma treated with cisplatin or cisplatin-curcumin or cisplatin-nanocurcumin as shown by (A) TNF-α and (B) TGF-β. N = 5 rats per group.
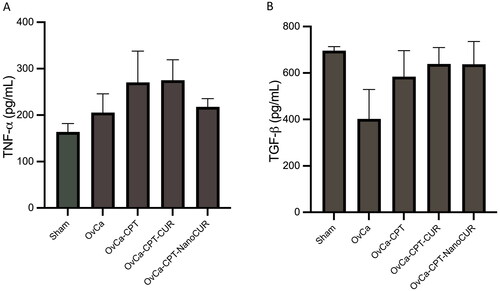
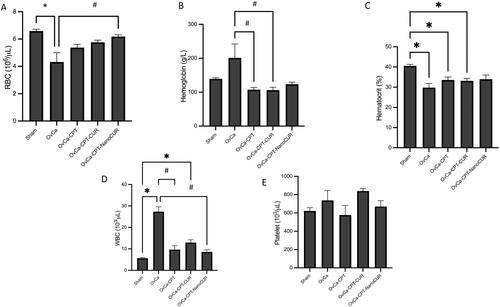
Figure 6. Haematologic parameters in the rats treated with cisplatin or cisplatin-curcumin or cisplatin-nanocurcumin as shown by (A) red blood cell counts; (B) haemoglobin; (C) haematocrit; (D) white blood cell count and (E) platelet count. N = 5 rats per group; *p < 0.05 vs. Sham; #p < 0.05 vs. OvCa.
FSH concentrations and histopathological features of the ovarian tissue in DMBA-induced ovarian cancer rats
The DMBA-induced rats that received no therapy at the end of the treatment had the highest FSH concentrations (Ov-Ca group). Plasma FSH concentrations were reduced during treatment with cisplatin, either alone or in combination with curcumin/nanocurcumin. The reductions were from 0.37 mIU/mL in OvCa group to 0.18 mIU/mL in cisplatin group, 0.18 mIU/mL in cisplatin-curcumin group and 0.20 mIU/mL in cisplatin-nanocurcumin group. However, no statistical significance was found between the three groups of treatment (). DMBA-induced rats presented with sarcomas, either with or without budding, on histological examination. The endometrioid neoplasm was the most common histological finding in the cisplatin-treated ovarian cancer group, whereas atypical hyper-proliferation was seen in the ovarian tissues of the cisplatin-curcumin and cisplatin-nanocurcumin groups ().
Nanocurcumin reduced urea, creatinine, and NGAL in cisplatin-treated ovarian cancer rats
Treatment of ovarian cancer with cisplatin has resulted in an increased kidney function parameter: urea, creatinine, and NGAL (). The addition of curcumin and nanocurcumin to cisplatin reduces urea and creatinine vs cisplatin alone. Surprisingly, curcumin and nanocurcumin have markedly reduced NGAL and have restored NGAL close to the normal value. Compared with cisplatin-treated rats, nanocurcumin reduced plasma urea levels from 66.4 to 47.7 mg/dL, plasma creatinine levels from 0.87 to 0.82 mg/dL and neutrophil gelatinase-associated lipocalin (NGAL) levels from 8.51 to 3.59 mIU/mg protein. Furthermore, compared with cisplatin alone, the combination therapy increases glutathione activities (from 2.02 to 3.23 U/µL), reduces lipid peroxidation (from 0.54 to 0.45 nmol/mL), and decreases plasma TNF-α (from 270.6 to 217.8 pg/mL).
Treatment with nanocurcumin limited the necrosis appearance in kidney histopathology
Microscopic observations of kidney tissues showed that rats treated with cisplatin indicate more severe damage than those treated with cisplatin-curcumin or cisplatin-nanocurcumin (). The kidney tissues of cisplatin-treated rats showed extensive necrosis and proximal tubular dilatation, and nephrosis. There were more minor areas of necrosis in cisplatin-curcumin and cisplatin-nanocurcumin treatment than in the cisplatin group—however, there were no notable differences in cisplatin-curcumin and cisplatin-nanocurcumin treatment.
Nanocurcumin increased glutathione, reduced lipid peroxidation, and decreased SOD
Treatment with nanocurcumin resulted in an enhanced antioxidant enzyme glutathione activity (from 2.02 to 3.23 U/µL) but decreased sodium dismutase (from 0.39 to 0.33 U/µL) as compared to cisplatin. Nevertheless, nanocurcumin reduced lipid peroxidation, as shown by malondialdehyde concentrations (from 0.54 to 0.45 nmol/mL) ().
Nanocurcumin reduced inflammatory markers TNF-α but not TGF-β
Combining nanocurcumin with cisplatin, not curcumin, reduces inflammatory marker TNF-α in plasma that was initially increased by cisplatin treatment only (from 270.6 to 217.8 pg/mL). (). However, both curcumin and nanocurcumin did not alter TGF-β concentrations.
Nanocurcumin restricted haematology abnormalities in rats subjected to cisplatin treatment
The addition of curcumin or nanocurcumin showed a tendency to normalize haematological abnormalities in rats administered with cisplatin and restores some of the haematological parameters closer to the normal values, as seen by the red blood cells, white blood cells, and platelet counts ().
Discussion
Our study group has successfully produced curcumin nanoparticles that resulted in a 16 times increase in the area under the curve of plasma curcumin concentrations and 10 times increase in maximum plasma concentrations (Arozal et al. Citation2021; Made Dwi Sandhiutami et al. Citation2021). Further pharmacodynamics in the rat ovarian cancer model also provided data of increased efficacy when nanocurcumin was given in combination with cisplatin by modulating PI3K/AKT and JAK/STAT3 cascades (Sandhiutami et al. Citation2020). However, it was unknown whether combining cisplatin and curcumin nanoparticles would also be beneficial in preserving renal function and haematological parameters.
Many studies have demonstrated the efficacy of curcumin or nanocurcumin as a nephroprotective agent in cisplatin-induced kidney injury (Chen et al. Citation2019; Soetikno et al. Citation2019; El-Gizawy et al. Citation2020). To our knowledge, none has provided a study utilizing the ovarian-cancer model. Kumar et al. (Citation2017) reported the favourable effect of curcumin as pretreatment to cisplatin in the breast cancer rat model before cisplatin therapy. In this study, our curcumin nanoformulation was combined with cisplatin and administered as treatment in an ovarian cancer model. Our result demonstrates that nanocurcumin provides protective efficacy against renal injury and haematological abnormalities when combined with cisplatin to treat the DMBA- induced ovarian cancer rat model. Nanocurcumin tends to normalize plasma urea and NGAL, along with reduced creatinine. Moreover, nanocurcumin tends to improve renal histopathological abnormalities, as shown by the reduced area of necrosis in the tubules.
Cisplatin was known to cause acute kidney injury after a single or multidose treatment, ranging from 4 to 10 mg/kg BW. In this study, the cisplatin dose selected was enough to produce antitumour efficacy yet still raised concerns regarding bone marrow suppression (Perše and Večerić-Haler Citation2018; Higuchi and Yanagawa Citation2019; Ben Ayed et al. Citation2020; Arita et al. Citation2021). Acute kidney injury in our experimental model was shown by the increase of plasma urea, creatinine, and NGAL in untreated and cisplatin-treated ovarian cancer rats.
When acute renal failure cannot be detected by plasma creatinine due to sustained loss of muscle mass, NGAL can be used as a reliable biomarker. The traditional acute kidney injury indicators, such as plasma urea and creatinine, do not rise until irreversible renal function (Antonucci et al. Citation2014; Shang and Wang Citation2017). Our study demonstrates that nanocurcumin has advantages in reducing urea and NGAL close to normal value, although there was no significant reduction in plasma creatinine. The NGAL lowering effect by nanocurcumin indicates the success of nanocurcumin in preventing further irreversible kidney damage.
In renal epithelial cells, cisplatin was then metabolically activated, which altered the cellular antioxidant system (as evidenced by decreased superoxide dismutase and glutathione levels). Furthermore, the toxic metabolites of cisplatin would interact with various cellular components and macromolecules, resulting in functional and structural damage, as shown by increased lipid peroxidation (Perše and Večerić-Haler Citation2018). Our experiment demonstrated that treatment with cisplatin causes an evident decline in glutathione but not superoxide dismutase and malondialdehyde. Nanocurcumin increased glutathione, but there was no notable difference when compared to curcumin.
In terms of superoxide dismutase, studies found the dual role of SOD in cancer (Amano et al. Citation2019, Citation2021). SOD enzymes regulate the levels of a range of reactive oxygen and nitrogen species, thus reducing the potential toxicity of cytotoxic drugs in cancer cells. On the other hand, mitochondrial superoxide dismutase (SOD2) is required for proper mitochondrial function and leads to chemotherapeutic resistance (Younus Citation2018; Amano et al. Citation2021). Amano et al.’s (Citation2019) reported that the increased superoxide dismutase expression in ovarian cancer has a poor prognosis. Their study showed that treatment with cisplatin resulted in a systemic increase in superoxide dismutase, which might signal drug resistance, and we demonstrate that the addition of curcumin or nanocurcumin tends to reduce SOD back to normal (Amano et al. Citation2019).
Treatment with cisplatin also increased the lipid peroxidation process, as indicated by the increase in malondialdehyde. Lipid peroxidation is a process of damaging cells that starts with the oxidation of polyunsaturated fatty acids and then continues with auto-catalytic chain reactions. MDA may also cause damage to membrane proteins by neutralizing enzymes and receptors bound to its membranes leading to polymerization of the membrane components (Mikuła-Pietrasik et al. Citation2019). Curcumin is known to have the ability to inhibit lipid peroxidation in various in vivo experiments (Lee et al. Citation2017; Alizadeh and Kheirouri Citation2019). However, in our study, nanocurcumin did not increase the efficacy of curcumin in decreasing lipid peroxidation.
Inflammation is one of the pathophysiological mechanisms in which cisplatin causes renal toxicity. Various pro-inflammatory cytokines and chemokines are secreted during the inflammatory process, such as TNF-α and TGF-β. TNF-α acts as a critical upstream regulator of the cisplatin-induced inflammatory response. TNF-α is involved in coordinating the activation of pro-inflammatory cytokines, an essential function of this protein. TNF-α also plays a role in inducing the expression of various molecules that play a role in the migration of inflammatory cells in the tissue, such as intercellular adhesion molecule 1 (ICAM-1), E-selectin, and vascular cell adhesion molecule 1 (VCAM-1). In addition, TNF-α triggers tissue damage and renal tubular cell death through its receptors, TNFR1 and TNFR2 (Qi et al. Citation2019; McSweeney et al. Citation2021). While TGF-β downstream cascades, Smad3 upregulation will increase necrosis and apoptosis after cisplatin treatment (Volarevic et al. Citation2019; Yang et al. Citation2021). Researchers suggested that TGF-β pathways might balance cisplatin’s antitumour efficacy and nephrotoxicity (Volarevic et al. Citation2019). Nanocurcumin protects organs in the present experimental model by reducing inflammatory markers TNF-α vs. cisplatin, not TGF-β. We propose that the effect of curcumin and nanocurcumin in modulating TNF-α and TGF-β is related to curcumin anticancer activities (Ashrafizadeh et al. Citation2020). Our previous study had shown that in the ovaries, curcumin, and nanocurcumin significantly reduce TGF-β concentrations (Made Dwi Sandhiutami et al. Citation2021), while in our present data, TGF-β in the plasma remained unchanged.
In cancer patients, haematological toxicity is also a frequent consequence of chemotherapy, including cisplatin (Vitzthum et al. Citation2020). Cisplatin haematological toxicity is reversible and dose-related. Generally, in some patients, the toxicity is mild but, in some cases, can be progressive upon dose accumulation. Previous studies reported anaemia, leucopoenia, and thrombocytopoenia (Ruggiero et al. Citation2019). Our present studies proved that nanocurcumin protects against haematotoxicity induced by ovarian cancer models and cisplatin treatment. Naik et al. (Citation2011) explained that the haematological protective effect of curcumin might be due to its inhibitory properties on iron-catalysed lipid peroxidation and further decreased haemolysis in erythrocytes.
Aside from the beneficial pharmacodynamic interaction between nanocurcumin and cisplatin, no study has reported the pharmacokinetic alterations between the two drugs. Curcumin has been described to interact with several anticancer drugs such as paclitaxel, docetaxel, etoposide, tamoxifen, and everolimus (Bahramsoltani et al. Citation2017). Several studies have shown that curcumin may function as an inhibitor for several CYP450 isoforms, particularly CYP1A2, CYP2D6, CYP2C9, and CYP3A4 (Appiah-Opong et al. Citation2007; Bamba et al. Citation2011; Shamsi et al. Citation2015). When given in combination with curcumin, other drugs that are substrates of CYPs will result in higher plasma concentrations due to the inhibition of CYPs (Bahramsoltani et al. Citation2017). Cisplatin is not metabolized via the cytochrome P450 (CYP450) enzyme; however, it may interact with CYP2E1 to produce ROS and cause organ toxicity (Quintanilha et al. Citation2017). Therefore, the curcumin and cisplatin combination will not cause any pharmacokinetic alterations.
Further analysis of the molecular mechanism regarding the upstream pathway leading to the antioxidative and anti-inflammatory effects of nanocurcumin should be further investigated using renal and ovarian tissue homogenates.
Conclusions
Our study confirmed that treating cisplatin and nanocurcumin in a rat model of ovarian cancer provides added benefit in preserving renal function and haematological parameters by increasing glutathione activities and reducing systemic anti-inflammatory markers. Further studies investigating the mechanism of nanocurcumin in providing protective effects to normal cells and antitumour efficacy in cancer models are needed to understand our findings better.
Acknowledgment
The authors thank Enago (www.enago.com) for the English language review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data supporting the findings of this study are available upon request to the corresponding author.
Additional information
Funding
References
- Alizadeh M, Kheirouri S. 2019. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: a comprehensive meta-analysis of randomized controlled trials. Biomedicine. 9(4):22–23.
- Amano T, Chano T, Isono T, Kimura F, Kushima R, Murakami T. 2019. Abundance of mitochondrial superoxide dismutase is a negative predictive biomarker for endometriosis-associated ovarian cancers. World J Surg Onc. 17(1):1–7.
- Amano T, Murakami A, Murakami T, Chano T. 2021. Antioxidants and therapeutic targets in ovarian clear cell carcinoma. Antioxidants. 10(2):115–187.
- Antonucci E, Lippi G, Ticinesi A, Pigna F, Guida L, Morelli I, Nouvenne A, Borghi L, Meschi T. 2014. Neutrophil gelatinase-associated lipocalin (NGAL): a promising biomarker for the early diagnosis of acute kidney injury (AKI). Acta Biomed. 85(3):289–294.
- Appiah-Opong R, Commandeur JNM, van Vugt-Lussenburg B, Vermeulen NPE. 2007. Inhibition of human recombinant cytochrome P450s by curcumin and curcumin decomposition products. Toxicology. 235(1-2):83–91.
- Arita M, Watanabe S, Aoki N, Kuwahara S, Suzuki R, Goto S, Abe Y, Takahashi M, Sato M, Hokari S, et al. 2021. Combination therapy of cisplatin with cilastatin enables an increased dose of cisplatin, enhancing its antitumor effect by suppression of nephrotoxicity. Sci Rep. 11(1):710–750.
- Arozal W, Louisa M, Rahmat D, Chendrana P, Sandhiutami NMD. 2021. Development, characterization, and pharmacokinetic profile of chitosan-sodium tripolyphosphate nanoparticle-based drug delivery systems for curcumin. Adv Pharm Bull. 11(1):77–85.
- Ashrafizadeh M, Zarrabi A, Hushmandi K, Zarrin V, Moghadam ER, Hashemi F, Makvandi P, Samarghandian S, Khan H, Hashemi F, et al. 2020. Toward regulatory effects of curcumin on transforming growth factor-beta across different diseases: a review. Front Pharmacol. 11:585413–585413.
- Bahramsoltani R, Rahimi R, Farzaei MH. 2017. Pharmacokinetic interactions of curcuminoids with conventional drugs: a review. J Ethnopharmacol. 209:1–12.
- Bamba Y, Yun YS, Kunugi A, Inoue H. 2011. Compounds isolated from Curcuma aromatica Salisb. inhibit human P450 enzymes. J Nat Med. 65(3-4):583–587.
- Ben Ayed W, Ben Said A, Hamdi A, Mokrani A, Masmoudi Y, Toukabri I, Limayem I, Yahyaoui Y. 2020. Toxicity, risk factors, and management of cisplatin-induced toxicity: a prospective study. J Oncol Pharm Pract. 26(7):1621–1629.
- Chen S, Gao W, Zhang MJ, Chan JY, Wong TS. 2019. Curcumin enhances cisplatin sensitivity by suppressing NADPH oxidase 5 expressions in human epithelial cancer. Oncol Lett. 18(2):2132–2139.
- Dwi Sandhiutami NM, Arozal W, Louisa M, Rahmat D, Wuyung PE, Ulum MF. 2018. Induction of epithelial ovarian cancer by implantation of 7,12-dimethylbenz(a)anthracene (DMBA) coated silk in rats. JYP. 11(1):56–61.
- El-Gizawy MM, Hosny EN, Mourad HH, Razik A-E, Amira N. 2020. Curcumin nanoparticles ameliorate hepatotoxicity and nephrotoxicity induced by cisplatin in rats. Naunyn Schmiedebergs Arch Pharmacol. 393(10):1941–1953.
- Fang C-y, Lou D-y, Zhou L-q, Wang J-c, Yang B, He Q-j, Wang J-j, Weng Q-j 2021. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 42(12):1951–1969.
- Higuchi K, Yanagawa T. 2019. Evaluating the dose of cisplatin responsible for causing nephrotoxicity. PLoS One. 14(4):e0215757.
- Huang Y, Jiang W, Wang Y, Zheng Y, Cong Q, Xu C. 2012. Enhanced efficacy and specificity of epithelial ovarian carcinogenesis by embedding a DMBA-coated cloth strip in the ovary of rat. J Ovarian Res. 5(1):1–8.
- Kumar P, Barua CC, Sulakhiya K, Sharma RK. 2017. Curcumin ameliorates cisplatin-induced nephrotoxicity and potentiates its anticancer activity in SD rats: potential role of curcumin in breast cancer chemotherapy. Front Pharmacol. 8(132):112–132.
- Lee H-Y, Kim S-W, Lee G-H, Choi M-K, Chung H-W, Lee Y-C, Kim H-R, Kwon HJ, Chae H-J. 2017. Curcumin and Curcuma longa L. extract ameliorates lipid accumulation by regulating the endoplasmic reticulum redox and ER stress. Sci Rep. 7(1):6513–6514. 1
- Lheureux S, Braunstein M, Oza AM. 2019. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 69(4):280–304.
- Made Dwi Sandhiutam N, Arozal W, Louisa M, Rahmat D. 2021. Determine curcumin concentration in organ rats and in ovaries at ovarian cancer model rats using ultra-performance liquid chromatography-mass spectrometry (MS)/MS. Pharm Sci Asia. 48(1):37–45.
- McSweeney KR, Gadanec LK, Qaradakhi T, Ali BA, Zulli A, Apostolopoulos V. 2021. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 13(7):1542–1572.
- Mikuła-Pietrasik J, Witucka A, Pakuła M, Uruski P, Begier-Krasińska B, Niklas A, Tykarski A, Książek K. 2019. Comprehensive review on how platinum- and taxane-based chemotherapy of ovarian cancer affects biology of normal cells. Cell Mol Life Sci. 76(4):681–697.
- Naik SR, Thakare VN, Patil SR. 2011. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity, and cardiotoxicity in rats: evidence of its antioxidant property. Exp Toxicol Pathol. 63(5):419–431.
- Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. 2017. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 34(6):103–110.
- Perše M, Večerić-Haler Ž. 2018. Cisplatin-induced rodent model of kidney injury: characteristics and challenges. Biomed Res Int. 2018(1462802):1462802–1462829.
- Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. 2019. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 32(8):1469–1486.
- Quintanilha J, Sousa V, Visacri M, Amaral L, Santos R, Zambrano T, Salazar L, Moriel P. 2017. Involvement of cytochrome P450 in cisplatin treatment: implications for toxicity. Cancer Chemother Pharmacol. 80(2):223–233.
- Rajendran G, Taylor JA, Woolbright BL. 2021. Natural products as a means of overcoming cisplatin chemoresistance in bladder cancer. Cancer Drug Resist. 4:69–84.
- Ruggiero A, Trombatore G, Triarico S, Capozza MA, Coccia P, Attina G, Mastrangelo S, Maurizi P. 2019. Cisplatin toxicity in children with malignancy. Biomed Pharmacol J. 12(04):1603–1611.
- Sandhiutami NMD, Arozal W, Louisa M, Rahmat D, Wuyung PE. 2020. Curcumin nanoparticle enhances the anticancer effect of cisplatin by inhibiting PI3K/AKT and JAK/STAT3 pathway in rat ovarian carcinoma induced by DMBA. Front Pharmacol. 11(603235):603213–603235.
- Shamsi S, Chen Y, Lim LY. 2015. Characterization and biological properties of NanoCUR formulation and its effect on major human cytochrome P450 enzymes. Int J Pharm. 495(1):194–203.
- Shang W, Wang Z. 2017. The update of NGAL in acute kidney injury. Curr Protein Pept Sci. 18(12):1211–1217.
- Shea K, Stewart S, Rouse R. 2014. Assessment standards: comparing histopathology, digital image analysis, and stereology for early detection of experimental cisplatin-induced kidney injury in rats. Toxicol Pathol. 42(6):1004–1015.
- Soetikno V, Sari SDP, Ul Maknun L, Sumbung NK, Rahmi DNI, Pandhita BAW, Louisa M, Estuningtyas A. 2019. Pretreatment with curcumin ameliorates cisplatin-induced kidney damage by suppressing kidney inflammation and apoptosis in rats. Drug Res. 69(2):75–82.
- Vitzthum LK, Heide ES, Park H, Williamson CW, Sheridan P, Huynh-Le M-P, Sirak I, Wei L, Tarnawski R, Mahantshetty U. 2020. Comparison of hematologic toxicity and bone marrow compensatory response in head and neck vs. cervical cancer patients undergoing chemoradiotherapy. Front Oncol. 10(1179):1–9.
- Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N. 2019. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 26(1):14–25.
- Webb PM, Jordan SJ. 2017. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 41:3–14.
- Yang Q, Gao L, Hu X, Wang J, Zhang Y, Dong Y, Lan HY, Meng X. 2021. Smad3-targeted therapy protects against cisplatin-induced AKI by attenuating programmed cell death and inflammation via a NOX4-dependent mechanism. Kidney Dis. 7(5):372–390.
- Younus H. 2018. Therapeutic potentials of superoxide dismutase. Int J Health Sci. 12(3):88–93.
