Abstract
Context
Sea fennel (Crithmum maritimum L. [Apiaceae]) is an aromatic herb rich in bioactive molecules, such as polyphenols, with potential positive effects on human health.
Objective
This study aimed at the characterization of sea fennel secondary metabolites, focusing on the phenolic fraction.
Materials and methods
Samples of whole sprouts, sole leaves and sole stems were subjected to accelerated solvent extraction with methanol, and the resulting extracts were analyzed by high‑performance thin‑layer chromatography, high-performance liquid chromatography, and liquid chromatography coupled with diode array detection and high-resolution mass spectrometry (LC-DAD-HRMS).
Results
HPTLC and HPLC analyses of sea fennel extracts showed similar chromatographic profiles among the tested samples, and the prevalence of chlorogenic acid within the phenolic fraction was verified. Ten hydroxycinnamic acids, including neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isochlorogenic acid B, isochlorogenic acid A and isochlorogenic acid C, 11 flavonoid glycosides, e.g., rutin, hyperoside, isoquercitrin, two triterpene saponins and two hydroxylated fatty acids, were detected and annotated via liquid chromatography coupled with diode array detection and high-resolution mass spectrometry.
Discussion and conclusions
The use of accelerated solvent extraction and LC-DAD-HRMS for the characterization of sea fennel secondary metabolites allowed the annotation of seven compounds newly detected in sea fennel, including triterpene saponins and hydroxylated fatty acids.
Introduction
Sea fennel (Crithmum maritimum L.) is an aromatic plant belonging to the Apiaceae family. The plant grows in coastal areas of Mediterranean and Black Sea and Atlantic Europe. It is rich in bioactive substances with nutritional and medicinal value (Alves-Silva et al. Citation2020). Its fleshy and succulent leaves are used for the preparation of cooked meals, salads, and pickles (Meot-Duros and Magné Citation2009; Generalić Mekinić et al. Citation2016). In folk medicine, they are applied as carminative, digestive, vermifuge, diuretic, depurative, anti-inflammatory, tonic, and antiscorbutic drug, as well as in the treatment of wounds and common cold (Atia et al. Citation2011; Zafeiropoulou et al. Citation2020). In recent years their ethyl acetate extracts showed activity against hepatocellular carcinoma (HCC) in vitro (Gnocchi et al. Citation2020), acting on the metabolic pathways (Gnocchi et al. Citation2021) and on the bioenergetic profile (Gnocchi et al. Citation2022) of HCC cells, and improving their sensitivity towards sorafenib, a commonly used chemotherapeutic drug (Gnocchi et al. Citation2023). Sea fennel is characterized by the presence of several bioactive constituents like vitamin C, essential fatty acids, essential oils, and polyphenols. Previous phytochemical studies revealed a high content of phenolic acids, mainly chlorogenic acids (Franke Citation1982; Cunsolo et al. Citation1993; Meot-Duros and Magné Citation2009; Generalić Mekinić et al. Citation2016, Citation2018; Pereira et al. Citation2017; Boutellaa et al. Citation2019; Najjaa et al. Citation2020).
Polyphenols represent an ubiquitous and large group of plant metabolites displaying key functions along their entire life cycle. In humans, they exhibit important physiological activities, mainly counteracting oxidative stress. They are therefore consided to be useful in the prevention of diabetes, cancer, cardiovascular diseases and neurological ailments (Han et al. Citation2007; Vrhovsek et al. Citation2012; Rodrigues et al. Citation2015).
Most of the studies dealing with sea fennel polyphenols have been performed using extracts produced by classical room temperature extraction (Nabet et al. Citation2017; Boutellaa et al. Citation2019; Zafeiropoulou et al. Citation2020; Souid et al. Citation2021), which was in some cases enhanced by the application of ultrasonic waves (ultrasound-assisted extraction, UAE) (Kumar et al. Citation2021; Martins-Noguerol et al. Citation2022), with methanol/water (Martins-Noguerol et al. Citation2022) or ethanol/water (Souid et al. Citation2021) mixtures. By the use of pressurized solvent, regularly at a pressure between 10 and 15 MPa, and high temperatures, regularly between 50 and 200 °C (Wang and Weller Citation2006), accelerated solvent extraction (ASE) is an advanced technique allowing a more efficient extraction of phenolic compounds than classical (Li et al. Citation2019) or ultrasound assisted methods (Pietrzak et al. Citation2014; Repajić et al. Citation2020).
Given these premises, the present study aimed at the characterization of the bioactive compounds of sea fennel with a focus on the polyphenolic fraction. Methanolic extracts from the whole sprouts, sole leaves and sole stems, obtained by accelerated solvent extraction, were analyzed by high‑performance thin‑layer chromatography (HPTLC), high-performance liquid chromatography (HPLC), and liquid chromatography coupled with diode array detection and high-resolution mass spectrometry (LC-DAD-HRMS) in order to identify the whole range of contained phenolic constituents.
Materials and methods
Test samples
Sea fennel cultivated in the Conero Natural Park, south of Ancona, Italy, was kindly supplied in June 2021 by a producer of sea fennel-based food preserves (Rinci S.r.l., Castelfidardo, Ancona, Italy). Fresh sea fennel sprouts (approximately 1 kg) were transported to the laboratory under cooled conditions (4 ± 2 °C), dried in a dehydrator (Captain Jerky 110, Klarstein, Berlin, Germany) at 30 °C, and stored in plastic bags under vacuum condition at room temperature (∼18–20 °C), prior to the analysis. Eight samples were prepared from the whole sprouts (S1, S2, S3, S4, S5, S6, S7, S8), one from the stems only (S9), and another one only from leaves (S10).
Chemicals and reagents
Methanol (ROTIPURAN® ≥ 99.9%, p.a., ACS, ISO), chloroform (≥99%, DAB, BP, pure), ethyl acetate (≥99.5%, Ph.Eur., pure), polyethylene glycol 4000, diphenylboric acid β-aminoethyl ester complex (natural product reagent A) (≥98%, p.a.), formic acid (ROTIPURAN® ≥ 98%, p.a., ACS), ortho-phosphoric acid (85% ROTIPURAN®, p.a., ISO), luteolin (ROTICHROM® 90%), hyperoside (ROTICHROM® TLC), chlorogenic acid (ROTICHROM® TLC), diosmin (purum), esculetin (purum CHR), (+)-catechin (pract CHR), (–)-quinic acid (purum CHR), (–)-epicatechin (∼95%), ferulic acid (≥99%), gallic acid (≥98%, p.a., ACS), hesperidin (CHR), protocatechuic acid (purum CHR), rutin (purum), syringic acid (purum CHR), kaempferol (ROTICHROM® CHR) and quercetin (puriss. CHR) were purchased from Carl Roth GmbH (Karlsruhe, Germany).
Neochlorogenic acid (≥98.0%), cryptochlorogenic acid (≥98.0%), isochlorogenic acid A (≥98.0%), isochlorogenic acid B (≥98.0%) and isochlorogenic acid C (≥98.0%) were purchased from Chengdu Push Bio-technology Co., Ltd (Wuhou Science Park, Chengdu, China).
Methanol (HiPerSolv, CHROMANORM®), acetonitrile (HiPerSolv, CHROMANORM®), and water (HiPerSolv, CHROMANORM®) were purchased from VWR International SAS (Fontenay Sous Bois, France), while glacial acetic acid (100% anhydrous for analysis) was bought from Merck KGaA (Darmstadt, Germany).
The reference compounds rosmarinic acid, p-coumaric acid (purum ≥ 98%, HPLC) and quercetin-3-O-glucoside were purchased from PhytoLab GmbH and Co. KG (Vestenbergsgreuth, Germany), Thermo Fisher Scientific, (Waltham, MA, USA), and Extrasynthese (Genay, France), respectively. Quercitrin CRS (European Pharmacopoeia Reference Standard) was purchased from EDQM (Strasbourg, France).
Accelerated solvent extraction
Dried sea fennel samples were ground using an analytical mill (A 11 basic, IKA®-Werke GmbH & Co. KG Staufen, Germany), mixed 4:1 (w/w) ratio with diatomaceous earth (Thermo Fisher Scientific, Waltham, MA, USA), and successively extracted with methanol by means of an accelerated solvent extractor (Dionex™ ASE™ 150, Thermo Fisher Scientific, Waltham, MA, USA). Extraction was performed setting the parameters as follows: heat time: 5 min; static time: 5 min; rinse volume: 40%; purge time: 60 s; cycles: 3; temperature: 68 °C. The extracts were dried under nitrogen flow and then stored at −20 °C until use.
Sample preparation
The dried extracts were dissolved in methanol at a concentration of 10 mg/mL, sonicated for 5 min at room temperature in an ultrasonic bath (Transsonic T 460/H, Elma Schmidbauer GmbH, Singen, Germany) and centrifuged for 15 min at 13.000 rpm with a centrifuge (Biofuge® pico, Heraeus, Hanau, Germany), to obtain a clear sample for further analyses. The reference compounds were dissolved in methanol at a concentration of 1 mg/mL. For high‑performance thin‑layer chromatography analysis, three separate solutions with a mix of reference compounds were prepared as follows: mix 1: esculetin, protocatechuic acid, gallic acid, and hyperoside; mix 2: ferulic acid, quercitrin, rosmarinic acid, quercetin-3-O-glucoside, and rutin; mix 3: kaempferol, quercetin, and chlorogenic acid.
High‑performance thin‑layer chromatography
High‑performance thin‑layer chromatographic (HPTLC) analyses were performed using a CAMAG-HPTLC system (CAMAG Chemie‑Erzeugnisse und Adsorptionstechnik AG, Muttenz, Switzerland) operated with winCATS software (CAMAG). Aliquots of samples (10 μL) and mixed reference compound solutions (5 μL) were applied to HPTLC glass plates coated with silica gel 60 F254 (Merck KGaA, Darmstadt, Germany) by a CAMAG Automatic TLC Sampler ATS 4. Two HPTLC separations were performed using two different mobile phase systems. In the first analysis, the application length for all the samples was set at 7 mm and a mobile phase consisting of chloroform-glacial acetic acid-methanol-water (64:32:12:8) was employed (Wagner and Bladt Citation1996). While, in the second analysis, the application length for all the samples was set at 8 mm and a mobile phase consisting of ethyl acetate-formic acid-glacial acetic acid-water (100:11:11:26) was used (Wagner and Bladt Citation1996). HPTLC plates were developed in a CAMAG Automatic Developing Chamber ADC2 after 20 min equilibration with saturation pad and 5 min plate preconditioning to a final migration distance of 75 mm. After drying, the plates were derivatized with natural products-polyethylene glycol reagent (NP/PEG). The plates were visualized and photographed with a CAMAG TLC visualizer 2 after development and after derivatization at UV 254 and 366 nm, and at white light.
Liquid chromatography‑diode array detection mass spectrometry
Fingerprint analyses and annotation of major compounds
Liquid chromatography‑diode array detection-high‑resolution mass spectrometry (LC-DAD-HRMS) analyses were performed using an Ultimate 3000 HPLC hyphenated with a Q Exactive™ hybrid quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific) in both HESI positive and negative mode. The separation was carried out on a Zorbax Extend-C18 column (3.5 μm, 4.6 mm × 150 mm, Agilent). The mobile phase consisted of water + 0.1% formic acid (A) and acetonitrile + 0.1% formic acid (B). A flow rate of 1.0 ml/min was applied and the gradient was set as follows: 0–4 min, 12% B in A; 4–5 min, 12%–20% B in A; 5–15 min, 20% B in A; 15–22 min, 20%–95% B in A; 22–24 min, 95% B in A; 24–25 min, 95%–12% B in A; 25–30 min, 12% B in A. The column temperature was set to 30 °C. The diode array detector was set to a range from 200 to 400 nm. The mass spectrometer was run in both, HESI positive and negative modes using the following parameters: probe heater temperature 350 °C; capillary temperature 330 °C; spray voltage 3.5 kV for positive and 3.1 kV for negative ion mode; sheath gas flow 65 arbitrary units; auxiliary gas flow 20 arbitrary units; resolution: 70.000 (full MS) and 17.500 (data‑dependent MS2). A volume of 5 μL was injected for the samples, reference and blank solutions. Data evaluation was performed with Thermo Xcalibur 2.2.44 (Thermo Fisher Scientific). Compounds were annotated by comparing retention time, precursor monoisotopic mass, and MS/MS fragment ion masses with authentic references, or by comparing MS/MS fragmentation patterns with literature. Molecular formulas were calculated from the exact mass using Thermo Xcalibur 2.2.44 software (Thermo Fisher Scientific).
Annotation of flavonoid aglycone moieties, triterpene saponins and hydroxylated fatty acids by LC-MSn
Identification of flavonoid aglycones was performed by an Ultimate 3000 HPLC hyphenated LTQ-XL linear ion trap mass spectrometer with HESI interface (Thermo Fisher Scientific) operated in negative mode. The HPLC separation was carried out as described in the section 'Fingerprint analyses and annotation of major compounds’. The mass spectrometer was run in the HESI negative mode using the following parameters: source heater temperature 350 °C; capillary temperature 330 °C; spray voltage 3.0 kV, sheath gas flow 50 arbitrary units; auxiliary gas flow 10 arbitrary units. The volume of 5 μL was injected for the samples, for reference and blank solutions. Flavonoid aglycone moieties were identified by comparison of the MS3 or MS4 fragmentation patterns in the respective glycosides to MS2 fragmentation patterns of authentic reference compounds of the respective aglycones. Additionally, these data were used for annotation of compounds 23–26.
Semiquantitative determination of hydroxycinnamic acid derivative and flavonoid levels by high performance liquid chromatography with diode array detection
High performance liquid chromatography (HPLC) analyses were performed by a 1260 Infinity HPLC-DAD system (Agilent Technologies, Inc., Santa Clara, CA, USA). Separation was performed with the same system described in the section 'Fingerprint analyses and annotation of major compounds’, with the exception that the mobile phase consisted of water + 0.1% ortho-phosphoric acid (A) and acetonitrile (B). DAD-detection was carried out at 320 and 360 nm. Chlorogenic acid and hydroxycinnamic acid derivatives were quantified using chlorogenic acid as external standard, while flavonoids were quantified using rutin as external standard. Calibration curves were prepared using six different concentrations of the reference compounds dissolved in methanol (1, 10, 50, 100, 500, 1000 μg/mL) and were injected in the same condition as the samples. For quantification, the peak area of each hydroxycinnamic acid derivative was recorded at 320 nm. For establishing the rutin calibration curve, detection was carried out at 360 nm. The results were calculated as g/100 g dry weight (DW) of sea fennel, and expressed as mean value of two replicates ± standard deviation. Chlorogenic acid concentration was calculated from the peak area of peak 3. The level of total hydroxycinnamic acid derivatives was calculated using the areas of peaks 2, 3, 4, 6, 7, 8, 9, 15, 16 and 18. The approximate flavonoid content was calculated from the areas of peaks 5, 10, 11, 12, 13, 14, 17, 19, 20, 21 and 22 (peak numbers as in ). Data evaluation was performed with Agilent ChemStation.
Statistical analysis
The results of the quantification of chlorogenic acid, hydroxycinnamic acid derivatives and flavonoids were subjected to one-way analysis of variance (ANOVA) through the Tukey-Kramer honest significant difference (HSD) test (p ≤ 0.05), to evaluate differences between the samples. The software JMP Version 11.0.0 (SAS Institute Inc., Cary, NC, USA) was used for the analysis.
Results
Accelerated solvent extraction
The extraction yields of each sample are listed in , as absolute (g) and relative (%) values. Stems (sample S9) provided higher extraction yields (19.01%) than leaves (sample S10, 14.30%).
Table 1. Absolute and relative extract yields obtained by accelerated solvent extraction (ASE) of sea fennel whole sprouts (sample S1, S2, S2, S3, S4, S5, S6, S7, S8), stems (S9) and leaves (S10).
Chemical profiling by high‑performance thin‑layer chromatography
The HPTLC analyses of sea fennel extracts highlighted similar chromatographic profiles among the tested samples. The use of a mobile phase generally employed for the analysis of polyphenols, namely ethyl acetate-formic acid-glacial acetic acid-water (100:11:11:26), allowed a proper chromatographic separation of rutin, chlorogenic acid, hyperoside, quercetin-3-O-glucoside, quercitrin, and rosmarinic acid, while the use of chloroform-glacial acetic acid-methanol-water (64:32:12:8) as mobile phase allowed the separation of gallic acid, protocatechuic acid, esculetin, quercetin, kaempferol and ferulic acid. By the use of corresponding reference compounds and derivatization with natural products-polyethylene glycol reagent (NP/PEG) () we could identify rutin (Rf = 0.34) and chlorogenic acid (Rf = 0.42).
Figure 1. High‑performance thin‑layer chromatography (HPTLC) carried out using a mobile phase consisting of ethyl acetate-formic acid-glacial acetic acid-water (100:11:11:26), derivatized with natural products-polyethylene glycol reagent (NP/PEG) and observed at UV 366 nm. S1, S2, S3, S4, S5, S6, S7, S8: sea fennel whole sprouts; S9 sea fennel stems; S10: sea fennel leaves. R1: reference compounds mix 1 (1: protocatechuic acid; 2: esculetin; 3: gallic acid; 4: hyperoside); R2: reference compounds mix 2 (5: ferulic acid; 6: rosmarinic acid; 7: quercitrin; 8: quercetin-3-O-glucoside; 9: rutin); R3: reference compounds mix 3 (10: kaempferol; 11: quercetin; 12: chlorogenic acid).
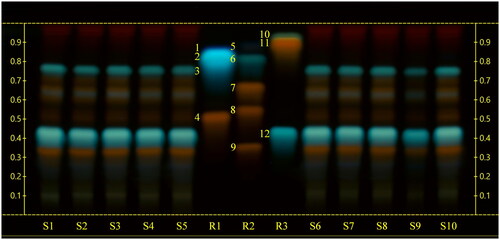
Three more compounds, namely hyperoside, quercetin-3-O-glucoside and quercitrin were tentatively allocated, but their bands in the samples were not well enough separated for unambiguous identification. Ferulic acid, kaempferol, quercetin, esculetin, protocatechuic acid, gallic acid and rosmarinic acid could not be detected in the extracts by HPTLC ().
Figure 2. High‑performance thin‑layer chromatography (HPTLC) carried out using a mobile phase consisting of chloroform-glacial acetic acid-methanol-water (64:32:12:8), derivatized with natural products-polyethylene glycol reagent (NP/PEG) and observed at UV 366 nm. S1, S2, S3, S4, S5, S6, S7, S8: sea fennel whole sprouts; S9 sea fennel stems; S10: sea fennel leaves. R1: reference compounds mix 1 (1: protocatechuic acid; 2: esculetin; 3: gallic acid; 4: hyperoside); R2: reference compounds mix 2 (5: ferulic acid; 6: rosmarinic acid; 7: quercitrin; 8: quercetin-3-O-glucoside; 9: rutin); R3: reference compounds mix 3 (10: kaempferol; 11: quercetin; 12: chlorogenic acid).
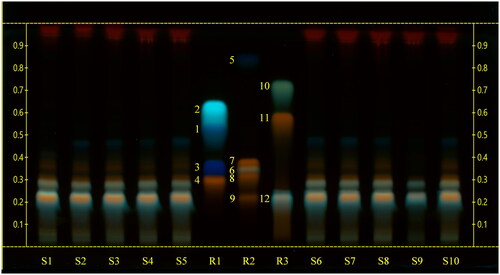
As far as the intensity of the bands was concerned, chlorogenic acid was the predominant compound in all the samples.
Chemical profiling of whole sea fennel sprouts methanolic extract by liquid chromatography‑diode array detection mass spectrometry
Preliminary HPLC analyses, performed as described in the section 'Semiquantitative determination of hydroxycinnamic acid derivative and flavonoid levels by high performance liquid chromatography with diode array detection’, had confirmed the presence of the same compounds in all the test samples (data not shown). Therefore, the sample S1, constituted by sea fennel whole sprouts, was chosen as representative for LC-DAD-HRMS analyses.
The chromatograms generated from sample S1 by HESI negative mode and DAD detection are depicted in .
Figure 3. Base peak chromatogram of sea fennel methanolic extract in HESI negative mode full MS [m/z 110–1650] (A) and DAD (200–400 nm) (B).
![Figure 3. Base peak chromatogram of sea fennel methanolic extract in HESI negative mode full MS [m/z 110–1650] (A) and DAD (200–400 nm) (B).](/cms/asset/3642e800-3166-4363-abe1-99bca3bd399c/iphb_a_2224820_f0003_b.jpg)
HESI negative mode was used for compound annotation because more compounds were detectable in the negative than in the positive mode. In sea fennel methanolic extracts, 26 compounds could be annotated, belonging to the following classes: organic acids, hydroxycinnamic acid derivatives, flavones, flavonols, triterpene saponins, and hydroxylated fatty acids ().
Table 2. Constituents annotated from sample S1, ordered by retention time (see ).
Eleven of these compounds were unambiguously identified by comparing their retention times, precursor monoisotopic mass and MS/MS fragmentation patterns with that of authentic reference substances, and 15 compounds were tentatively annotated by comparing precursor monoisotopic mass, MS/MS fragmentation patterns and the molecular formulas calculated from the exact mass with existing data from literature. In cases where MS/MS fragmentation was not sufficient for annotation, MS3 and MS4 fragmentation patterns generated in a linear ion trap mass spectrometer were additionally used.
Ten hydroxycinnamic acids were detected in the extract, six of them were unambiguously identified as neochlorogenic acid (2), chlorogenic acid (3), cryptochlorogenic acid (4), isochlorogenic acid B (15), isochlorogenic acid A (16) and isochlorogenic acid C (18).
The fragmentation pattern of compound 6 indicated that it is composed of a caffeic acid and a quinic acid moiety. The MS/MS fragmentation pattern is the same as 5-caffeoylquinc acid (chlorogenic acid; compound 3) but it eluted significantly later. Accordingly, it was assigned as cis-5-caffeoylquinic acid (Clifford et al. Citation2005, Citation2008).
The isomeric compounds 7 and 9 exhibited a MS/MS fragment with m/z 191, matching quinic acid, that was obviously generated by the neutral loss of a coumaroyl moiety. The MS/MS base peak at m/z 191 indicated a 5-O-coumaroylquinic acid (Clifford et al. Citation2003). According to retention times, compounds 7 and 9 were annotated as trans-5-O-p-coumaroylquinic acid and cis-5-O-p-coumaroylquinic acid, respectively (Jaiswal et al. Citation2014; Nabet et al. Citation2017).
Compound 8 also exhibited a MS/MS fragment at m/z 191 [M-H-C10H8O3]–. The neutral loss of C10H8O3 indicated a feruloyl moiety that was obviously attached to quinic acid. The MS/MS base peak at m/z 191 indicated that the ferulic acid moiety is attached at position 5 (Clifford et al. Citation2003). Hence the compound was annotated as 5-O-feruoylquinic acid.
In total, 11 flavonoid glycosides were annotated: three flavone and eight flavonol glycosides. Flavones were represented by two O-glycosides and one C-glycoside. Among O-glycosidic flavones, compound 11 generated a parent ion at m/z 593.1514 [M-H]–, corresponding to the calculated molecular formula C27H30O15. The fragment at m/z 447 was caused by the loss of the deoxyhexose, and the combined loss of a hexose and a deoxyhexose moiety caused the fragment at m/z 285 [M-H-hexose-deoxyhexose]–. MSn fragmentation patterns indicated a 7-O-diglycoside moiety (Cuyckens and Claeys Citation2004; Vukics and Guttman Citation2010). The aglycone was annotated as luteolin by comparison with an authentic luteolin reference (). Accordingly, compound 11 was tentatively annotated as luteolin-7-O-hexosyl-deoxyhexoside.
Table 3. MSn fragmentation data of selected compounds.
Compound 17 presented a [M-H]– ion at m/z 607.1672, corresponding to the molecular formula C28H32O15.The fragment at m/z 299 derived from the combined loss of a hexose and a deoxyhexose. A neutral loss of 15 Da indicates the presence of a cleavable methyl group within the aglycone. By comparison with an authentic reference, compound 17 was assigned as diosmin (diosmetin 7-O-rutinoside), previously reported in sea fennel (Cornara et al. Citation2009).
Compound 5 showed a parent ion at m/z 593.1513 [M-H]–, corresponding to a calculated neutral formula of C27H30O15. Fragment ions at m/z 503 [M-H-90]–, 473 [M-H-120]–, 383 [M-H-90-120]–, 353 [M-120-120]– indicated the presence of two C-glycosidic hexose moieties (Cuyckens and Claeys Citation2004). By comparison with literature data, compound 5 was assigned vicenin 2 (apigenin-6,8-di-C-glycopyranoside), previously reported from sea fennel (Gouvea et al. Citation2017; Zafeiropoulou et al. Citation2020).
All annotated flavonols were O-glycosides, including rutin, (10), hyperoside (12) and isoquercitrin (13), which were unambiguously identified by comparison of their retention times and MS/MS fragmentation patterns with reference standards.
The parent ion of compound 14 was detected at m/z 433.0773 [M-H]–. The neutral loss of 132 Da indicated the presence of an O-glycosidic pentose moiety. By comparison of its MS3 fragmentation pattern with the MS2 fragmentation patterns of an authentic flavonoid aglycone reference, the aglycone was identified as quercetin (). Additionally, the fragment at m/z 271 [Aglycone-H-2H-CO]– indicated that the pentose moiety was attached at position 3 (Vukics and Guttman Citation2010). Hence, compound 14 was tentatively annotated as quercetin-3-O-pentoside.
Beside flavonol monoglycosides, four flavonol-hydroxycinnamoyl-glycosides were annotated. Compounds 20 and 21 both exhibited parent ions at m/z 609.1256 and a calculated molecular formula of C30H26O14. Both compounds exhibited an MS/MS fragment ion at m/z 463. Neutral loss of 146 Da and calculated molecular formula of the neutral fragment (C9H6O2) indicated the cleavage of a coumaroyl moiety. Subsequent loss of 162 Da and the absence of a fragment at m/z 447 [M-Hex-H]– led to the conclusion that both compounds contain O-coumarylhexose moieties (Kachlicki et al. Citation2016). In analogy to compound 14, the aglycone was assigned to quercetin and the fragment at 271 indicated glycosylation in position 3, leading to the assignment of compound 20 and 21 to two isomeric quercetin-3-O-coumaroylhexosides.
Similarly, compound 19 and 22 consisted of quercetin and an O-glycosidic hexose. In these cases, calculated molecular formulas and neutral losses of C9H6O3 and C10H8O3 led to the assumption that compounds 19 and 22 contained a caffeic acid and a ferulic acid moiety, respectively. Accordingly, compound 19 was annotated as quercetin-3-O-caffeoyl-hexoside and compound 22 as quercetin-3-O-feruloylhexoside.
Two triterpene saponins were also annotated. MS/MS fragmentation patterns of both, compounds 23 and 24, indicated the presence of three hexose and a glucuronic acid moiety. The aglycones of compounds 23 and 24 were found to possess calculated molecular formulas of C31H50O4 and C30H46O3, respectively.
Finally, two hydroxylated fatty acids were annotated. The calculated molecular formula (C18H32O5) and the fragmentation pattern of compound 25 indicated a trihydroxy-octadecadienoic acid (FA 18:2; O3). The calculated molecular formula (C18H34O5) and the fragmentation pattern of compound 26 indicated a trihydroxy-octadecenoic acid (FA 18:1; O3). By comparing MS/MS data of 26 with literature, the compound was annotated tianshic acid (Gao et al. Citation2016; Kothari et al. Citation2020).
Semiquantitative determination of hydroxycinnamic acid derivative and flavonoid levels
The results of the semiquantitative determination of hydroxycinnamic acid derivatives and flavonoids are reported in .
Table 4. Concentration of chlorogenic acid, hydroxycinnamic acid derivatives and flavonoids in sea fennel.
One-way analysis of variance (ANOVA) highlighted significant differences in flavonoid and hydroxycinnamic acid derivative levels among the whole sprout samples. All the compounds resulted to be less concentrated in stems (S9) than in leaves (S10). Whereas, chlorogenic acid resulted to be the most abundant polyphenol with contents ranging from 0.81 (S1) to 1.19 (S3) g/100 g DW sea fennel in the whole sprouts.
Discussion
Starting from the premise that sea fennel is rich in several classes of bioactive compounds and taking into consideration the increasing interest in polyphenols for their health benefits (Vrhovsek et al. Citation2012), this study aimed at a holistic characterization of sea fennel phenolic constituents. Accelerated solvent extraction using pure methanol as solvent was performed, in order to recover the phenolic compound-enriched fraction (Alonso-Salces et al. Citation2001; Sun et al. Citation2012) from the whole sprouts, leaves, and stems, to be subjected to further characterization.
The extraction yield is a parameter influenced not only by the extraction solvent and method, but also by the vegetable matrix (Pferschy-Wenzig and Bauer Citation2015). This assumption is in agreement with the different extraction yields obtained between leaves and stems.
The combination of two HPTLC methods with different mobile phases allowed a good separation for all the reference compounds of different polarity (Jesionek et al. Citation2015). The derivatization of the plates with natural products-polyethylene glycol reagent (NP/PEG), regularly employed for polyphenol analysis, and the observation at UV 366 nm returned structure-dependent typical fluorescence (Wagner and Bladt Citation1996).
Nineteen of the compounds annotated in sample S1 on the basis of LC-HRMS data have been detected in sea fennel in previous phytochemical studies (Nabet et al. Citation2017; Alves-Silva et al. Citation2020; Sánchez-Faure et al. Citation2020; Souid et al. Citation2020; Zafeiropoulou et al. Citation2020; Piatti et al. Citation2023), while seven compounds, namely luteolin-7-O-hexosyl-deoxyhexoside, quercetin-caffeoyl-hexoside, quercetin-feruloyl-hexoside, the two hydroxylated fatty acids and the two triterpene glycosides, were newly found in sea fennel.
Saponins are a group of compounds consisting of a triterpene or steroid aglycone and one or more sugar chains. These compounds, traditionally considered as ‘anti-nutritional factors’ in food, are recognized, nowadays, as the active principles in many herbs used in traditional medicine. Saponins exhibit a wide range of biological activities behaving as hypocholesterolemic, anti-mutagenic, anti-inflammatory, antioxidant, immunomodulatory, hepatoprotective and neuroprotective agents (Liu and Henkel Citation2002; Sparg et al. Citation2004; Güçlü-Üstündağ and Mazza Citation2007). Furthermore, among the tentatively identified compounds new for sea fennel, hydroxylated fatty acids are described in literature as bioactive compounds with antimicrobial, cytotoxic and anti-neuroinflammatory properties (Masoodi et al. Citation2008; Serag et al. Citation2020). Therefore, comprehensive analysis of sea fennel extract by LC-HRMS provided evidence that next to phenolic constituents, other potentially bioactive compounds like triterpene glycosides and hydroxy fatty acids are present that may deserve more in-depth evaluation.
As far as the semiquantitative determination of hydroxycinnamic acid derivatives and flavonoids is concerned, the lower concentration detected in stems than in leaves and the presence of chlorogenic acid as the most concentrated polyphenol are in agreement with previous studies by Pereira et al. (Citation2017) and Meot-Duros and Magné (Citation2009), respectively. Furthermore, the results herein collected agree with available data related to sea fennel growing in different geographical areas. In more detail, concentrations of chlorogenic acid of 0.64 g/100 g DW (Nabet et al. Citation2017), 0.73 g/100 g DW (Souid et al. Citation2021), and between 0.56 and 1.63 g/100 g DW (Generalić Mekinić et al. Citation2018) were reported in sea fennel harvested in Algeria, France, and Croatia, respectively. Chlorogenic acid is a hydroxycinnamic acid described in literature as one of the most widely distributed and functional polyphenols in the human diet, displaying health beneficial effects, behaving as antioxidant, anti-inflammatory, antimicrobial, antimutagenic, cardiovascular protective, neuroprotective, renoprotective, gastrointestinal tract-protective and hepatoprotective agent, and modulating lipid and glucose metabolism (Naveed et al. Citation2018; Lu et al. Citation2020).
Conclusions
In conclusion, the characterization of constituents with potential pharmacological activity performed on sea fennel (Crithmum maritimum) cultivated in the Conero Natural Park highlighted similar polyphenolic profiles among different sprouts despite slight differences in the concentration of the single compounds, and confirmed the predominance of chlorogenic acid in the phenolic fraction. Moreover, the use of accelerated solvent extraction and a comprehensive characterization by HPLC-DAD-HRMS allowed the annotation of a wide range of phenolic constituents, some of them new for sea fennel, and triterpene saponins as well as hydroxylated fatty acids, again newly detected in this plant.
Acknowledgments
We thank NAWI Graz for supporting Central Lab Environmental, Plant & Microbial Metabolomics and Rinci S.r.l for the providing of sea fennel crop.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adouani I, Du M, Hang TJ. 2013. Identification and determination of related substances in diosmin bulk drug and pharmaceutical formulations by HPLC and HPLC-MS. Chromatographia. 76(9–10):499–508. doi: 10.1007/s10337-013-2404-z.
- Alonso-Salces RM, Korta E, Barranco A, Berrueta LA, Gallo B, Vicente F. 2001. Pressurized liquid extraction for the determination of polyphenols in apple. J Chromatogr A. 933(1–2):37–43. doi: 10.1016/s0021-9673(01)01212-2.
- Alves-Silva JM, Guerra I, Gonçalves MJ, Cavaleiro C, Cruz MT, Figueirinha A, Salgueiro L. 2020. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind Crops Prod. 149:112329. doi: 10.1016/j.indcrop.2020.112329.
- Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A. 2011. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J Med Plants Res. 5:3564–3571.
- Boutellaa S, Zellagui A, Öztürk M, Bensouici C, Ölmez ÖT, Menakh M, Duru ME. 2019. HPLC-DAD profiling and antioxidant activity of the n-butanol extract from aerial parts of Algerian Crithmum maritimum L. Acta Sci Nat. 6(1):8–16. doi: 10.2478/asn-2019-0002.
- Clifford MN, Johnston KL, Knight S, Kuhnert N. 2003. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 51(10):2900–2911. doi: 10.1021/jf026187q.
- Clifford MN, Kirkpatrick J, Kuhnert N, Roozendaal H, Salgado PR. 2008. LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 106(1):379–385. doi: 10.1016/j.foodchem.2007.05.081.
- Clifford MN, Knight S, Kuhnert N. 2005. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J Agric Food Chem. 53(10):3821–3832. doi: 10.1021/jf050046h.
- Cornara L, D’Arrigo C, Pioli F, Borghesi B, Bottino C, Patrone E, Mariotti MG. 2009. Micromorphological investigation on the leaves of the rock samphire (Crithmum maritimum L.): occurrence of hesperidin and diosmin crystals. Plant Biosyst. 143(2):283–292. doi: 10.1080/11263500902722527.
- Cunsolo F, Ruberto G, Amico V, Piattelli M. 1993. Bioactive metabolites from Sicilian marine fennel, Crithmum maritimum. J Nat Prod. 56(9):1598–1600. doi: 10.1021/np50099a022.
- Cuyckens F, Claeys M. 2004. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 39(1):1–15. doi: 10.1002/jms.585.
- De Andrade Neves N, Stringheta PC, Gómez-Alonso S, Hermosín-Gutiérrez I. 2018. Flavonols and ellagic acid derivatives in peels of different species of jabuticaba (Plinia spp.) identified by HPLC-DAD-ESI/MSn. Food Chem. 252:61–71. doi: 10.1016/j.foodchem.2018.01.078.
- Farag NF, Farag MA, Abdelrahman EH, Azzam SM, El-Kashoury ESA. 2015. Metabolites profiling of Chrysanthemum pacificum Nakai parts using UPLC-PDA-MS coupled to chemometrics. Nat Prod Res. 29(14):1342–1349. doi: 10.1080/14786419.2015.1025396.
- Franke W. 1982. Vitamin C in sea fennel (Crithmum maritimum), an edible wild plant. Econ Bot. 36(2):163–165. doi: 10.1007/BF02858711.
- Gao X, Ma YL, Zhang P, Zheng XP, Sun BL, Hu FD. 2016. Chemical characteristics combined with bioactivity for comprehensive evaluation of tumuxiang based on HPLC-DAD and multivariate statistical methods. World J Tradit Chinese Med. 2(2):36–47. doi: 10.15806/j.issn.2311-8571.2015.0035.
- García-Villalba R, Espín JC, Tomás-Barberán FA, Rocha-Guzmán NE. 2017. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J Food Compos Anal. 63(Oct. 2017):38–46. doi: 10.1016/j.jfca.2017.07.034.
- Generalić Mekinić I, Blažević I, Mudnić I, Burčul F, Grga M, Skroza D, Jerčić I, Ljubenkov I, Boban M, Miloš M, et al. 2016. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J Food Sci Technol. 53(7):3104–3112. doi: 10.1007/s13197-016-2283-z.
- Generalić Mekinić I, Šimat V, Ljubenkov I, Burčul F, Grga M, Mihajlovski M, Lončar R, Katalinić V, Skroza D. 2018. Influence of the vegetation period on sea fennel, Crithmum maritimum L. (Apiaceae), phenolic composition, antioxidant and anticholinesterase activities. Ind Crops Prod. 124:947–953. doi: 10.1016/j.indcrop.2018.08.080.
- Gnocchi D, Cesari G, Calabrese GJ, Capone R, Sabbà C, Mazzocca A. 2020. Inhibition of hepatocellular carcinoma growth by ethyl acetate extracts of Apulian Brassica oleracea L. and Crithmum maritimum L. Plant Foods Hum Nutr. 75(1):33–40. doi: 10.1007/s11130-019-00781-3.
- Gnocchi D, Del Coco L, Girelli CR, Castellaneta F, Cesari G, Sabbà C, Fanizzi FP, Mazzocca A. 2021. 1H-NMR metabolomics reveals a multitarget action of Crithmum maritimum ethyl acetate extract in inhibiting hepatocellular carcinoma cell growth. Sci Rep. 11(1):1259. doi: 10.1038/s41598-020-78867-1.
- Gnocchi D, Sabbà C, Mazzocca A. 2022. The edible plant Crithmum maritimum shows nutraceutical properties by targeting energy metabolism in hepatic cancer. Plant Foods Hum Nutr. 77(3):481–483. doi: 10.1007/s11130-022-00986-z.
- Gnocchi D, Sabbà C, Mazzocca A. 2023. Crithmum maritimum improves sorafenib sensitivity by decreasing lactic acid fermentation and inducing a pro-hepatocyte marker profile in hepatocellular carcinoma. Plant Foods Hum Nutr. 78(1):230–232. doi: 10.1007/s11130-022-01037-3.
- Gouvea DR, Buqui GA, Lopes JLC, Diniz A, Lopes NP. 2017. An UPLC-MS/MS method for determination of vicenin-2 and lychnopholic acid in rat plasma and its application to a pharmacokinetic study. J Braz Chem Soc. 28:427–434. doi: 10.21577/0103-5053.20160249.
- Granato D, do Prado-Silva L, Alvarenga VO, Zielinski AAF, Bataglion GA, de Morais DR, Eberlin MN, Sant’Ana A de S. 2016. Characterization of binary and ternary mixtures of green, white and black tea extracts by electrospray ionization mass spectrometry and modeling of their in vitro antibacterial activity. LWT – Food Sci Technol. 65:414–420. doi: 10.1016/j.lwt.2015.08.037.
- Güçlü-Üstündağ Ö, Mazza G. 2007. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 47(3):231–258. doi: 10.1080/10408390600698197.
- Han X, Shen T, Lou H. 2007. Dietary polyphenols and their biological significance. Int J Mol Sci. 8(9):950–988. doi: 10.3390/i8090950.
- Jaiswal R, Müller H, Müller A, Karar MGE, Kuhnert N. 2014. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry. 108:252–263. doi: 10.1016/j.phytochem.2014.08.023.
- Jesionek W, Majer-Dziedzic B, Choma IM. 2015. Separation, identification, and investigation of antioxidant ability of plant extract components using TLC, LC-MS, and TLC-DPPH•. J Liq Chromatogr Relat Technol. 38(11):1147–1153. doi: 10.1080/10826076.2015.1028295.
- Kachlicki P, Piasecka A, Stobiecki M, Marczak Ł. 2016. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules. 21(11):1494. doi: 10.3390/molecules21111494.
- Kothari D, Lee W, Do Jung ES, Niu KM, Lee CH, Kim SK. 2020. Controlled fermentation using autochthonous Lactobacillus plantarum improves antimicrobial potential of Chinese chives against poultry pathogens. Antibiotics. 9(7):386. doi: 10.3390/antibiotics9070386.
- Kumar K, Srivastav S, Sharanagat VS. 2021. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem. 70:105325. doi: 10.1016/j.ultsonch.2020.105325.
- Li J, Zhang S, Zhang M, Sun B. 2019. Novel approach for extraction of grape skin antioxidants by accelerated solvent extraction: box–Behnken design optimization. J Food Sci Technol. 56(11):4879–4890. doi: 10.1007/s13197-019-03958-5.
- Liu J, Henkel T. 2002. Traditional Chinese Medicine (TCM): are polyphenols and saponins the key ingredients triggering biological activities? Curr Med Chem. 9(15):1483–1485. doi: 10.2174/0929867023369709.
- Lu H, Tian Z, Cui Y, Liu Z, Ma X. 2020. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr Rev Food Sci Food Saf. 19(6):3130–3158. doi: 10.1111/1541-4337.12620.
- Martins-Noguerol R, Matías L, Pérez-Ramos IM, Moreira X, Muñoz-Vallés S, Mancilla-Leytón JM, Francisco M, García-González A, DeAndrés-Gil C, Martínez-Force E, et al. 2022. Differences in nutrient composition of sea fennel (Crithmum maritimum) grown in different habitats and optimally controlled growing conditions. J Food Compos Anal. 106:104266.,. doi: 10.1016/j.jfca.2021.104266.
- Masoodi M, Mir AA, Petasis NA, Serhan CN, Nicolaou A. 2008. Simultaneous lipidomic analysis of three families of bioactive lipid mediators leukotrienes, resolvins, protectins and related hydroxy-fatty acids by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun Mass Spectrom. 22(2):75–83. doi: 10.1002/rcm.3331.
- Meot-Duros L, Magné C. 2009. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol Biochem. 47(1):37–41. doi: 10.1016/j.plaphy.2008.09.006.
- Mhlongo MI, Piater LA, Steenkamp PA, Madala NE, Dubery IA. 2015. Metabolomic fingerprinting of primed tobacco cells provide the first evidence for the biological origin of cis-chlorogenic acid. Biotechnol Lett. 37(1):205–209. doi: 10.1007/s10529-014-1645-8.
- Nabet N, Boudries H, Chougui N, Loupassaki S, Souagui S, Burló F, Hernández F, Carbonell-Barrachina ÁA, Madani K, Larbat R. 2017. Biological activities and secondary compound composition from Crithmum maritimum aerial parts. Int J Food Prop. 20(8):1843–1855. doi: 10.1080/10942912.2016.1222541.
- Najjaa H, Abdelkarim BA, Doria E, Boubakri A, Trabelsi N, Falleh H, Tlili H, Neffati M. 2020. Phenolic composition of some Tunisian medicinal plants associated with anti-proliferative effect on human breast cancer MCF-7 cells. EuroBiotech J. 4(2):104–112. doi: 10.2478/ebtj-2020-0012.
- Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, Ahmad F, Babazadeh D, FangFang X, Modarresi-Ghazani F, et al. 2018. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed Pharmacother. 97:67–74. doi: 10.1016/j.biopha.2017.10.064.
- Pereira CG, Barreira L, da Rosa Neng N, Nogueira JMF, Marques C, Santos TF, Varela J, Custódio L. 2017. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: in vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem Toxicol. 107(Pt B):581–589. doi: 10.1016/j.fct.2017.04.018.
- Pferschy-Wenzig EM, Bauer R. 2015. The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav. 52(Pt B):344–362. doi: 10.1016/j.yebeh.2015.05.037.
- Piatti D, Angeloni S, Maggi F, Caprioli G, Ricciutelli M, Arnoldi L, Bosisio S, Mombelli G, Drenaggi E, Sagratini G. 2023. Comprehensive characterization of phytochemicals in edible sea fennel (Crithmum maritimum L., Apiaceae) grown in central Italy. J Food Compos Anal. 115:104884. doi: 10.1016/j.jfca.2022.104884.
- Pietrzak W, Nowak R, Olech M. 2014. Effect of extraction method on phenolic content and antioxidant activity of mistletoe extracts from Viscum album subsp. abietis. Chem Pap. 68:976–982.
- Repajić M, Cegledi E, Kruk V, Pedisić S, Çinar F, Kovačević DB, Žutić I, Dragović-Uzelac V. 2020. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments from wild nettle leaves. Processes. 8(7):803. doi: 10.3390/pr8070803.
- Rodrigues MJ, Soszynski A, Martins A, Rauter AP, Neng NR, Nogueira JMF, Varela J, Barreira L, Custódio L. 2015. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind Crops Prod. 77:315–322. doi: 10.1016/j.indcrop.2015.08.061.
- Sánchez-Faure A, Calvo MM, Pérez-Jiménez J, Martín-Diana AB, Rico D, Montero MP, Gómez-Guillén M del C, López-Caballero ME, Martínez-Alvarez O. 2020. Exploring the potential of common ice plant, seaside Arrowgrass and sea fennel as edible halophytic plants. Food Res Int. 137:109613. doi: 10.1016/j.foodres.2020.109613.
- Serag A, Baky MH, Döll S, Farag MA. 2020. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: a prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 10(1):76–85. doi: 10.1039/c9ra07841j.
- Souid A, Croce CM, Della Frassinetti S, Gabriele M, Pozzo L, Ciardi M, Abdelly C, Hamed K, Ben Magné C, Longo V, et al. 2021. Nutraceutical potential of leaf hydro-ethanolic extract of the edible halophyte Crithmum maritimum L. Molecules. 26(17):5380. doi: 10.3390/molecules26175380.
- Souid A, Croce CM, Della Pozzo L, Ciardi M, Giorgetti L, Gervasi PG, Abdelly C, Magné C, Hamed K, Ben Longo V, et al. 2020. Antioxidant properties and hepatoprotective effect of the edible halophyte Crithmum maritimum L. against carbon tetrachloride-induced liver injury in rats. Eur Food Res Technol. 246(7):1393–1403. doi: 10.1007/s00217-020-03498-9.
- Sparg SG, Light ME, Van Staden J. 2004. Biological activities and distribution of plant saponins. J Ethnopharmacol. 94(2–3):219–243. doi: 10.1016/j.jep.2004.05.016.
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW-M, Fiehn O, Goodacre R, Griffin JL, et al. 2007. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics. 3(3):211–221. doi: 10.1007/s11306-007-0082-2.
- Sun H, Ge X, Lv Y, Wang A. 2012. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J Chromatogr A. 1237:1–23. doi: 10.1016/j.chroma.2012.03.003.
- Vrhovsek U, Masuero D, Gasperotti M, Franceschi P, Caputi L, Viola R, Mattivi F. 2012. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J Agric Food Chem. 60(36):8831–8840. doi: 10.1021/jf2051569.
- Vukics V, Guttman A. 2010. Structural characterization of flavonoid glycosides by multi‐stage mass spectrometry. Mass Spectrom Rev. 29(1):1–16. doi: 10.1002/mas.20212.
- Wagner H, Bladt S. 1996. Plant drug analysis: a thin layer chromatography Atlas. Berlin: Springer Science & Business Media.
- Wang L, Weller CL. 2006. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol. 17(6):300–312. doi: 10.1016/j.tifs.2005.12.004.
- Zafeiropoulou V, Tomou E-M, Ioannidou O, Karioti A, Skaltsa H. 2020. Sea fennel: phytochemical analysis of Greek wild and cultivated Crithmum maritimum L. populations, based on HPLC-PDA-MS and NMR methods. J Pharmacogn Phytochem. 9:998–1004.
