Abstract
Context
Kazinol B (KB), an isoprenylated flavan derived from Broussonetia kazinoki Sieb. (Moraceae) root, has long been used in folk medicine.
Objective
This study examines the protective effects of KB and its underlying mechanisms in hypoxia and reoxygenation (H/R)-induced cardiac injury in H9c2 rat cardiac myoblasts.
Materials and methods
H9c2 cells were incubated with various concentrations of KB (0, 0.3, 1, 3, 10 and 30 μM) for 2 h and then subjected to H/R insults. The protective effects of KB and its underlying mechanisms were explored.
Results
KB significantly elevated cell viability (1 μM, 1.21-fold; 3 μM, 1.36-fold, and 10 μM, 1.47-fold) and suppressed LDH release (1 μM, 0.77-fold; 3 μM, 0.68-fold, and 10 μM, 0.59-fold) in H/R-induced H9c2 cells. Further, 10 μM KB blocked apoptotic cascades, as shown by the Annexin-V/PI (0.41-fold), DNA fragmentation (0.51-fold), caspase-3 (0.52-fold), PARP activation (0.27-fold) and Bax/Bcl-2 expression (0.28-fold) assays. KB (10 μM) downregulated reactive oxygen species production (0.51-fold) and lipid peroxidation (0.48-fold); it upregulated the activities of GSH-Px (2.08-fold) and SOD (1.72-fold). KB (10 μM) induced Nrf2 nuclear accumulation (1.94-fold) and increased ARE promoter activity (2.15-fold), HO-1 expression (3.07-fold), AKT (3.07-fold) and AMPK (3.07-fold) phosphorylation. Nrf2 knockdown via using Nrf2 siRNA abrogated KB-mediated protective effects against H/R insults. Moreover, pharmacological inhibitors of AKT and AMPK also abrogated KB-induced Nrf2 activation and its protective function.
Discussion and conclusions
KB prevented H/R-induced cardiomyocyte injury via modulating the AKT and AMPK-mediated Nrf2 induction. KB might be a promising drug candidate for managing ischemic cardiac disorders.
Introduction
Coronary heart disease is one of the most common causes of mortality and morbidity worldwide (Moran et al. Citation2014; Thomas et al. Citation2018). Myocardial ischemia injury is the primary factor causing cardiovascular dysfunctions in patients suffering from coronary heart diseases (Moran et al. Citation2014; Thomas et al. Citation2018). Extreme intracellular reactive oxygen species (ROS) production and cellular injury directly affect cellular structures and functions in myocardial tissues with ischemic and reperfusion insults (Kalogeris et al. Citation2012; Chatauret et al. Citation2014). Thus, preventing oxidative stress and cardiomyocyte injury is one of the effective strategies for treating myocardial ischemia and coronary heart disease (Kalogeris et al. Citation2012; Chatauret et al. Citation2014).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive transcriptional factor that modulates cellular antioxidant defence and maintains redox homeostasis (Bubb et al. Citation2017; Strom and Chen Citation2017; Chen G et al. Citation2019). Under stress or stimulation, Nrf2 dissociates, translocates into the nucleus, and binds to the antioxidant response element (ARE) in the promoters of genes encoding for antioxidants and detoxifications that protect against oxidative stress damage, including heme oxygenase-1 (HO-1), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) (Bubb et al. Citation2017; Strom and Chen Citation2017; Chen G et al. Citation2019). The phosphatidylinositol 3-hydroxy kinase/protein kinase B (PI3K/AKT) axis is an essential signalling pathway involved in myocardial ischemia–reperfusion injury (Li X et al. Citation2018; Huang J et al. Citation2019). The AMP-activated protein kinase (AMPK) signalling pathway, a critical regulator of energetic stress, controls glucose uptake and glycolysis and protects myocardial tissue from ischemic injury (Shibata et al. Citation2005; Penumathsa et al. Citation2009; Feng et al. Citation2018). The phosphorylation of AKT and AMPK induces the activation of Nrf2 pathways (Chen X et al. Citation2018; Nudelman et al. Citation2020). Thus, Nrf2, AKT and AMPK are targeted to develop novel agents for myocardial ischemia and coronary heart disease.
Kazinol B (KB, ) is an isoprenylated flavan that is derived from the root of Broussonetia kazinoki Sieb. (Moraceae) (Ryu et al. Citation2003; Lee H et al. Citation2016). The B. kazinoki plant is widely distributed in the East Asia, especially in China, Korea and Japan. This plant has a long history of being used as a folk medicine (Zhang PC et al. Citation2001; Lee DY et al. Citation2010). Previous studies demonstrate that KB suppresses oxidative stress and inflammatory response in lipopolysaccharide-induced macrophages (Ryu et al. Citation2003). Moreover, KB confers antidiabetic effects by modulating the AKT and AMPK pathways in mouse 3T3-L1 preadipocytes (Lee H et al. Citation2016). Thus, KB-mediated cytoprotective effects and activation of AKT and AMPK pathways protect against myocardial ischemia. Hence, in this study, we investigate whether KB could protect hypoxia and reoxygenation (H/R)-induced ischemic injury in H9c2 rat cardiac myoblasts. We also explored how KB modulates the Nrf2, AKT and AMPK signalling pathways to mediate its protective effect.
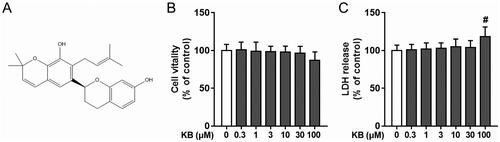
Figure 1. Chemical structure of KB and its effects on cell viability and cytotoxicity in H9c2 cells. (A) Chemical structure of KB. (B, C) H9c2 cells were treated with various doses of KB for 24 h, and cell viability and cytotoxicity were measured using the CCK8 and LDH assays, respectively. The cell viability and LDH release are expressed as % of vehicle control. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). #p< 0.05 vs. the vehicle group.
Materials and methods
Chemicals and reagents
Kazinol B (purity in HPLC ≥ 98%, the chemical structure is shown in ) was purchased from Weikeqi Biotechnology (Chengdu, China). LY294002 and Compound-C were purchased from Selleck (Houston, TX). DMEM and FBS were obtained from Gibco (Grand Island, NY).
Cell culture and treatment
The H9c2 rat cardiac myoblasts were obtained from the American Type Culture Collection or ATCC (Manassas, VA) and maintained in DMEM with 10% FBS in a humidified atmosphere at 37 °C with 5% CO2 (95% air). The chemical compound, KB, was first dissolved in dimethyl sulphoxide (DMSO) and then added to the medium. The vehicle control was dissolved with DMSO (final concentration was 0.1%, v/v).
Hypoxia and reoxygenation
Hypoxia and reoxygenation were induced according to previous studies (Peng et al. Citation2019; Jia et al. Citation2022). Briefly, the H9c2 cells were incubated with the serum-free and glucose-free medium and cultured in a hypoxic chamber (95% N2 and 5% CO2, STEMCELL Technologies, Vancouver, Canada) for 6 h. Next, the cells were subjected to reoxygenation with a normal culture medium in a normal incubator (95% air and 5% CO2) for 24 h.
Cell viability and cytotoxicity
Viability and toxicity were measured using the CCK8 (Dojindo, Kumamoto, Japan) and LDH assay kits (Roche, Mannheim, Germany). In brief, cells were seeded into the culture plate for 24 h and treated with KB or specific inhibitors followed by the H/R insults. Subsequently, the supernatant was removed for the LDH assay, while the cells were subjected to the CCK8 assay. The absorbances were measured at 450 nm and 490 nm for the CCK-8 and LDH assay, respectively.
Annexin V-FITC and PI apoptosis analysis
Cell apoptosis and death were measured using the Annexin V-FITC and PI apoptosis detection kits (Beyotime, Shanghai, China). Quantitative analysis was performed using a C6 flow-cytometer (BD Biosciences, San Jose, CA).
Determination of DNA fragmentation and caspase-3 activity
DNA fragmentation was measured using the DNA Fragmentations ELISA kit (Roche, Mannheim, Germany). The absorbance (490 nm) was detected and represented as DNA fragmentations. Caspase-3 activity was analysed using the caspase-3 activity assay kit (Beyotime, Shanghai, China). The absorbance at 405 nm was measured, and the data were normalized to the protein concentrations.
Measurement of cytochrome c release
The cytoplasm and mitochondria of cells were separated using the mitochondria isolation kit (Beyotime, Shanghai, China). Cytochrome c release in the cytoplasm and mitochondria was measured using the cytochrome C Quantikine ELISA kit (R&D Systems, Minneapolis, MN). The result was normalized to the protein concentration.
Measurement of ATP synthesis
ATP synthesis was measured using the luciferin-luciferase-based enhanced ATP assay kit (Beyotime, Shanghai, China). In brief, cells were collected and prepared with lysis buffers, centrifuged, and the supernatants were collected for ATP synthesis assay. The luminescence was monitored for 3 min using a FlexStation3 multi-mode microplate-reader (Molecular Devices, Sunnyvale, CA).
Measurements of Δψm, ROS, lipid peroxidation, GSH-Px and SOD
The mitochondrial membrane potential (Δψm) was monitored using the fluorescent probe JC-1 (Invitrogen, Carlsbad, CA). The fluorescence intensity was detected, and the Δψm was represented by the ratio of JC-1 red/green intensity. ROS production was measured using the fluorescent probe CM-H2DCFDA (Invitrogen, Carlsbad, CA). The lipid peroxidation was determined by malondialdehyde (MDA) levels using the MDA assay kits (Beyotime, Shanghai, China).
ARE luciferase activity assay
H9c2 cells were transfected with the pARE-Luc reporter plasmid (SABiosciences, Frederick, MD) for 24 h and then treated with KB followed by the H/R insults. The cell samples were prepared, and the luciferase activity of the samples was measured using the Dual-Luciferase reporter assay systems (Promega, Madison, WI).
Nrf2 siRNA transfection
The cells were transfected with the Nrf2 siRNA (100 nM) or scrambled control siRNA (Santa Cruz, Santa Cruz, CA) for 36 h using Lipofectamine 3000 (Invitrogen, Carlsbad, CA), after which the cells were collected for further tests.
Quantitative PCR assay
Total RNA and cDNA were prepared using the HighPure RNA isolation and Transcriptor cDNA synthesis kits (Roche, Mannheim, Germany), respectively, according to the manufacturer’s protocol. qPCR was performed using the FastStart Universal SYBR Green Master reagents in the 7900 HT System (Applied Biosystems, Foster City, CA). The relative fold changes were normalized to Gapdh and calculated as folds of the control group using the 2–ΔΔCt method. The primer sequences used were as follows: HO-1, forward primer: CTGGAAGAGGAGATAGAGCGAA, reverse primer: TCTTAGCCTCTTCTGTCACCCT; Gapdh, forward primer: GACATGCCGCCTGGAGAAAC, reverse primer: AGCCCAGGATGCCCTTTAGT.
Western blot assay
The cells were lysed using the RIPA lysis buffer. The protein concentration was measured by the Pierce BCA protein assay kit (Thermo Scientific, San Diego, CA). Same amounts of protein were electrophoresed on SDS-PAGE and then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA). The membrane was then incubated with different primary and secondary antibodies at room temperature for 1 h. The primary antibodies, including cleaved-caspase 3, cleaved-PARP, Bax, Bcl-2, p-AKT, t-AKT, p-AMPKα, t-AMPKα, Lamin B1, GAPDH and β-actin, were obtained from Cell Signaling Technology (Boston, MA). The Nrf2 and HO-1 antibodies were obtained from Abcam (Waltham, MA). The western blot band was visualized using the ECL assay kit (GE Healthcare, Milwaukee, WI). Finally, the blot was analysed and quantified using the Image Lab software (Bio-Rad, Hercules, CA).
Statistical analysis
Statistical analyses were performed with one-way ANOVA followed by Bonferroni’s multiple comparisons test using GraphPad Prism 7.00 software (GraphPad Prism, La Jolla, CA). All experiments were performed three times and in triplicates. Data are presented as mean ± SEM. A p value < 0.05 was considered statistically significant.
Results
Effects of KB on cell viability and cytotoxicity in H9c2 cells
We measured the cytotoxic effects of KB on H9c2 cells using the CCK8 and LDH assays. As shown in , no cytotoxicity was observed in cells treated with KB up to 30 µM for 24 h. However, we observed cytotoxicity at a KB dose of 100 µM. Hence, we chose KB doses up to 30 µM (0.3, 1, 3, 10 and 30 μM) for further experiments.
KB exerted protective effects against H/R-induced cell death and apoptosis in H9c2 cells
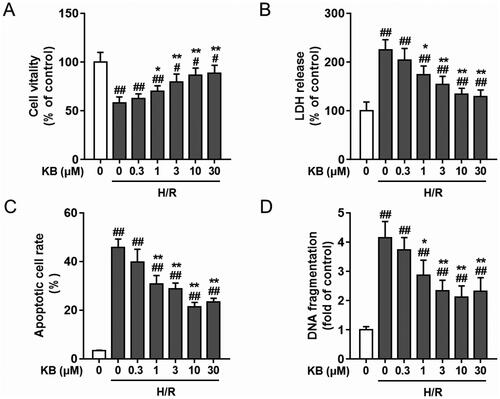
Next, we investigated the effects of KB on cell death and apoptosis in H9c2 cells subjected to H/R insults. Based on previous reports and our preliminary experiments, we induced cardiac injury in H9c2 cells by subjecting the cells to hypoxia for 6 h, followed by reoxygenation for 24 h (Peng et al. Citation2019; Jia et al. Citation2022). First, KB (1, 3, 10 and 30 μM) inhibited the H/R-induced decline in cell viability and H/R-induced LDH release in a dose-dependent manner (, respectively). The inhibitory effects of KB against cell injury were detected by Annexin-V/PI and DNA fragmentation assays. As shown in , the apoptotic and dead populations decreased upon KB treatment in a dose-dependent manner (1, 3, 10 and 30 μM, all p< 0.05 compared with the H/R-treated group). KB (1, 3, 10 and 30 μM) also alleviated H/R-induced DNA fragmentation in a dose-dependent manner in H9c2 cells (). However, 10 and 30 μM of KB exerted almost the same protective effects on H9c2 cells with H/R insults. Hence, we selected a KB dose of 10 μM for our further experiments.
Figure 2. KB protected H9c2 cells against H/R-induced cell death and apoptosis. H9c2 cells were treated with various doses of KB for 2 h and then subjected to H/R insults. (A) The cell viability was measured via CCK8 assay. (B) Cytotoxicity was measured via the LDH assay. The cell viability and LDH release are expressed as % of vehicle control. (C) Cell apoptosis was measured by Annexin-V-FITC/PI flow cytometry assay. (D) The cytoplasmic histone-associated DNA fragmentation was measured by ELISA. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). #p< 0.05 and ##p< 0.01 vs. the vehicle group. *p< 0.05 and **p< 0.01 vs. the H/R-treated group.
KB blocked apoptotic cascades in H/R-induced H9c2 cells
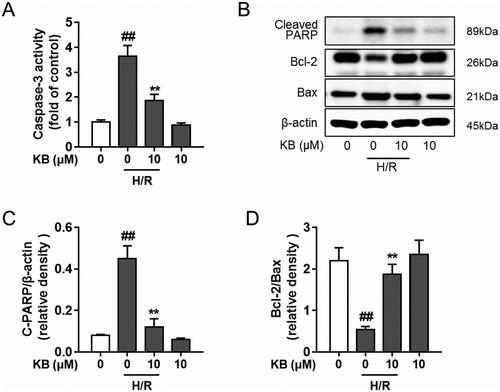
As shown in , the H/R insult significantly increased caspase-3 activity in H9c2 cells, whereas KB significantly suppressed the caspase-3 activity. KB treatment also significantly down-regulated the protein level of cleaved PARP in cells with H/R insults (). Further, KB reversed the H/R-induced decline in the ratio of Bcl-2/Bax ().
Figure 3. KB blocked H/R-induced apoptotic cascades in H9c2 cells. H9c2 cells were treated with KB and then subjected to H/R insults. (A) The caspase-3 activity was measured by the caspase-3 assay. The results are expressed as fold of control. (B–E) Protein was prepared, and the level of cleaved PARP, Bcl-2 and Bax was detected by western blot. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group.
KB alleviated H/R-induced mitochondrial dysfunction in H9c2 cells
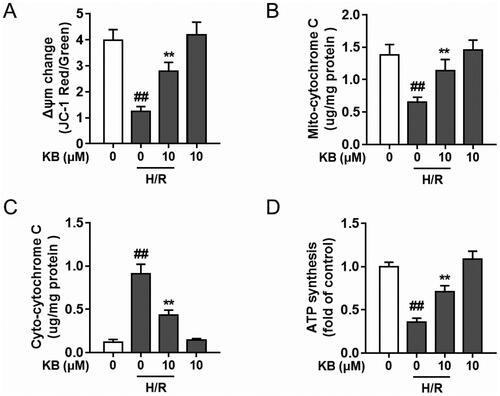
The mitochondrial membrane potential (Δψm) collapses during an apoptotic cascade (Mansingh et al. Citation2018). As shown in , KB significantly attenuated Δψm disruption by H/R insults in H9c2 cells, as evidenced by the increase in the ratio of red to green fluorescence. Further, KB significantly inhibited H/R-induced cytochrome c release from mitochondria into the cytoplasm in H9c2 cells (). KB also rescued the decrease in H/R-induced dysfunction in ATP production in H9c2 cells ().
Figure 4. KB alleviated H/R-induced mitochondrial dysfunction in H9c2 cells. H9c2 cells were treated with KB and then subjected to H/R insults. (A) The Δψm change was determined by the JC-1 assay, and quantification is expressed as a ratio of JC-1 aggregate to monomer (red to green) fluorescence intensity. (B, C) The mitochondria and cytoplasm were isolated and the mitochondrial cytochrome c (B) and cytoplasmic cytochrome c (C) were measured. (D) The ATP synthesis levels were measured. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group.
KB attenuated H/R-induced oxidative stress in H9c2 cells
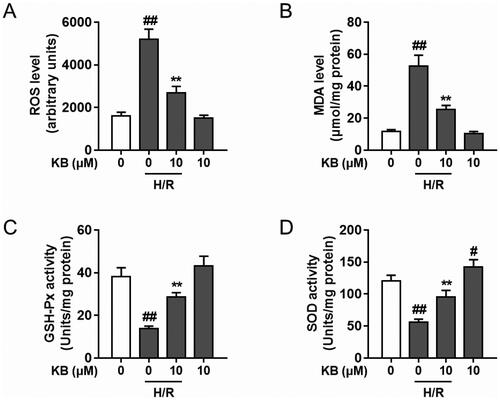
Oxidative stress is critical in H/R-induced cellular damage (Zhou P et al. Citation2022). Therefore, we further investigated the antioxidant capability of KB. KB significantly suppressed ROS production and H/R-aroused lipid peroxidation (MDA production) in H9c2 cells subjected to H/R insults (, respectively). Furthermore, KB improved GSH-Px and SOD activity in H/R-induced H9c2 cells ().
Figure 5. KB attenuated H/R-induced oxidative stress in H9c2 cells. H9c2 cells were treated with KB and then subjected to H/R insults. (A) ROS generation was determined, and fluorescence intensity was measured and expressed as an arbitrary unit. (B) The lipid peroxidation level was measured by determining the MDA level. (C–E) The activity of GSH-Px and SOD was determined and expressed as Unit/mg protein. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). #p< 0.05 and ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group.
KB promoted Nrf2 nuclear translocation and activated the Nrf2/ARE/HO-1 pathway in H/R-induced H9c2 cells
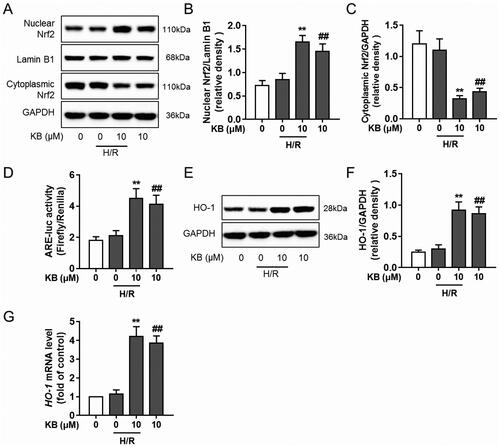
The Nrf2/ARE pathway is crucial for the protective effects of various natural products in cardiomyocyte injury models (Lu et al. Citation2022). Thus, we examined the effects of KB on the Nrf2/ARE axis. KB significantly facilitated Nrf2 nuclear accumulation () and improved ARE promoter activity () in H/R-induced H9c2 cells. Further, KB up-regulated the HO-1 protein () and mRNA expression () in H/R-induced H9c2 cells.
Figure 6. KB promoted the Nrf2 nuclear translocation and activated the Nrf2/ARE/HO-1 pathway in H/R-induced H9c2 cells. H9c2 cells were treated with KB and then subjected to H/R insults. (A–C) The nuclear and cytoplasmic protein fractions were prepared, and the Nrf2 level was analysed using a western blot. (D) Cell samples were subjected to a dual-luciferase reporter assay for determining luciferase activity and expressed as the ratio of Firefly/Renilla intensity. (E, F) The total protein was extracted, and the expression of HO-1 was measured. (G) The mRNA level of HO-1 was measured. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 9). ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group.
KB-induced Nrf2/ARE activation protected the cells against H/R-induced cardiac injury
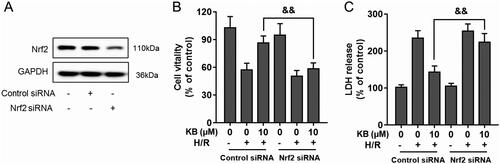
Nrf2 siRNA was used to investigate the specific role of Nrf2 in KB-mediated protection against H/R-induced injury. H9c2 cells were transfected with Nrf2 siRNA, which significantly abrogated the Nrf2 expression in H9c2 cells (). As expected, Nrf2 siRNA abrogated the protective effect of KB against H/R-induced toxicity, as shown by the decreased cell viability and increased LDH release in Nrf2 siRNA-treated groups ().
Figure 7. KB-induced Nrf2/ARE activation was responsible for the protective effect of KB against H/R-induced cardiac injury. H9c2 cells were transfected with Nrf2 siRNA or scrambled control siRNA for 24 h. (A) The silencing efficiency was detected using a western blot. (B, C) The transfected H9c2 cells were treated with KB and then subjected to H/R insults. The cell viability and cytotoxicity were determined by CCK8 assay and LDH release, respectively. Data are expressed as % of vehicle control. Results are shown as mean ± SEM (n = 8). The vehicle control group was treated with DMSO only. &&p< 0.01, between two groups.
KB up-regulated phosphorylation of AKT and AMPK in H/R-induced H9c2 cells
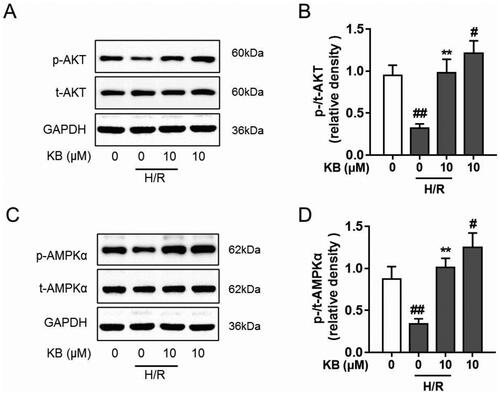
KB confers beneficial functions via modulating several pathways, including AKT and AMPK (Mansingh et al. Citation2018; Huang J et al. Citation2019). Thus, we explored the effect of KB on AKT and AMPK signalling to reveal its underlying mechanisms in H/R-induced cardiomyocyte injury. As shown in , H/R insults decreased the phosphorylation of AKT and AMPKα in H9c2 cells. However, KB significantly restored AKT phosphorylation in H9c2 cells under the H/R lesioning condition. Further, KB up-regulated AMPKα phosphorylation in H/R-induced H9c2 cells (). Thus, our results showed that KB alone could promote and facilitate AKT and AMPKα phosphorylation in H9c2 cells without H/R insults ().
Figure 8. KB up-regulated the phosphorylation of AKT and AMPK in H/R-induced H9c2 cells. The H9c2 cells were treated with KB and then subjected to H/R insults. (A–D) The levels of phosphorylated and total AKT and AMPKα were analysed using western blot. GAPDH served as a loading control. The vehicle control group was treated with only DMSO. Results are shown as mean ± SEM (n = 8). #p< 0.05 and ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group.
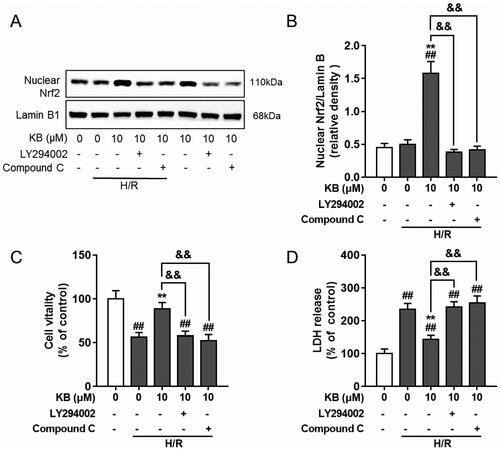
KB-induced AKT and AMPK were involved in KB-mediated Nrf2/ARE activation in H/R-induced H9c2 cells
Several protein kinases, including PI3K/AKT and AMPK, have been implicated in Nrf2 induction (Huang J et al. Citation2019). Therefore, we used specific inhibitors to block AKT and AMPK activity and determine whether AKT and AMPK were involved in KB-mediated Nrf2 activation. Interestingly, AKT and AMPK inhibitors abrogated Nrf2 nuclear translocation () in H/R-induced H9c2 cells. Moreover, AKT and AMPK inhibitors abolished the protective effect of KB against H/R-induced decline in cell viability and the increase in LDH release (). Thus, these results indicated that AKT and AMPK activation by KB was associated with the induction of the Nrf2/ARE/HO-1 pathway, which contributed to the protective effect of KB on H/R-induced H9c2 cells.
Figure 9. KB-mediated AKT and AMPK were involved in KB-induced Nrf2/ARE activation and its protective effect on H/R-induced H9c2 cells. H9c2 cells were pre-incubated with inhibitors for 1 h and treated with KB for 2 h, followed by H/R insults. (A, B) The nuclear protein was prepared, and the Nrf2 level was analysed using a western blot. (C, D) The cell viability and cytotoxicity were determined by the CCK8 assay and LDH release, respectively. Data are expressed as % of vehicle control. Results are shown as mean ± SEM (n = 8). The vehicle control group was treated with only DMSO. ##p< 0.01 vs. the vehicle group. **p< 0.01 vs. the H/R-treated group. &&p< 0.01 between two groups.
Discussion
In this study, we explored the protective effect of KB on H/R-induced cardiac injury and its underlying mechanisms. We found that KB improved cell survival and decreased cell death against H/R damage. KB also reversed H/R-induced apoptotic cascades, as evidenced by the decrease in the ratio of apoptotic populations in the DNA fragmentation assay. Further, KB reversed the decline of Δψm in H/R-treated H9c2 cells. The collapse of Δψm leads to mitochondrial membrane depolarization, which usually serves as a marker of early apoptotic events (Chen Y et al. Citation2018; Mansingh et al. Citation2018). Thus, the effects of KB on the collapse of Δψm could also confirm its inhibitory actions on H/R-mediated cell apoptosis. H/R insult modulates other intrinsic apoptotic cascades, including caspase-3, PARP, Bcl-2 and Bax (Kaushal et al. Citation2004). Caspase-3 activation leads to the execution phase of apoptotic cascades and mediates cellular degradation. Moreover, caspase-3 also interacts with other apoptosis markers, including the PARP and Bcl-2 family proteins (Elmore Citation2007). KB also modulates Bcl-2 and Bax (Kaushal et al. Citation2004; Chen Y et al. Citation2018; Mansingh et al. Citation2018; Kalpage et al. Citation2019; Peng et al. Citation2019). The Bcl-2 and Bax are two important proteins of the Bcl protein family, which execute diverse functions in intrinsic apoptosis (Kaushal et al. Citation2004; Elmore Citation2007; Peng et al. Citation2019). Bcl-2 is anti-apoptotic while Bax is pro-apoptotic (Kaushal et al. Citation2004). Thus, in this study, the modulation of Bcl-2 and Bax by KB confirmed that KB could inhibit H/R-induced apoptotic cascades, which demonstrated the protective effect of KB on H/R-induced H9c2 cells.
Cardiac injury resulting from H/R-aroused cell damage is closely related to ROS productions and oxidative stress in cardiomyocytes (Hayyan et al. Citation2016; Cadenas Citation2018; Zhou P et al. Citation2022). The myocardial cells are more susceptible to free radical damage because they have lesser antioxidants and antioxidant enzymes like GSH, SOD and catalase (Valko et al. Citation2007; Zhou P et al. Citation2022). KB also acts as an indirect antioxidant that induces endogenous antioxidants and enzymes. In this study, we also confirmed that KB could suppress H/R-mediated ROS generation and lipid peroxidation inhibition in H9c2 cells. Further, the up-regulation of antioxidant enzymes was also observed in KB-treated cells. Thus, based on these results, we suggested that KB could suppress H/R-induced oxidative damages in H9c2 cells.
H/R insults induce mitochondria dysfunction injury in cardiomyocytes (Solaini and Harris Citation2005; Kang et al. Citation2017; Jia et al. Citation2022). Myocardial cells have abundant mitochondria, which mainly contribute to the principal ROS generation, and are usually exposed to high oxygen tension (Solaini and Harris Citation2005; Kang et al. Citation2017; Jia et al. Citation2022). In this study, KB alleviated H/R-induced mitochondrial dysfunction. Accumulating evidence indicates that H/R could induce mitochondrial dysfunction followed by the prompt efflux of ROS, which leads to apoptosis (Huang CH et al. Citation2015; Quan et al. Citation2021). Hence, the inhibition of H/R-induced mitochondrial dysfunction by KB might be essential in protecting against H/R-induced apoptosis.
Enhancing Nrf2 transcriptional activity and promoting the transcription of genes encoding endogenous antioxidants is a promising strategy to attenuate H/R-induced oxidative stress and damage (Fan et al. Citation2018; Li CW et al. Citation2018; Qiu et al. Citation2018; Lv et al. Citation2019; Zhou F et al. Citation2019). Thus, in this study, we investigated the effects of KB on the Nrf2 pathway. The Nrf2/ARE pathway is pivotal in regulating cellular defences against oxidative damages, and the Nrf2/ARE pathway is also associated with H/R-induced cardiac injury (Bubb et al. Citation2017; Strom and Chen Citation2017; Chen G et al. Citation2019; Lu et al. Citation2022). Nrf2 is one of the redox-sensitive transcription factors that modulate cellular antioxidant defences and maintain redox homeostasis (Bubb et al. Citation2017; Strom and Chen Citation2017; Chen G et al. Citation2019; Lu et al. Citation2022). The expression of target genes in the Nrf2/ARE axis could inhibit intracellular ROS generation (Bubb et al. Citation2017; Strom and Chen Citation2017; Chen G et al. Citation2019). In this study, we found that KB led to significant Nrf2 nuclear accumulation and enhanced ARE promoter activity in H9c2 cells. Moreover, KB significantly increased HO-1 expression, which could directly protect against oxidative damage. Notably, the up-regulation of HO-1 promoted cellular resistance to H/R-induced damage. Therefore, our data demonstrated that KB could activate the Nrf2/ARE pathway, which might contribute to KB-mediated protective effects. Next, we conducted Nrf2 gene silencing using specific Nrf2-siRNA. Nrf2-siRNA transfection could abolish KB-mediated Nrf2 nuclear activations and ARE promoter activities in H/R-treated H9c2 cells. As expected, we confirmed that Nrf2 knockdown could abrogate KB-mediated anti-apoptotic actions in cells with H/R treatments. Thus, the activation of the Nrf2/ARE/HO-1 pathway contributed to KB-mediated protective effects against H/R insults.
KB is beneficial to the cell since it modulates several pathways, including AKT and AMPK (Mansingh et al. Citation2018; Huang J et al. Citation2019). The AKT pathway is involved in myocardial ischemia–reperfusion injury (Mansingh et al. Citation2018; Huang J et al. Citation2019). In addition, the AMPK signalling pathway serves as a critical regulator in modulating energetic stress, controls glucose uptake and glycolysis, and protects myocardial tissue from ischemic injury (Cui et al. Citation2013; Nagaoka et al. Citation2015; Thirunavukkarasu et al. Citation2015; Venardos et al. Citation2015; Kosuru et al. Citation2018; Potenza et al. Citation2019; Tian et al. Citation2019; Zhang BF et al. Citation2019). Therefore, we hypothesized that the cardioprotective actions of KB against H/R-induced injury were due to the AKT and AMPK axis. Notably, the activation of AKT and AMPK pathways was observed in KB-treated H9c2 cells with or without H/R insults. AKT or AMPK inhibitors also abrogated KB-induced protection against H/R injury. Therefore, we suggested the up-regulation of AKT and AMPK activities might be essential for the protective effect of KB. Further, several kinases, including AKT and AMPK, could modulate the activation of the Nrf2 pathway (Fan et al. Citation2018; Li CW et al. Citation2018; Lv et al. Citation2019; Zhou F et al. Citation2019). In this study, we not only found that KB promoted the phosphorylation of AKT and AMPK but also observed that blocking AKT and AMPK pathways via pharmacological inhibitors abolished the KB-mediated Nrf2 activation. Thus, we suggested that AKT and AMPK pathways were involved in Nrf2 activation and contributed to KB-induced protection against H/R-induced cardiac injury.
Conclusions
This study showed that KB alleviated cardiomyocyte injury in H/R-induced H9c2 rat cardiac myoblasts. Further, KB inhibited ROS production and lipid peroxidation but promoted antioxidant enzyme activity and the activation of the Nrf2/ARE/HO-1 pathway in H9c2 cells against H/R insults. Moreover, the KB-mediated activation of Nrf2 pathways was associated with the AKT and AMPK signal cascades. In conclusion, this study revealed that KB confers protection to cardiomyocytes against hypoxia/reoxygenation insult via the AKT and AMPK-mediated activation of the Nrf2/ARE/HO-1 signalling pathway. We believe that KB has the potential to become a promising drug candidate for managing ischemic cardiac disorders.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data in the current study are available from the corresponding author upon request.
Additional information
Funding
References
- Bubb KJ, Kok C, Tang O, Rasko NB, Birgisdottir AB, Hansen T, Ritchie R, Bhindi R, Reisman SA, Meyer C, et al. 2017. The NRF2 activator DH404 attenuates adverse ventricular remodeling post-myocardial infarction by modifying redox signalling. Free Radic Biol Med. 108:585–594.
- Cadenas S. 2018. ROS and redox signaling in myocardial ischemia–reperfusion injury and cardioprotection. Free Radic Biol Med. 117:76–89.
- Chatauret N, Badet L, Barrou B, Hauet T. 2014. Ischemia–reperfusion: from cell biology to acute kidney injury. Prog Urol. 24(Suppl. 1):S4–S12.
- Chen G, Liu G, Cao D, Jin M, Guo D, Yuan X. 2019. Polydatin protects against acute myocardial infarction-induced cardiac damage by activation of Nrf2/HO-1 signaling. J Nat Med. 73(1):85–92.
- Chen X, Jiang Z, Zhou C, Chen K, Li X, Wang Z, Wu Z, Ma J, Ma Q, Duan W. 2018. Activation of Nrf2 by sulforaphane inhibits high glucose-induced progression of pancreatic cancer via AMPK dependent signaling. Cell Physiol Biochem. 50(3):1201–1215.
- Chen Y, Chen S, Liang H, Yang H, Liu L, Zhou K, Xu L, Liu J, Yun L, Lai B, et al. 2018. Bcl-2 protects TK6 cells against hydroquinone-induced apoptosis through PARP-1 cytoplasm translocation and stabilizing mitochondrial membrane potential. Environ Mol Mutagen. 59(1):49–59.
- Cui GZ, Shan LC, Hung MW, Lei SW, Choi IL, Zhang ZJ, Yu P, Hoi PM, Wang YQ, Lee SM. 2013. A novel Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2 pathways. Int J Cardiol. 168(2):1349–1359.
- Elmore S. 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol. 35(4):495–516.
- Fan X, Lv H, Wang L, Deng X, Ci X. 2018. Isoorientin ameliorates APAP-induced hepatotoxicity via activation Nrf2 antioxidative pathway: the involvement of AMPK/Akt/GSK3β. Front Pharmacol. 9:1334.
- Feng Y, Lu Y, Liu D, Zhang W, Liu J, Tang H, Zhu Y. 2018. Apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside pretreatment attenuates myocardial ischemia/reperfusion injury via activating AMPK signaling. Life Sci. 203:246–254.
- Hayyan M, Hashim MA, AlNashef IM. 2016. Superoxide ion: generation and chemical implications. Chem Rev. 116(5):3029–3085.
- Huang CH, Tsai MS, Chiang CY, Su YJ, Wang TD, Chang WT, Chen HW, Chen WJ. 2015. Activation of mitochondrial STAT-3 and reduced mitochondria damage during hypothermia treatment for post-cardiac arrest myocardial dysfunction. Basic Res Cardiol. 110(6):59.
- Huang J, Sun Y, Chen L, Ma G. 2019. The lymphocyte adapter protein: a negative regulator of myocardial ischemia/reperfusion injury. J Mol Cell Cardiol. 134:107–118.
- Jia X, Shao W, Tian S. 2022. Berberine alleviates myocardial ischemia–reperfusion injury by inhibiting inflammatory response and oxidative stress: the key function of miR-26b-5p-mediated PTGS2/MAPK signal transduction. Pharm Biol. 60(1):652–663.
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. 2012. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 298:229–317.
- Kalpage HA, Bazylianska V, Recanati MA, Fite A, Liu J, Wan J, Mantena N, Malek MH, Podgorski I, Heath EI, et al. 2019. Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 33(2):1540–1553.
- Kang PT, Chen CL, Lin P, Chilian WM, Chen YR. 2017. Impairment of pH gradient and membrane potential mediates redox dysfunction in the mitochondria of the post-ischemic heart. Basic Res Cardiol. 112(4):36.
- Kaushal GP, Liu L, Kaushal V, Hong X, Melnyk O, Seth R, Safirstein R, Shah SV. 2004. Regulation of caspase-3 and -9 activation in oxidant stress to RTE by forkhead transcription factors, Bcl-2 proteins, and MAP kinases. Am J Physiol Renal Physiol. 287(6):F1258–F1268.
- Kosuru R, Cai Y, Kandula V, Yan D, Wang C, Zheng H, Li Y, Irwin MG, Singh S, Xia Z. 2018. AMPK contributes to cardioprotective effects of pterostilbene against myocardial ischemia–reperfusion injury in diabetic rats by suppressing cardiac oxidative stress and apoptosis. Cell Physiol Biochem. 46(4):1381–1397.
- Lee DY, Kim DH, Lee HJ, Lee Y, Ryu KH, Jung BI, Song YS, Ryu JH. 2010. New estrogenic compounds isolated from Broussonetia kazinoki. Bioorg Med Chem Lett. 20(12):3764–3767.
- Lee H, Li H, Jeong JH, Noh M, Ryu JH. 2016. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia. 112:90–96.
- Li CW, Tang BQ, Feng Y, Tang F, Hoi MPM, Su ZR, Lee SMY. 2018. Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson’s disease models through Nrf2 induction. J Agric Food Chem. 66(31):8307–8318.
- Li X, Hu X, Wang J, Xu W, Yi C, Ma R, Jiang H. 2018. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int J Mol Med. 42(4):1917–1924.
- Lu H, Xiao H, Dai M, Xue Y, Zhao R. 2022. Britanin relieves ferroptosis-mediated myocardial ischemia/reperfusion damage by upregulating GPX4 through activation of AMPK/GSK3beta/Nrf2 signaling. Pharm Biol. 60(1):38–45.
- Lv H, Hong L, Tian Y, Yin C, Zhu C, Feng H. 2019. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway. Cell Commun Signal. 17(1):2.
- Mansingh DP, Sunanda OJ, Sali VK, Vasanthi HR. 2018. [6]-Gingerol-induced cell cycle arrest, reactive oxygen species generation, and disruption of mitochondrial membrane potential are associated with apoptosis in human gastric cancer (AGS) cells. J Biochem Mol Toxicol. 32(10):e22206.
- Moran AE, Roth GA, Narula J, Mensah GA. 2014. 1990–2010 global cardiovascular disease atlas. Glob Heart. 9(1):3–16.
- Nagaoka K, Matoba T, Mao Y, Nakano Y, Ikeda G, Egusa S, Tokutome M, Nagahama R, Nakano K, Sunagawa K, et al. 2015. A new therapeutic modality for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin induces cardioprotection from ischemia–reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat model. PLOS One. 10(7):e0132451.
- Nudelman V, Zahalka MA, Nudelman A, Rephaeli A, Kessler-Icekson G. 2020. Cardioprotection by AN-7, a prodrug of the histone deacetylase inhibitor butyric acid: selective activity in hypoxic cardiomyocytes and cardiofibroblasts. Eur J Pharmacol. 882:173255.
- Peng N, Jin L, He A, Deng C, Wang X. 2019. Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing ischemia/reperfusion injury. Pharm Biol. 57(1):753–759.
- Penumathsa SV, Thirunavukkarasu M, Samuel SM, Zhan L, Maulik G, Bagchi M, Bagchi D, Maulik N. 2009. Niacin bound chromium treatment induces myocardial Glut-4 translocation and caveolar interaction via Akt, AMPK and eNOS phosphorylation in streptozotocin induced diabetic rats after ischemia–reperfusion injury. Biochim Biophys Acta. 1792(1):39–48.
- Potenza MA, Sgarra L, Nacci C, Leo V, De Salvia MA, Montagnani M. 2019. Activation of AMPK/SIRT1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia–reperfusion (I/R) injury in rats. PLOS One. 14(1):e0210654.
- Qiu YL, Cheng XN, Bai F, Fang LY, Hu HZ, Sun DQ. 2018. Aucubin protects against lipopolysaccharide-induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomed Pharmacother. 106:192–199.
- Quan W, Liu HX, Zhang W, Lou WJ, Gong YZ, Yuan C, Shao Q, Wang N, Guo C, Liu F. 2021. Cardioprotective effect of rosmarinic acid against myocardial ischemia/reperfusion injury via suppression of the NF-kappaB inflammatory signaling pathway and ROS production in mice. Pharm Biol. 59:222–231.
- Ryu J-H, Ahn H, Jin Lee H. 2003. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia. 74(4):350–354.
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. 2005. Adiponectin protects against myocardial ischemia–reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 11(10):1096–1103.
- Solaini G, Harris DA. 2005. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 390(Pt 2):377–394.
- Strom J, Chen QM. 2017. Loss of Nrf2 promotes rapid progression to heart failure following myocardial infarction. Toxicol Appl Pharmacol. 327:52–58.
- Thirunavukkarasu M, Selvaraju V, Tapias L, Sanchez JA, Palesty JA, Maulik N. 2015. Protective effects of Phyllanthus emblica against myocardial ischemia–reperfusion injury: the role of PI3-kinase/glycogen synthase kinase 3β/β-catenin pathway. J Physiol Biochem. 71(4):623–633.
- Thomas H, Diamond J, Vieco A, Chaudhuri S, Shinnar E, Cromer S, Perel P, Mensah GA, Narula J, Johnson CO, et al. 2018. Global atlas of cardiovascular disease 2000–2016: the path to prevention and control. Glob Heart. 13(3):143–163.
- Tian L, Cao W, Yue R, Yuan Y, Guo X, Qin D, Xing J, Wang X. 2019. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. 139(4):352–360.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39(1):44–84.
- Venardos KM, Rajapakse NW, Williams D, Hoe LS, Peart JN, Kaye DM. 2015. Cardio-protective effects of combined l-arginine and insulin: mechanism and therapeutic actions in myocardial ischemia–reperfusion injury. Eur J Pharmacol. 769:64–70.
- Zhang BF, Jiang H, Chen J, Guo X, Li Y, Hu Q, Yang S. 2019. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int Immunopharmacol. 73:98–107.
- Zhang PC, Wang S, Wu Y, Chen RY, Yu DQ. 2001. Five new diprenylated flavonols from the leaves of Broussonetia kazinoki. J Nat Prod. 64(9):1206–1209.
- Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao X, Yan Y, Yan S, Luo X, Fang Y. 2019. Schizandrin A protects against cerebral ischemia–reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 11:199–209.
- Zhou P, Gao G, Zhao CC, Li JY, Peng JF, Wang SS, Song R, Shi H, Wang L. 2022. In vivo and in vitro protective effects of shengmai injection against doxorubicin-induced cardiotoxicity. Pharm Biol. 60(1):638–651.
