Abstract
Context
Polygonum hydropiper L. (Polygonaceae) (PH) is a traditional Chinese traditional medicine with a pungent flavor and mild drug properties. PH is mainly distributed in the channel tropism in the stomach and large intestine. PH has multiple uses and can be used to treat a variety of diseases for a long time.
Objective
This review summarizes the phytochemical and pharmacological activities, and applications of PH from 1980 to 2022. We also provide suggestions for promoting further research and developing additional applications of PH.
Methods
The data and information on PH from 1980 to 2022 reviewed in this article were obtained from scientific databases, including Science Direct, PubMed, Science Citation Index, SciFinder Scholar (SciFinder), Springer, American Chemical Society (ACS) Publications, and China National Knowledge Infrastructure (CNKI), etc. Some information was obtained from classic literature on traditional Chinese medicines. The search terms were Polygonum hydropiper, phytochemistry compositions of Polygonum hydropiper, pharmacological activities of Polygonum hydropiper, and applications of Polygonum hydropiper.
Results
The comprehensive analysis of the literature resulted in 324 compounds being isolated, identified, and reported from PH. Regarding traditional uses, the majority of phytochemical and pharmacological studies have indicated the diverse bioactivities of PH extracts, flavonoids, and volatile oil elements, including antibacterial, antifungal, insecticidal, antioxidant, and anti-inflammatory.
Conclusions
PH has a long history of diversified medicinal uses, some of which have been verified in modern pharmacological studies. Further detailed studies are required to establish scientific and reasonable quality evaluation standards and action mechanisms of active constituents from PH.
Introduction
The Polygonaceae family consists of 50 genera and 1120 species; of these, approximately 13 genera and 238 species are found in China. Polygonum is the major Polygonaceae genus for medicinal purposes, with approximately 300 species worldwide. In total, there are 131 species and 31 varieties of Polygonum in China; 72 species have medicinal value, are distributed in many provinces in northern and southern China, and are commonly found on roadsides or in watery wet places (Wang et al. Citation1996; Wang Citation2008; Wang et al. Citation2012).
Polygonum hydropiper (PH) is the whole plant and stem of Polygonum hydropiper L., an annual herb (Wang Citation1996). PH was first recorded in Tang Materia Medica (Cheng et al. Citation1999; Li et al. Citation2003), and the 1977 Edition of the Chinese Pharmacopoeia included the whole herb of PH. The leaves of PH have deep red spots, which are exclusive characteristics of PH and are distinguished from other Polygonaceae plants. PH, known as laliao in Chinese, is widely used as a traditional herbal medicine. Among the Chinese people, PH is also called shuiliao, liaoyacai, liuliao, laliaocao, liaozicao, banjiaocao, litongcao, etc. (Wang, Liu et al. Citation1996; Wang Citation2014). PH often grows in patches in low mountain areas, hills, plain hillsides, riverbanks, and other moist places. PH is widely distributed in the northern and southern provinces of China, including Hebei, Henan, Shanxi, Jiangsu, Zhejiang, Hubei, Fujian, Jiangxi, Guangdong, Guangxi, and Yunnan (Zhang Citation2004). According to the theory of traditional Chinese medicine, PH has a pungent flavor and mild drug properties. The meridian tropism of PH is the stomach and large intestine. PH has many traditional effects, such as dampness-resolving, stagnation-removing, wind-expelling and detumescence; it is mainly used to treat and relieve some diseases including dysentery, enter gastritis, diarrhea, dermatophytosis beriberi, itch, rheumatoid arthritis, hemostasis swelling pain, and functional uterine hemorrhage. Topical treatment includes snakebite and skin eczema, etc. (Li Citation2017). At present, PH is often used in combination with other traditional Chinese medicines in the clinic to treat rheumatism, traumatic injury, skin diseases, acute and chronic gastroenteritis, chronic rhinitis, gynecological diseases, ophthalmological diseases, and sebaceous cysts, etc. (Huang and Zhen Citation2013).
Previous studies in the 1990s found that PH has antimicrobial, insecticidal, antioxidant, antitumor, and other biological activities (Haraguchi et al. Citation1992, Citation1993, Citation1996; Yagi et al. Citation1994). The active ingredients are flavonoids and volatile oils. In recent years, there have been many reports about the phytochemical composition and pharmacological activities of PH worldwide. Flavonoids have antimicrobial and anti-inflammatory effects, and volatile terpenoids have insecticidal activities, which can provide some references for the development of new drugs and new plant-based pesticides. Moreover, there is a certain development prospect in the fields of medicine, food, botanical pesticides, veterinary medicine, and food additives (Zeng et al. Citation2006; Huang and Zhen Citation2013). However, an overall review of these factors is lacking. In this review, the phytochemical constituents, pharmacological activities, and applications of PH have been summarized for the further detailed research, with the hope that PH can be fully and effectively developed and utilized.
Methods
This literature review of PH covers the reports from 1980 to 2022. The data and information were obtained from multiple scientific databases, including Science Direct, PubMed, Science Citation Index, Sci Finder, Springer, ACS Publications, Innojoy, Google Scholar, Baidu Scholar, and China National Knowledge Infrastructure (CNKI). Additional information was obtained from classic literature on traditional Chinese medicines, as well as PhD and MSc theses in the school library, and downloaded from CNKI, read manually and analyzed in groups. The search terms were Polygonum hydropiper, phytochemistry compositions of Polygonum hydropiper, pharmacological activities of Polygonum hydropiper, applications of Polygonum hydropiper and other related search terms. We excluded reports and articles that appeared in some news media or newspapers, as well as literature that was not published in formal professional magazines or periodicals.
Phytochemistry
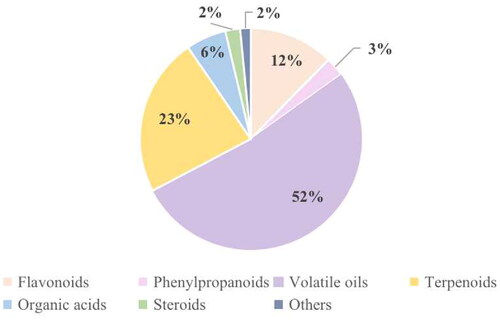
To date, 324 compounds have been isolated and identified from PH, and researchers have adopted multiple separation techniques for the isolation and purification of chemical constituents from PH, including 40 flavonoids, 9 phenylpropanoids, 169 volatile oils, 75 terpenoids, 19 organic acids, 7 steroids, and 5 others. The percentages of chemical constituents are shown in .
Flavonoids
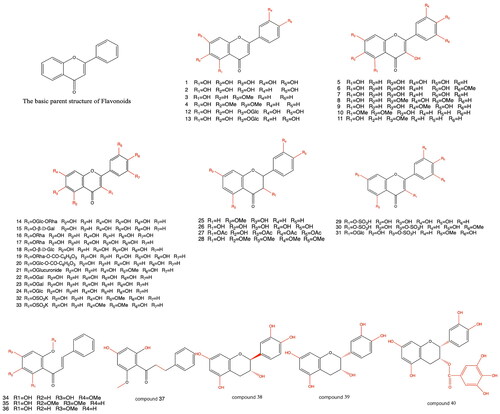
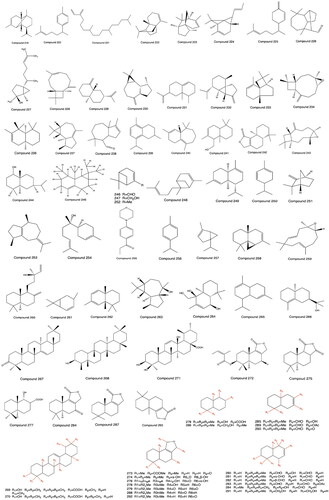
Flavonoid ingredients are the main active ingredients of PH. In addition, flavonoid glycosides, flavonol and its glycosides, flavone and its glycosides, chalcone, and dihydrochalcone are found in PH. The species, compounds, and molecular formulas of PH are shown in . The basic parent nuclei and structures of flavonoids 1 to 40 are shown in .
Table 1. The species, compounds, and molecular formulas of flavonoids.
Phenylpropanoids
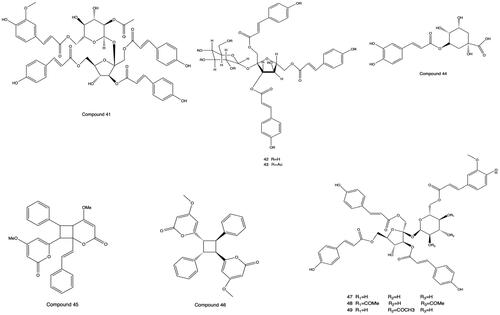
Most of the phenylpropanoids contained in PH are simple phenylpropanoids and coumarins. Vanicoside A′ (41), hydropiperoside B (42), and hydropiperoside A (43) were isolated, purified and distinguished from PH by column chromatography with silica gel and Sephadex, preparative high performance liquid chromatography (pre-HPLC), NMR, and ESI-MS (Fukuyama et al. Citation1983; Kiem et al. Citation2008; Wang et al. Citation2018). Chlorogenic acid (44) was separated and identified from PH by chromatography with silica gel and MCI, physicochemical properties, and spectral analysis for the first time (Xu et al. Citation2017). Aniba-dimer A (45) and 6,6′-((1 R,2R,3S,4S)-2,4-diphenylcyclobutane-1,3-diyl) bis (4-methoxy-2H-pyran-2-one) (46) were separated from the dichloromethane part of PHL (Xiao Citation2018). Vanicoside B (47), vanicoside E (48) and vanicoside F (49) were isolated from the dichloromethane-soluble portion of the ethanol extract of PH (Xiao et al. Citation2017). The structures of phenylpropanoids from 41 to 49 are shown in .
Volatile oils
The volatile oils stored in PH are extremely complex, mainly sesquiterpenoids, and contain enol tautomerisms and sterols. Due to the volatility and instability of the volatile oils, different origins, different months, and different extraction methods can affect the volatile components of PH.
Eight volatile oil components were first separated and identified by the supercritical CO2 technique and combined GC–MS coupling technique (Zhang and Zeng Citation2005). A total of 125 volatile oils from the aboveground parts of PH grown in May, August, and November in Guizhou in 2018 were obtained and identified by steam distillation and GC–MS coupling techniques; they had 15 similar components () (Yu et al. Citation2018). Volatile oils of PH were first extracted by the methods of steam distillation and CO2 supercritical extraction, and identified by the GC–MS coupling technique, which yielded 14 and 4 volatile oil-like components, respectively, including 2 similar compounds (Li Citation2007). Seventy-five volatile components from PH in Guizhou contained the same 5 compounds, and they were obtained and distinguished by solid phase extraction, steam distillation and GC–MS coupling techniques (Lin et al. Citation2012). Fifty-three volatile components of PH from Hunan were obtained by steam distillation combined with GC–MS (Yao et al. Citation1999). Six volatile components of PH were first found first from an ether extraction part of alcohol extraction by GC–MS (Zeng Citation2007). Altogether, 103 volatile components of PH were isolated and identified by steam distillation and GC–MS coupling techniques (Wu et al. Citation2007; Liu, Zhang et al. Citation2009; Wang et al. Citation2017). The volatile oil compounds (from 50 to 218) of PH are shown in . The structures of phenylpropanoids 50 to 218 are shown in .
Table 2. Compounds of volatile oils.
Terpenoids
The terpenoid compounds of PH are almost all monoterpenes and triterpenes, including α-copaene (219), curcumene (220), neophytadiene (221), 8-(3-methy-2-butanol)-tricyclene (222), cedrene (223), 8-(3-methyl-2-butenyl)-α-pinene (224), β-sesquiphellandrene (225), longifolene aldehyde (226), 7-epi-cis-sesquisabinene hydrate (227), β-caryophyllene (228), selinene (229), aromadendrene (230), eremophilene (231), cubebene (232), α-panasinsene (233), oxide caryophyllene (234), chamigrene (235), widdrene (236), ledene (237), 1,5,5,8a-tetramethyl (238), 8-isopropyl-2,5-dimethyl-1,2,3,4-tetrahydronaphthalene (239), cis-himachalene (240), drimenol (241), naphthol-[1,2-c]-furan-1-(3H)-one-4,5,5a,6,7,8,9,9a-octahydro-6,6,9a-trimethy-(-)-drimenin (242), caryophyllene oxide (243), eudesmol (244), aristolene (245), myrtanal (246), myrtanol (247), trans-α-bergmotene (248), α-muurolene (249), 1-phellandrene (250), camphene (251), α-pinene (252), guaiene (253), α-bisabolol (254), elemol (255), γ-terpinene (256), α-thujene (257), thujopsene (258), humulene epoxide II (259), 1-naphthalenepropanol (260), trans-carene (261), thujopsene-I3 (262), globulol (263), 1,4,4α,5,6,7,8,8a-octahydro-2,5,5,8α-tetramethyl-β-eudesmol (264), 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1(1-methylethyl) naphthalene (265), 10-epi-γ-eudesmol (266), taraxerone (267), friedelinol (268), ursolic acid (269), oleanolic acid (270), 3β,13β-dihydroxyl-11-ene-28-ursolic acid (271), 3β-angeloyloxy-7-epifutronolide (272), polygonumate (273), dendocarbin L (274), (+) winterin (275), (+) fuegin (276), changweikangic acid A (277), futronolide (278), 7-ketoisodrimenin (279), warburganal (280), polygodial (281), isopolygodial (282), ugandensidal (283), muzigadial (284), polygonal (285), drimenol (286), isodrimeninol (287), octylene (288), monoacetate (289), α,β,β′-disubstituted furano (290), drimanediol (291), isodrimenin (292), and confertifolin (293) (Fukuyama et al. Citation1980, Citation1985; Yao et al. Citation1999; Zhang and Zeng Citation2005; Li Citation2007; Wu et al. Citation2007; Huang et al. Citation2012; Lin et al. Citation2012; Goswami et al. Citation2014; Wang et al. Citation2017; Xu et al. Citation2017; Yu et al. Citation2018). The structures from 219 to 293 are shown in .
Organic acids
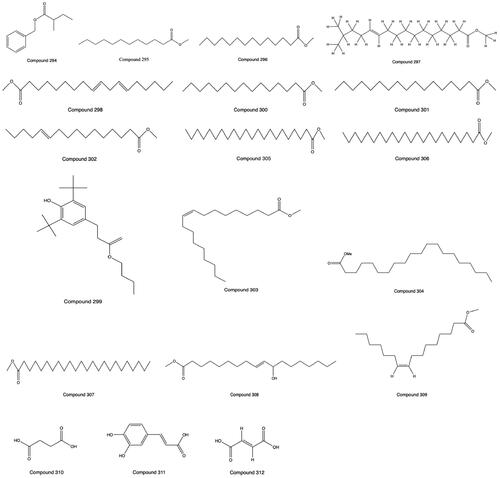
Multiple compounds of the organic acid are found in PH, such as fatty acids, polyphenols and carboxylic acids. The fatty acid-like components are mostly unsaturated fatty acids. Sixteen constituents of organic acids were isolated and identified by GC–MS (Liu, Qin et al. Citation2009), and 3 organic acids were purified and isolated by column chromatography (Li et al. Citation2017; Xu et al. Citation2017). The compounds and molecular formulas from 309 to 327 are shown in . The structures from 294 to 312 are shown in .
Table 3. Compounds and molecular formulas of organic acids.
Steroids
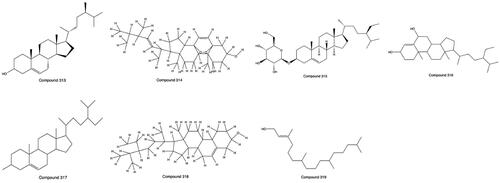
A variety of sterols and phytosterols exist in PHL. At present, 7 steroid compounds have been isolated and identified independently, including β-sitosterol (313), ergosterol-5,8-peroxide (314), daucosterol (315), stigmast-4-ene-3β,6a-diol (316), γ-sitosterol (317), 22,23-dihydrostigmasterol (318) and phytol (319) (Liu, Qin et al. Citation2009; Li et al. Citation2017; Wang et al. Citation2018). The structures from 313 to 319 are shown in .
Others
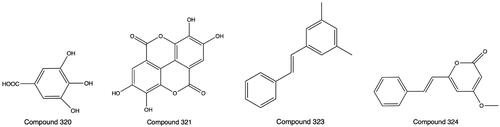
Gallic acid (320), which is the tannin monomer, was isolated from PH extract with 75% ethanol (Huang et al. Citation2012). Ellagic acid (321), a tannin component, was obtained from PH (Li et al. Citation2017). An acidic polysaccharide named PFMP (322) was separated by DEAE column chromatography and authenticated to consist of d-mannose, l-rhamnose, d-glucuronic acid, d-galactose, d-glucose, and l-arabinose by HPLC (Zhu Citation2020). Pinosylvin (323) and 5,6-dehydrokawain (324) were isolated from chloroform extract (Xiao Citation2018). Rich metallic elements, such as Ca, Mg, Al, K, Fe, Mn, Ag, and Zn, and several harmful metals, such as Pb, As, Cu, Hg, and Cd, were discovered by microscopic with identification combined inductively coupled emission spectrometry (Wang et al. Citation2019). The contents of four heavy metals, Pb, Cd, As, and Hg, were detected by flame atomic absorption spectroscopy, and all met the Green Trade Standard (Lai et al. Citation2011). The structures of 320, 321, 323 and 324 are shown in .
Pharmacological activities
Antibacterial and antifungal effects
The extracts from different parts of PH have some antibacterial and antifungal effects, especially in vitro, showing more extensive antibacterial and antifungal effects. The components of PH, including volatiles, flavones and carboxylic acids, have good broad-spectrum inhibition of bacterial activity (Lin et al. Citation2012; Li et al. Citation2017; Ma et al. Citation2017). The ethanol extract and acetone extract of PH have antifungal activity and may be used in the treatment of fungal infections, such as Trichospora photospora and Pestalotia funerea in poultry. In conclusion, the active ingredients of PH with antibacterial and antifungal activities may be flavonoids and volatile oils. The active fractions and antibacterial and antifungal species of PH with antibacterial and antifungal activities are shown in .
Table 4. Active fractions with antibacterial and antifungal activity, and antibacterial and antifungal species.
Antiviral effects
Both the ethyl acetate and n-butanol portions of PH have antiviral effects. The main active component with antiviral effects is flavonoids (Zhou et al. Citation2020; Lu et al. Citation2021). The antiviral species and mechanisms of PH are shown in .
Table 5. Antiviral species and mechanisms of PH.
Antifeedant and insecticide effects
Because PH has strong antifeedant and insecticidal effects, it could be used in the development of novel pesticides and insecticides in the future. The active constituents of PH with antifeedant and insecticidal effects may be terpenoids. The active ingredients of PH with antifeedant or insecticidal and insect categories are shown in .
Table 6. The active ingredients and insect categories of PH with antifeeding and insecticidal effects.
Antioxidant activity
The flavonoids from PH have good antioxidant activity, and the antioxidant activity of PH is different because of its different structure. Flavonoids with more enol structures have stronger antioxidant capacity, and quercetin has stronger antioxidant activity than vitamin C (Li et al. Citation2017; Ma et al. Citation2017). The alcohol extracts from different parts of PH have the ability to scavenge DPPH in the order of caffeic acid > rutin > PH flowers > PH leaves > PH stems > PH roots and the ability to scavenge OH- in the order of PH leaves > PH stems of > PH roots > PH flowers > caffeic acid > rutin (Yang et al. Citation2014). Muhammad et al. (Citation2014) found that n-hexane, chloroform, ethyl acetate, n-butanol, water and saponins of PH all had certain antioxidant activities, which were all concentration-dependent on DPPH and could inhibit the activity of acetylcholinesterase. The hexane, chloroform, ethyl acetate, and water fractions of PH had inhibitory activity on butyrylcholinesterase. Yagi et al. (Citation1994) determined the antioxidant activity of flavonoids in PH by the ferric thiocyanate method and discovered that PH had a high eradication rate of superoxide ions in a dose-dependent manner. It was speculated that flavonoids might play a role in scavenging superoxide anions by inhibiting xanthine and xanthine oxidase, thus having antioxidant activity. Sharif et al. (Citation2013) discovered that methanol, ethanol, chloroform, petroleum ether and n-hexane fractions of PH all had antioxidant activity, and methanol, ethanol, and petroleum ether had the strongest activity. Overall, PH has good antioxidant activity and can become a natural antioxidant, probably because it contains a large number of flavonoids, terpenoids, and tannins.
Anti-inflammatory effects
Both the aqueous and alcoholic extracts of PH herbs have certain anti-inflammatory effects. Their active ingredients and their anti-inflammatory effects, inflammation categories, inflammatory modeling methods and anti-inflammatory mechanisms or pathways are shown in .
Table 7. Anti-inflammatory active ingredients, dosage, mode of administration, inflammatory categories, effects, modeling methods and anti-inflammatory mechanisms or pathways of PH.
Impact on the immune system
PH can inhibit Escherichia coli diarrhea and hepatitis B virus to some extent and can resist immunosuppression caused by cyclophosphamide in mice, which proves that it has a certain immunomodulatory effect. The active components of PH with immunomodulatory effects, the impacts on the immune system and the mechanisms of action are shown in .
Table 8. Active components with immunomodulatory effects, dosage, mode of administration, modeling methods, the impact on the immune system and the mechanisms of action.
Protection of the gastrointestinal tract
PH aqueous extract has a good therapeutic effect on E. coli diarrhea (Xiao et al. Citation2018), and acute gastric mucosal injury of rats caused by ethanol (Ren et al. Citation2018). The mechanism of treatment of E. coli diarrhea may be related to reducing the release of inflammatory factors in intestinal tissues, improving the intestinal mucosal barrier and intervening with TGF-β/Smad signal transduction. The mechanism of treating acute gastric mucosal injury induced by ethanol may be related to increasing the content of Nrf 2 and the activity of SOD in gastric mucosal tissues (Ayaz, Junaid, Ullah, Sadiq, et al. Citation2017).
Others
In addition, PH has analgesic (Sharif et al. Citation2013), hypoglycemic (Oany et al. Citation2016), hypotensive (Muhammad et al. Citation2016; Devarajan Citation2018), antiangiogenic (Muhammad et al. Citation2016), antitumor (Muhammad et al. Citation2016; Ayaz et al. Citation2019), antifertility and embryo implantation inhibition effects (Daniyal and Akram Citation2015). PH also has a therapeutic effect on the reproductive system and influences the expression of insulin-like growth factor in the uterus of early pregnancy rats (Goswami et al. Citation2014). The volatile composition of PH, β-sitosterol, can improve memory deficits and disorders such as Alzheimer’s disease, and is mainly manifested in the aspects of anticholinesterase, improvement of working memory, spontaneous alternating behavior, motor coordination, etc. of transgenic animals (Ayaz et al. Citation2015, Ayaz, Junaid, Ullah, Subhan, et al. Citation2017). PH water extracts alleviate hepatic and duodenal injury induced by enteropathogenic E. coli. The mechanism of E. coli infection is that PH inhibits the secretion and expression of inflammatory cytokines and regulates the expression level of CYPs (Huang et al. Citation2022).
Applications and prospects
Traditional applications
Many ancient medical books in China described the traditional effects of PH. As recorded in Mingyi Bielu: ‘Polygonum leaves, the meridian tropism is tongue, could remove the small and large intestinal evil, also benefit in the intelligence.’ As recorded in Tang Materia Medica: ‘PH main treatment snake bite, mashed to apply; juice to take, treatment of snake venom caused by abdominal stuffiness; soak feet in water decocted solution of PH, followed by massage, remove beriberi swelling.’ As recorded in Bencao Shiyi: ‘PH, the main treatment of periumbilical and hypochondriac firmness and pain, takes 60 g daily after boiling; PH treats cholera with muscular spasm and to massage feet with hot water decoction; the leaves are crushed and applied to treating the huci nevus; the leaves are also used for treating the head sores in children.’ As recorded in Lingnan Caiyaolu: ‘PH can be applied to the injury site, can be used to clean the surface of naevus scabies, and also can itch and swell.’ (Zhang Citation2004).
Modern applications
PH can be used for preventing and treating enteritis in black carp and grass carp in the high temperature season (July to September every year). The feed method comprises chopped herb PH hay or fresh herb PH decocted with an appropriate amount of water, mixing that filtrate with bait, and continuously feeding for 3-6 days (250 g hay or 1.5 kg fresh herb PH per 50 kg) (Wang et al. Citation2021). Planting PH on the mud bank of eel ponds can prevent red-skin disease for a long time (Liu Citation2020). Cleaning and mashing fresh stems and leaves of PH, adding water to soak for 24 h, filtering to remove residues, diluting with water and spraying a solution that is five times that of the original on leaf surfaces helps to prevent and control plant hoppers, aphids, rice leafhoppers, tea caterpillars, etc. The fresh stems and leaves of PH can be washed, dried, powdered and sprinkled to prevent and control cutworms and grubs (Ao Citation2019; Zhou and Du Citation2019). Intramuscular injection of the PH liquid can be used for treating porcine epidemic diarrhea (Ren Citation2017). After drying, PH is crushed and mixed into grain at a ratio of 1:1000, which can keep the grain free of insects for a year. The mung bean weevil can be controlled by putting PH into mung beans at a ratio of 9:1000 (Peng Citation2016). Feeding dried or fresh PH to fish can prevent and cure rotten gill disease (Wang Citation2016). PH has been boiled in water and mixed with chopped tender grass or bait to treat fish enteritis (Liu and Wang Citation2017). PH has also been ground into powder, mixed with bait and fed to cure fish enteritis, rotten gill and red-skin disease (Liu Citation2017). The stem or whole plant of fresh herba PH can be mashed into mud and applied to the affected part to treat livestock pellagra (Zhang Citation2014). Clinically, PH and Gardenia jasminoides Ellis (Rubiaceae) can treat ovarian cysts (Li and Gu Citation2018).
There are few studies on the toxicology of PH. At present, only Zhang et al. (Citation2022) and Zhu et al. (Citation2020) found that the flavonoid extract of PH at 5 g/(kg·BW) and below had no acute toxic effects or side effects in mice, has no subchronic toxic effects after long-term continuous medication, and has good safety.
Prospects
Since PH contains many active ingredients, each with different medicinal effects, it also has more prospects for development and application. The polysaccharide isolated from PH can be used as an immunomodulator in health food. PH can be made into new anti-trypanosomal drugs due to its anti-trypanosomal activity. In places where mosquitoes are very likely to breed, such as cesspools and gutters, a certain amount of volatile oil extract of PH can be added as an insecticide and mite killer. In addition, PH can be made into plant pesticides, antibacterial agents, and natural antioxidants.
Conclusions
As a traditional herbal medicine with a long history, PH can be used in a single or compound prescription. Because of its multiple effects and medicinal history, many researchers have a keen interest in PH. This review comprehensively summarizes the phytochemical constituents, pharmacological activities, and applications of PH, which could offer ideas and foundations for the further studies.
To date, a total of 324 compounds have been isolated and identified from PH, mainly flavonoids, phenylpropanoids, volatile oils, terpenoids and organic acids. Among them, flavonoids and volatile oil components have certain antibacterial and antiviral effects, but the active ingredients and pharmacodynamic substance basis have not been reported. The volatile oil component ‘eugenol’ was found to be a pesticide, and the insecticidal mechanism was that eugenol significantly inhibited the activities of acetylcholinesterase and glutathione-S-transferase (Zeng Citation2007). The second volatile oil component ‘confertifolin’ was considered for use in the control of human vector mosquitoes, but the mechanism is unclear (Maheswaran and Ignacimuthu Citation2013). Confertifolin is very promising for formulating a potent and affordable natural product to control dreadful disease transmission and nuisance-creating human vector mosquitoes. The other active insecticidal components were crude extracts. In general, the study of PH remains inadequate. Quercetin is an anti-inflammatory active ingredient, and its mechanism is that it inhibits the Src/Syk/NF-κB and IRAK/AP-1/CREB signaling pathways. It has been suggested that quercetin should be developed as a novel anti-inflammatory remedy (Yang et al. Citation2012). In particular, there is a lack of toxicity research. To date, only a few studies have reported that the flavonoids of PH have no acute toxicity and are nontoxic after long-term use. However, there is a lack of reports of other active components, especially volatile oil and terpenoid components. When used as pesticides, there is also a lack of reports on the optimal concentration and dose of pesticides for various pests and whether there is harm to the human body when used as pesticides. In ancient China, people often burned PH at night to repel mosquitoes and insects, and this practice could match the insecticidal effect in modern pharmacological research. The polygodial contained in PH is a volatile terpenoid, has good antifeedant activity to insects, and has antioxidation, good stability to air and light, and a half-life of more than one month. It is less stable to heat but has a half-life of more than 15 days, which is different from other herbs (Zhang Citation2004). While the current study only reported that the active ingredients of PH with insecticidal effects are volatile oils and some crude extracts, the active compounds have not been determined. High-temperature burning has a pungent odor, and whether the insecticidal mechanism is related to the pungent odor is unknown, so the subsequent research should involve more in-depth screening of the active substances with insecticidal effects, which can be developed into pesticides and insecticides of plant origin.
As a common traditional herbal medicine, PH is often grown in ditches and depressions, probably because of its ability to resist the growth of weeds. Currently, with the continuous modernization, an increasing number of skyscrapers are rising from the ground, and the germplasm resources of PH are gradually decreasing. We must pay attention to the conservation of this natural medicinal resource. Since PH grows in gullies and depressions, such places are hotbeds of bacteria and many kinds of microorganisms; however, PH resists the growth of miscellaneous bacteria, probably because it has some important endophytic bacteria of its own. However, no research has been done on this topic yet, which could be another new research direction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ao LL. 2019. Self- made environment-friendly and efficient plane pesticide (1). Ke Xue Zhong Yang. (2):40–42.
- Ayaz M, Junaid M, Ullah F, Sadiq A, Azam M, Waqar K, Muhammad A, Shah R, Sajjad I. 2015. Comparative chemical profiling, cholinesterase inhibitions and anti-radicals properties of essential oils from Polygonum hydropiper L: a preliminary anti-Alzheimer’s study. Lipids Health Dis. 14:141–153.
- Ayaz M, Junaid M, Ullah F, Sadiq A, Shahid M, Ahmad W, Ullah I, Ahmad A, Syed NH. 2017. GC-MS Analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Front Chem. 5:58–68.
- Ayaz M, Junaid M, Ullah F, Subhan F, Sadiq A, Ali G, Ovais M, Shahid M, Ahmad A, Wadood A, et al. 2017. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front Pharmacol. 8:697.
- Ayaz M, Sadiq A, Wadood A, Junaid M, Ullah F, Khan NZ. 2019. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids. 141:30–35.
- Cao HL. 2006. Observation on the killing effect of different concentrations of Polygonum hydropiper on the larvar of Culex vulgaris. Chin J Vector Biol Control. 6:464.
- Chen CL, Zheng H, Cao GW, Li CH. 2012. Experiment on the antibacterial effect of compound Polygonum hydropiper on Escherichia coli. Shanghai J Anim Husb Vet Med. 3:52.
- Chen JZ, Tan JC, Li XW, Zeng WA, Yang JL. 2007. Bioactivity of the Polygonum hydropiper L. extracts against Ectropis obliqua Prout. J Tea Commun. 1:7–9.
- Cheng ZN, Li CY, Huang CJ. 1999. China’99 Kunming world horticultural expo horticultural encyclopedia. Beijing: China Forestry Press.
- Cheng ZH, Liu YH, Cao H, Li R, Song BZ, Li SC. 2014. Studies on acaricidal bioactivities of six plants extracts against the Tetranychus Cinnabarinus Bois. J Shanxi Agric Univ (Nat Sci Ed). 34:403–406.
- Daniyal M, Akram M. 2015. Antifertility activity of medicinal plants. J Chin Med Assoc. 78(7):382–388.
- Devarajan S, Yahiro E, Uehara Y, Kuroda R, Hirano Y, Nagata K, Miura S, Saku K, Urata H. 2018. Depressor effect of the young leaves of Polygonum hydropiper Linn. in high-salt induced hypertensive mice. Biomed Pharmacother. 102:1182–1187.
- Fukuyama Y, Sato T, Asakawa Y, Takemoto T. 1980. A potent cytotoxic warburganal and related drimane-type. sesquiterpenoids from Polygonum hydropiper. Pergamon. 21(12):2895–2898.
- Fukuyama Y, Sato T, Miura I, Asakawa Y. 1985. Drimane-type sesqui- and norsesquiterpenoids from Polygonum hydropiper. Planta Med. 77:1848–1851.
- Fukuyama Y, Sato T, Miura I, Asakawa Y, Takemoto T. 1983. Hydropiperside, a novel coumaryl glycoside from the root of Polygonum hydropiper. Phytochemistry. 22(2):549–552.
- Goswami P, Hazarika A, Sarma HN. 2014. Inhibition of Insulin like growth factor-I expression by chromatographic fraction of Polygonum hydropiper root reduces implantation preference in rat. Middle East Fertility Soc J. 19(1):34–41.
- Gu LY, Tao JY, Wu WY, Song ML, Hu TJ. 2018. Effects of FEA from Polygonum hydropiper L. on biochemical indexes and inflammatory cytokines in mice with endotoxemia induced by lipopolysaccharide. Chin J Inf Tradit Chin Med. 25(04):40–45.
- Gu LY, Tao JY, Yang J, Zeng Y, Wei YY, Hu TJ. 2018. Protective effects of FNB from Polygonum hydropiper on endotoxemia induced by lipopolysaccharides in mice. Prog Vet Med. 39(02):84–90.
- Guan JY, Li L, Yu ML, Wei YY, Hu TJ. 2021. Study on effect of anti-inflammatory of flavonoids from Polygonum hydropiper L. in mice. Mod J Anim Husb Vet Med. 2:1–4.
- Haraguchi H, Hashimoto K, Yagi A. 1992. Antioxidative substances in leaves of Polygonum hydropiper. J Agric Food Chem. 40(8):1349–1351.
- Haraguchi H, Matsuda R, Hashimoto K. 1993. High-performance liquid chromatographic determination of sesquiterpene dialdehydes and antifungal activity from Polygonum hydropiper. J Agric Food Chem. 41(1):5–7.
- Haraguchi H, Ohmi I, Sakai S, Fukuda A, Toihara Y, Fujimoto T, Okamura N, Yagi A. 1996. Effect of Polygonum hydropiper flavonoids on lens aldose reductase and related enzymes. J Nat Prod. 59(4):443–445.
- He LM, Chen YT, Liu HM, Peng XY, Pan YF, Yuan MG, Luo SJ, Wei GW. 2014. Simultaneous determination of six kinds of flavonoid in extract of Polygonum hydropiper L. by HPLC. Guangdong Agric Sci. 41(13):94–98.
- Huang HH, Zhen HS. 2013. Recent research progress of the Chinese herb Polygonum hydropiper. Chin J Ethnomed Ethnopharm. 22(01):38–40.
- Huang J, Hou PY, Wu LJ, Gao HY. 2012. Isolation and identification of chemical constituents of Polygonum hydropiper. J Shenyang Pharm Univ. 29(01):22–25.
- Huang JH, Yue L, Zhang MR, Yang Q, Cheng XX. 2022. Effects of Polygonum hydropiper on the expressions of inflammatory cytokines and cytochrome P450 enzymes in mice with E. coli-induced diarrhea. J Chin Pharm Sci. 31:622–633.
- Kiem PV, Nhiem NX, Cuong NX, Hoa TQ, Huong HT, Huong LM, Minh CV, Kim YH. 2008. New phenylpropanoid esters of sucrose from. Polygonum hydropiper and their antioxidant activity. Arch Pharm Res. 31(11):1477–1482.
- Kurkina AV, Ryazanova TK, Kurkin VA. 2013. Flavonoids from the aerial part of Polygonum hydropiper. Chem Nat Compd. 49(5):830–832.
- Lai WY, Yang WL, Liu MS, Zhang JQ. 2011. Detection of heavy metal contents in four li nationality medicine. China Trop Med. 11:579–581.
- Li AR, Bao BJ, Alisa EGB. 2003. Urticaceae (Flora of China). Beijing: Science Press, Beijing & Missouri Botanical, Garden Press. p. 277–278.
- Li M, Liu XF, Zhang KF. 2014. Effect of Polygonum perfoliatum L. on the expression of HBsAg and HBeAg in HepG2. 2.15 cells. Chin J Ethnomed Ethnopharm. 23(13):2–3.
- Li MY. 2017. Chemical compositions of Polygonum hydropiper L. and their bioactivities [master’s thesis]. Xi’an: shanxi Univ Sci Technol.
- Li MY, Ma YM, Qiao K, Guo LX. 2017. Chemical constituents from Polygonum hydropiper. Chin Tradit Pat Med. 39:769–773.
- Li Q. 2007. Extraction, analysis of chemical composition and determination of insecticidal activity of volatile oil from Polygonum hydropiper [master’s thesis]. Zhanzhou: south China Univ Trop Agr.
- Li Q, Gu ZX. 2018. Gu Zhongxin’s clinical experience in treating cyst with Polygonum hydropiper and Gardenia jasminoides Ellis. China’s Naturop. 26(11):14–15.
- Lin CM, Wang DP, Cui FZ, Zhu HY, Yang XS. 2012. Volatile components of Polygonum hydropiper distributed in Guizhou Province. Guihaia. 32(03):410–414.
- Lin YW. 2011. Antibacterial experiment of two Polygonaceae plants in vitro. J Microbiol. 31(03):102–105.
- Liu FR. 2017. Chinese herbal medicine for fishery (2). Hunan Agric. 8:25.
- Liu MQ, Tao JY, Hu WY, Wei YY, Hu TJ. 2021. Anti-inflammatory mechanism of FEA from Polygonum hydropiper L. in LPS-treated RAW264.7 cells. Prog Vet Med. 42(06):40–45.
- Liu QF, Luyten W, Pellens K, Wang YM, Wang W, Thevissen K, Liang QL, Cammue BPA, Schoofs L, Luo GA. 2012. Antifungal activity in plants from Chinese traditional and folk medicine. J Ethnopharmacol. 143(3):772–778.
- Liu SQ, Wang GR. 2017. Prevention and control techniques of fish diseases in spring. Fishery Guidetobe Rich. 3:57–58.
- Liu WZ. 2020. Using Chinese herbal medicine to treat eel disease. New Countrys. 12:28.
- Liu XP, Zhang C, Tan ZW, Liu YX, Yu AN. 2009. Study on chemical components for volatile oil from Polygonum flaccidum Messin. Chin J Mod Appl Pharm. 26:285–288.
- Liu YX, Qin JF, Zhang BB, Tang WY. 2009. Fatty acids from Polygonum hydropiper L. J Henan Univ Med Sci. 28(01):32–34.
- Lu CX, Huang HC, Tu LQ, Xie J, Ren CZ. 2021. Study on anti-PRV activity ethyl acetate of flavonoid from. Polygonum hydropiper L. in vitro. Heilongjiang J Anim Sci Vet Med. 5:131–135.
- Luo WJ, Tao JY, Yang J, Zeng Y, Wei YY, Hu TJ. 2017. Effects of flavonoids from Polygonum hydropiper on levels of reactive oxygen species and inflammatory factors in RAW264.7 cells induced by lipopolysaccharide. Prog Vet Med. 38(08):1–6.
- Luo XY. 2017. Study on quality evaluation and antibacterial activity in vitro of Polygonum hydropiper [master’s thesis]. Guangzhou: Guangdong Pharmaceut Univ. p. 36–38.
- Luo XY, Cheng XX, Yang HW, Wen ST, Yang Q. 2017. Study on extraction technology of total flavonoids from Polygonum hydropiper and antimicrobial susceptibility of different polarity fractions. Mod Chin Med. 19:839–844.
- Ma YM, Li MY, Guo LX, Qiao K, Fan C. 2017. Antibacterial and antioxidant activity of the chemical compositions of Polygonum hydropiper L. J Shanxi Univ Sci Technol. 35(01):120–123.
- Maheswaran R, Ignacimuthu S. 2013. Bioefficacy of essential oil from Polygonum hydropiper L. against mosquitoes, Anopheles stephensi and Culex quinquefasciatus. Ecotoxicol Environ Saf. 97:26–31.
- Miyazawa M, Tamura N. 2007. Inhibitory compound of tyrosinase activity from the sprout of Polygonum hydropiper L. (Benitade). Biol Pharm Bull. 30(3):595–597.
- Muhammad A, Muhammad J, Farhat U, Abdul S, Fazal S, Azam KM, Waqar A, Gowhar A, Muhammad I, Sajjad A. 2016. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Front Pharmacol. 7:74.
- Muhammad A, Muhammad J, Jawad A, Farhat U, Abdul S, Imran S. 2014. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BioMed Central. 14:145.
- Oany AR, Siddikey AA, Hossain MU, Islam R, Emran AA. 2016. A preliminary evaluation of cytotoxicity, antihyperglycemic and antinociceptive activity of Polygonum hydropiper L. ethanolic leaf extract. Clin Phytosci. 2(1):1–6.
- Ono K, Nakao M, Toyota M, Terashi Y, Yamada M, Kohno T, Asakawa Y. 1998. Catechin production in cultured Polygonum hydropiper cells. Phytochemistry. 49(7):1935–1939.
- Peng WD. 2016. Characteristics and applications of botanical insecticides. Gansu Sci Technol. 32(19):143–144.
- Ren CZ, Lu CX, Wei YY, Yu ML, Hu TJ. 2021. Effect of ethyl acetate fraction of flavonoids from Polygonum hydropiper L. on cytokines secretion in RAW264.7 cells induced by Pseudorabies virus. China Anim Husb Vet Med. 48:1132–1140.
- Ren SZ, Su WQ, Zhu HR, Wang N, Niu HY, Zhao YM, Ma ZJ. 2018. Study on protective effects of Polygonum hydropiper extract on acute gastric mucosal injury in rats. China Pharm. 29:955–958.
- Ren XD. 2017. Diagnosis and treatment of porcine epidemic diarrhea. Chin J Anim Husb Vet Med. (12):83.
- Sharif S, Shahriar M, Haque M, Chowdury ZS, Islam M, Bhuiyan MA. 2013. In-vitro antioxidant activities, anti-nociceptive and neuropharmacological activities of Polygonum hydropiper. J Biol Agric Healthc. 19(3):62–70.
- Shi YW, Liu QZ, Jiang H, Wang HJ, Liu Y. 2017. Extraction processed antibacterial activity on total flavonoids from Polygonum hydropiper. Mod J Anim Husb Vet Med. 4:5–8.
- Tao JY, Wei YY, Hu TJ. 2016. Flavonoids of Polygonum hydropiper L. attenuates lipopolysaccharide-induced inflammatory injury via suppressing phosphorylation in MAPKs pathways. BMC Complement Altern Med. 16:25.
- Tripathi AK, Jain DC, Singh SC. 1999. Persistency of bioactive fractions of Indian plant, Polygonum hydropiper as an insect feeding deterrent. Phytother Res. 13(3):239–241.
- Wang DY, Tang C, Liao BX, Luo J, Pan NS. 2019. Microscopic identification and detection of metallic, elements in Polygonum flaccidum Meissn. in Guizhou. Food Drug. 21(01):24–27.
- Wang GQ. 2014. National Compendium of Chinese Herbal Medicine (Volume II). Beijing: people’s Health Publishing House. p. 1110–1111. Chinese.
- Wang HC. 2008. Germplasm resources and prospect of utilization and exploitation about Polygonum L. Qinghai Prataculture. 17(4):17–21.
- Wang JH, Liu ZT, Zhao XR. 1996. An initial study on chemical composition and tissue of 6 medicinal plants of Polygonum. Henan Sci. 109–111.
- Wang JX. 2016. Application of Chinese herbal medicine in aquaculture. Shandong J Anim Sci Vet Med. 37(11):56–57. Chinese.
- Wang PQ, Zhang XN, Liu YX, Li CL, Kang WY. 2013. Antibacterial activities of nine Polygonaceae plants. Chin J Exp Tradit Med Formulae. 19(17):109–112.
- Wang W, Xia XH, Zeng R, Chen SH, Fu RG, Li B, Xie Q, Hu J, Xie LJ. 2018. Scientific and technological achievements. Chasngsha: Hunan University of Traditional Chinese Medicine. Chinese.
- Wang XY, Li YM, Sun QX. 2021. How to prevent and cure fish disease with Chinese herbal medicine in high temperature season. J Aquac. 42(9):71–72.
- Wang Y, Yu TY, Wang ZB, Wang QH, Kuang HX. 2017. GC-MS analysis and antibacterial effect of volatile. oil in Polygonum hydropiper. Chem Eng. 31(12):26–29.
- Wang YC, Wei K, Lin J. 2012. Recent advances of chemical constituents and pharmacological activities of genus Polygonum. Guangdong Chem Ind. 39(09):16–17.
- Wang YP, Wang QY, Wang YY. 1996. A review of Chinese medicinal plants of the genus Polygonum. Lishizhen Med Mater Med Res. (03):47–48.
- Wang ZX. 1996. Newly complied Latin Chinese-English Plant Names. Beijing: Aviation Press. p. 569.
- Wu LY, Li Q, Cang T. 2007. Study on the extraction technology and insecticidal activities of volatile oil of Polygonum hydropiper Linn. Guangdong Agric Sci. 9:72–73.
- Xiao H. 2018. Study on the chemical composition of dichloromethane fraction and its active diversity in Polygonum hydropiper [master’s thesis]. Changsha: Hunan University of Traditional Chinese Medicine.
- Xiao H, Ravu RR, Tekwani BL, Li W, Liu WB, Jacob MR, Khan SI, Ca X, Peng CY, Khan IA, et al. 2017. Biological evaluation of phytoconstituents from Polygonum hydropiper. Nat Prod Res. 31(17):2053–2057.
- Xiao ZH, Luo XY, Cheng XX, Zheng SJ, Yang Q, Yang HW. 2018. Effect of polygoni hydropiperis herbal on intestinal mucosal repair in diarrhea mice induced by Escherichia coli. Chin J Exp Med Formulae. 24(22):120–126.
- Xie YH, Li L, Xiao Q, Yu ML, Hu TJ. 2021. Effect of flavonoids from Polygonum perfoliatum L. on immune regulation in mice. Mod J Anim Husb Vet Med. 1:28–32.
- Xu R, Xiong W, Long ZB, Tang L. 2017. The chemical constituent study of Polygonum hydropiper L. Guangdong Chem Ind. 44(05):22–23.
- Yagi A, Uemura T, Okamura N, Haraguchi H, Imoto T, Hashimoto K. 1994. Antioxidative sulphated flavonoids in leaves of Polygonum hydropiper. Phytochemistry. 35(4):885–887.
- Yang XZ, Hao ZY, Zhu YC, Dong Y, Lin HK. 2014. Antioxidant activity of different parts of Polygonum hydropiper. Jiangsu Agric Sci. 42:284–285.
- Yang YY, Yu T, Jang HJ, Byeon SE, Song SY, Lee BH, Rhee MH, Kim TW, Lee J, Hong S, et al. 2012. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol. 139(2):616–625.
- Yao ZF, Zhou LC, Liu JX. 1999. Study on the chemical composition of volatile oil of Polygonum hydropiper in Hunan. Nat Prod Res Dev. 2:37–40.
- Yu XL, Luo J, Yang Y, Tang C, Wang DY, Pan NS, Liu YB, Liu L. 2018. Comparison of volatile constituents of Polygonum hydropiper in May, August and November produced in Guizhou China. J Guizhou Med Coll. 43(02):144–153.
- Yue L. 2020. Effects of Polygonum hydropiper on the expressions levels of inflammatory cytokines and cytochrome P450 enzymes in the diarrhea mice induced by E. coli. [master’s thesis]. Guangzhou: Guangdong Pharmaceutical University. p. 54–70.
- Zeng WA. 2007. Study on insecticidal constituents of Polygonum hydropiper Linn. [dissertation]. Changsha: Hunan Agricultural University.
- Zeng WA, Tan JC, Tan L, Chen JZ. 2007. Biological activities of crude extracts from Polygonum hydropiper L. against Pieris rapae L. J Hunan Agric Univ. 33(01):76–78.
- Zeng WA, Tan JC, Tan L, Zhang CY, Chen JZ, Li XW, Li M. 2006. Application and efficacy of Polygonum hydropiper L. Chin Agric Sci Bull. 8:369–372.
- Zhang GY. 2004. Extraction, isolation, and identification of major components [master’s thesis]. Nanjing: Nanjing Forestry University.
- Zhang GY, Zeng T. 2005. Study on chemical constituents of Polygonum hydropiper Linn. Chem Ind for Prod. 25(03):21–24.
- Zhang HQ. 2014. Fresh Polygonum hydropiper L. to treat livestock mange. Chin J Tradit Vet Sci. (5):74.
- Zhang S, Guo Y, Zhao QH, Xue WH, Li YR, Wu XJ, Huo SY. 2020. Study on the bacteriostatic action of Chinese herbal medicine on avian Trichosporon. Poult Sci. 99(9):4530–4538.
- Zhang S, Zhao QH, Xue WH, Li YR, Guo Y, Wu XJ, Huo SY, Li Y, Li CY. 2021. The isolation and identification of Candida glabrata from avian species and a study of the antibacterial activities of Chinese herbal medicine in vitro. Poult Sci. 100(4):1–10.
- Zhang W, Pan YN, Qu SH, Wang DM, Cheng S, Liu XQ. 2018. Anti-inflammatory effects of an extract of Polygonum hydropiper stalks on 2,4,6-trinitrobenzenesulphonic acid-induced intestinal inflammation in rats by inhibiting the NF-κB pathway. Mediators Inflamm. 2018:6029135.
- Zhang W, Tan ZB, Cheng J, Yu ML, Wei YY, Li J, Hu TJ. 2022. Study on acute and subchronic toxicity of Polygonum hydropiper L. extract. China Anim Husb Med. 49(4):1532–1544.
- Zhao FP, Strack D, Baumert A, Subramaniam R, Goh NK, Chia TF, Tan SN, Chia LS. 2003. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry. 62(2):219–228.
- Zhou SM, Cao MX, Hu TJ. 2020. Study on effect of anti-PRRSV of n-butanol part of flavonoids from Polygonum. hydropiper L. in vitro. China Anim Husb Vet Med. 47(02):605–611.
- Zhou SM, Du ZJ. 2019. The preparation of 30 kinds of botanical liquid medicine and the control method of insect pests. Plant Dr. 32(4):37–47.
- Zhu JG, Li L, Wei YY, Yu ML, Chen LX, Hu TJ. 2020. Preliminary study on toxicology of total flavonoids from Polygonum hydropiper L. Mod J Anim Husb Vet Med. (10):19–23.
- Zhu YD. 2020. Chemical characterization and immunomodulatory activity of Polygonum odoratum polysaccharides [master’s thesis]. Kunming: Kunming Medical University. p. 10–17.