Abstract
Context
Semen cuscutae is commonly used to treat male infertility (MI), and semen cuscutae flavonoid (SCF) is the main active component of semen cuscutae. The therapeutic mechanism of SCF on MI is still unclear.
Objective
To clarify the mechanisms of SCF against MI.
Materials and methods
Network pharmacology and molecular docking were used to predict the potential pathways of SCF against MI. Primary Sertoli cells (SCs) were extracted from testis of 60-day-old rats and divided into Control, Model, and 3 treatment groups. The Control and Model groups were given normal medium, the treatment groups were treated with various concentrations of SCF-containing medium (200, 400, and 800 μg/mL). After 24 h, the Model and treatment groups were exposed to heat stress at 43 °C for 15 min. Western blotting and immunohistochemistry were used to detect the expression of targets.
Result
Network pharmacology indicated that the treatment of SCF on MI was closely related to PI3K-AKT signaling pathway. The in vitro experiments showed that SCF could up-regulated the expression of AKT, AR, occludin, and Ki67, and down-regulated the expression of CK-18 in SCs after heat stress. The AKT inhibitor could block this process.
Conclusions
SCF can treat MI by regulating the proliferation and differentiation of SCs and the integrity of the blood-testis barrier. The study could provide experimental basis for clinical research.
Introduction
Male infertility (MI) refers to the condition where couples live together without contraception for more than 1 year, and the woman fails to conceive because of the male factor, caused by multiple factors (Jungwirth et al. Citation2012). According to the World Health Organization (WHO), about 15% of couples of childbearing age suffer from infertility, with about 50% caused by MI. The incidence of MI is increasing, seriously threatening human reproductive health (Agarwal et al. Citation2015; Min and Min Citation2017).
MI is closely associated with abnormalities in the structure and function of the testicles. The testicles are mainly composed of seminiferous tubules and Leydig cells, and the seminiferous tubules include Sertoli cells (SCs) and spermatogenic cells at different stages of spermatogenesis. Abnormal spermatogenesis caused by damaged spermatogenic cells is the primary culprit of MI. Interestingly, SCs are essential for maintaining the structure and function of spermatogenic cells. SCs are adjacent to the spermatogenesis cells and are vital to providing morphological and nutritional support for spermatogenesis, which have been widely studied in recent years (Yang et al. Citation2022). In addition, SCs participate in the formation of the blood-testis barrier (BTB), made of tight, adherens and gap-junctions between SCs (Mruk and Cheng Citation2015). The primary function of the BTB is to restrict the paracellular flow of water and nutrients across the SCs epithelia, avoid the passage of toxic agents from the blood into the tubules, and protect the developing haploid forms of male germ cells by establishing an immune-privileged environment (Möller et al. Citation2021). It can be seen that the SCs and BTB play important roles in maintaining normal spermatogenesis and fertility. However, the function of SCs depends on their normal proliferation, differentiation, and tight junctions between the cells. If SCs are damaged or lose their functions, they cannot provide the necessary energy for sperm generation and maintain the integrity of BTB. This can hinder the spermatogenesis process and cause sperm abnormalities and infertility, which further highlights the importance of SCs in maintaining testicular tissue structure and male reproductive function.
The PI3K-AKT signaling pathway and pathway-related proteins, such as AR, Ki67, CK-18, and occludin, are closely related to the proliferation, differentiation and tight junction function of SCs. PI3K-AKT signaling pathway is involved in regulating cell division, proliferation, apoptosis, and metabolism, and has a wide range of biological effects. AR is a hormone-induced transcription factor. Androgen must bind to AR to show its effects, which is very important for maintaining male characteristics and reproductive ability. Ki67 is a nuclear protein associated with the cell cycle, and it is present during the active phases of the cell cycle but is absent during the inactive phases. So, Ki67 is a reliable indicator of cell proliferation (Rangel-Gamboa et al. Citation2013). CK-18 is a cytoskeletal protein that plays an important role in maintaining cell shape and integrity, cell differentiation, stability of the intracellular structure, and intracellular signal transduction (Turczyn et al. Citation2021). CK-18 is highly expressed in SCs before puberty and disappears after puberty (Zhang et al. Citation2006), which is an indicator of cell differentiation and maturity. Occludin is an important tight junction-associated protein between SCs. It participates in the formation of BTB and provides a stable environment and nutritional support during spermatogenesis (Wang et al. Citation2019). Decreased occludin expression cannot guarantee the integrity and normal function of BTB, and ultimately affect spermatogenesis. This study was also centered on the above proteins.
The structure and function of the testis and SCs are greatly affected by temperature. Normally, the testicles of male mammals are located in the lower temperature scrotum, and the higher temperature of the scrotum can destroy the structure and function of the testicular tissue, resulting in infertility (Barazani et al. Citation2014; Al-Otaibi Citation2018). Sitting for a long time, working in high temperatures, and wearing tight pants, which are commonly observed today, are factors increasing testicular heat and leading to MI (Erkanli Şentürk et al. Citation2012). It has been shown that heat exposure (≥43 °C) can destroy the morphology, function, and tight junctions between SCs, damage the integrity of BTB, and thus fail to provide nutritional support and necessary structure for spermatogenesis, resulting in abnormal spermatogenesis and infertility.
The theory of Traditional Chinese Medicine (TCM) emphasizes that the ‘kidney dominates reproduction’, indicating that MI is closely related to the deficiency of ‘kidney essence’ and ‘kidney qi’, and that the treatment should be based on tonifying the kidney. Semen cuscutae is one of the common drugs used for the treatment of MI in TCM, it is the seed of Cuscuta chinensis Lam. (Convolvulaceae). The theory of TCM suggests that SCF enters the kidney meridian and performs certain functions, such as tonifying yang and yin, nourishing the liver and kidney, consolidating essence and shrinking urine, protecting the fetus, etc. Semen cuscutae contains flavonoids, chlorogenic acids, polysaccharides, alkaloids, steroids, etc. Flavonoids, including hyperoside, astragalin, isoquercitrin, quercitrin, quercetin, kaempferol, etc., are the main bioactive components of Semen cuscutae (Zhang W et al. Citation2020). Pharmacological studies have shown that SCF can regulate and protect the male reproductive system (Zhang et al. Citation2019), but the underlying mechanism is not clear, and worthy of further exploration.
Network pharmacology is an approach to explore the mechanism of drugs by constructing the interrelated network of ‘drugs-target-diseases’, and provides new methods for studying the complex pharmacological mechanisms of Chinese herbs (Zhu et al. Citation2018). Network pharmacology systematically combines the biological and the drug networks to comprehensively analyze the mechanism of Chinese herbs and prescribe them in the treatment, which is similar to the ‘overall concept’ of TCM theory (Baptista et al. Citation2021).
This study was designed to determine the pharmacological mechanisms of major active compounds in SCF in treating MI by utilizing network pharmacology and in vitro experiments. The findings of this study might provide evidence for SCF in the treatment of MI.
Materials and methods
Network pharmacology
Screening the components of SCF and predicting targets of the components
The components of Semen cuscutae were obtained from Traditional Chinese Medicine Systems Pharmacology Database (TCMSP, https://tcmspw.com/tcmspsearch.php) database (Ru et al. Citation2014) and The Encyclopedia of Traditional Chinese Medicine (ETCM, http://www.tcmip.cn/ETCM/index.php/) database (Xu et al. Citation2019). Oral bioavailability (OB) and Drug-likeness (DL) are important pharmacokinetic parameters to evaluate the druggability of compounds in the process of absorption, distribution, metabolism, and excretion (ADME). The parameters of active compounds for selection were set as follows: OB > 30%, DL > 0.18 (Zhang et al. Citation2022). In addition, China National Knowledge Infrastructure (CNKI) and PubMed were used to search more components to supplement the results of TCMSP and ETCM. We used Pubchem (https://pubchem.ncbi.nlm.nih.gov/) to obtain the 2D structure of all components, then, imported it to SwissTarget Prediction (http://www.swisstarget prediction.ch/) and Pharmmapper (http://www.lilab-ecust.cn/pharmmapper/) (Wang et al. Citation2017) and set Probability > 0, Normalized Fit Score ≥ 0.9 to obtain the targets of SCF. Then we integrated the targets obtained from each database and removed the duplicate targets. The UniProt database (http://www.uniprot.org/) was used to acquire the standard gene symbol with the organism selected as ‘Homo sapiens’. Finally, Cytoscape 3.8.2 was used to construct a drug-components-targets network.
Collecting the MI-related targets and screening drug-disease intersection target genes
With ‘male infertility’, ‘man infertility’, ‘men infertility’ as the keywords, we searched the Online Mendelian Inheritance in Man (OMIM, http://omim.org/) (Amberger et al. Citation2015), GeneCards (https://www.genecards.org/) (Safran et al. Citation2010), and DisGeNET (https://www.disgenet.org/) (Piñero et al. Citation2017). The targets obtained from the three databases were integrated and the duplicate targets were removed. SCF-MI intersection targets were obtained through Venny 2.1 (http://bioinfogp.cnb.csic.es/tools/venny).
Build protein–protein interaction (PPI) network and screen the core targets
Search Tool for the Retrieval of Interacting Genes (STRING, https://www.string-db.org/) includes both direct physical interactions and indirect functional correlations between proteins. The SCF-MI intersection targets were imported to the STRING platform to build the Common Target PPI network (Szklarczyk et al. Citation2019). Network visualization was performed by Cytoscape 3.8.2. CytoNCA in Cytoscape software was used to analyze the Topological parameters of these nodes such as the degree, betweenness and closeness, and screened out the nodes whose degree, betweenness and closeness are greater than or equal to the median as the core target.
Enrichment Analyses
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed based on integrated visualization and comments from The Database for Annotation, Visualization and Integrated Discovery (DAVID) 6.8 database (https://david.ncifcrf.gov/summary.jsp) (Huang da et al. Citation2009). The potential therapeutic targets of SCF against MI were input into the DAVID platform, and the species was set as ‘Homo sapiens’. The biological processes (BP), cell components (CC), molecular functions (MF), and KEGG pathway enrichment analysis were carried out, respectively. Finally, the GO bubble map and KEGG signal pathway enrichment map of the top 20 enrichment genes were drawn using Bioinformatics (www.bioinformatics.com.cn).
Molecular docking
To further verify the reliability of the target prediction results, the main relevant targets were subjected to molecular docking verification. The 3D structure of the key target protein was obtained from the Protein Data Bank (PDB) database of Research Collaboratory for Structural Bioinformatics (RCSB) (http://www.rcsb.org/). The 2D structure of compounds was downloaded from the PubChem database. The crystal structure of the target protein was pretreated in Maestro 11.9 platform, including the removal of water molecules (organic and heteroatom), adding hydrogenation (charge and atom type), Minimizing energy and optimizing geometry (Rajeswari et al. Citation2014; Fazi et al. Citation2015). Finally, molecular docking was done by the Glide module in Schrodinger Maestro software.
In vitro experiments
Animals, drugs and main reagents
Animals: Specific Pathogen Free (SPF) grade, 60-day-old male Sprague-Dawley (SD) rats were provided by Beijing Huafukang Biotechnology Co., Ltd., the animal batch number was SYXK (Beijing) 2011-0024. All animal experiments were carried out in accordance with the recommendations of the institutional and national guidelines for animal care and use. The protocol was approved by the Laboratory Animal Welfare and Ethics Committee of the Beijing University of Chinese Medicine, Beijing, China (NO. BUCM-4-2017010801-1027).
Drug and Reagents: SCF powder was purchased from Nanjing Zelang Biotechnology Co., Ltd. (batch number: zlzt20210918091). Before administration, we dissolved the SCF powder in dimethyl sulfoxide (DMSO) solution, prepared it into 80 mg/mL solution, and stored it at −20 °C for standby.
The anti-Ki67 (ab16667) and anti-AR (ab133273) were purchased from Abcam (Cambridge, UK). The anti-occludin (PA5-20755) was obtained from Thermo Fisher Scientifific (Waltham, MA, USA). The anti-Phospho-AKT (Ser473) (#4060) and anti-AKT (#4691) were obtained from Cell Signaling Technology (Boston, MA, USA). The anti-CK-18 antibody (10830-1-AP) and anti-β-actin antibody (60008-1-lg) were obtained from Proteintech (Chicago, IL, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), DMSO, phosphate buffered saline (PBS), and the bicinchoninic acid (BCA) protein assay kit were purchased from Solarbio Life Sciences (Beijing, China).
Isolation, culture and grouping of SCs and heat stress
Isolation and culture of SCs was according to Guo et al. (Citation2007). The rats were anesthetized intraperitoneally, the testicular tissue was obtained from the testis and washed twice in PBS, the testicular capsule and parenchyma were separated with ophthalmic scissors, then the testicular parenchyma tissue was cut into fragments. Then the SCs were separated with collagenase IV and trypsin and cultured in a culture dish with a diameter of 100 mm. The SCs were randomly divided into Control group, Model group, Low dose group, Medium dose group and High dose group, and 3 repetitions were conducted for each group in the following experiment. The culture medium was changed on second day and forth day, respectively, and drug intervention was carried out on fifth day. A certain dose of SCF solution was added to the Low, Medium, and High dose group so that the final drug concentrations were 200, 400, and 800 μg/mL, respectively. The same volume of medium was added to the Control group and Model group as in 3 treatment groups. After 24 h, the Model group and the 3 treatment groups were placed in a 43 °C constant temperature water bath. After 15 min of heat stress, the following detection was performed.
Evaluation of cell viability
MTT assay has been widely used for measuring cell viability (Huang and Zheng Citation2022). The optical density (OD) obtained under the specific wavelength is directly proportional to the number of living cells. The cell suspension (5 × 104/mL, 200 μL) was inoculated into 96 well plates, and 5 multiple Wells were set in each group, then, cultured in the incubator for 24 h. After the SCs was completely adhered to the wall, the culture medium was discarded, and the corresponding solution or drug solution was added to the wells. The cells were cultured for 24 h, then 100 μL medium was removed from each well and MTT regent (5 mg/mL, 15 μL) was added. The cells were cultured in incubator for 4 h again, the culture medium was discarded, and 150 μL of DMSO solution was added. After 15 min of shaking, the OD value was measured (wavelength: 570 nm) using the microplate reader FLUO Star Omega (BMG Labtech, Offenburg, Germany).
Immunohistochemistry
The immunohistochemistry was performed as Zhang et al. (Citation2013). The cell suspension (5 × 104/mL, 500 μL) was inoculated into 48 well plates, and 3 multiple wells were set in each group, then, cultured in the incubator for 24 h. After the SCs were completely adhered to the wall, the culture medium was discarded, and the corresponding solution or drug solution was added to each group of wells. After 24 h, SCs were fixed in 4% paraformaldehyde and treated with 0.5% Triton X-100 and 0.3% H2O2, respectively. After washing with PBS for 3 times, cells were blocked with 10% goat serum to suppress the nonspecific antigen and then incubated in the primary antibody of Ki67 (1:200) or AR (1:200) at 4 °C overnight. The next day, the primary antibody was discarded, the biotinylated secondary antibody was added and incubated for 15 min at 37 °C. After washing with PBS for 3 times, the horseradish enzyme labeling streptavidin working solution was added for 15 min at 37 °C. After washing with PBS for 3 times again, DAB chromogenic agent was used for 1 min, and after washing with tap water, hematoxylin was redyed for 20 s, and microscope was used to observe and take pictures. Six non-overlapping fields were selected for each group to take pictures. Image-Pro Plus (Version 6.0, Media Cybernetics, Bethesda, MD, USA) software was used to process the images and count the positive cells for statistical analysis.
Western blotting
Western blotting was performed as Zhang et al. (Citation2016). The expression of AR, P-AKT, CK-18 and occludin in SCs was detected by Western blotting. In order to prove that SCF could indeed act on the AKT signaling pathway and regulate the expression of CK-18 and occludin, we conducted an AKT inhibitor test (10 µM, 2 h, AT7867, APEXBIO) in the presence of heat stress and SCF. The regulatory effect of SCF on AKT and the reliability of the network pharmacology results were confirmed by observing the expression of CK-18 and occludin.
Statistical analysis
Image J V8.0 was used to analyze the gray value of the images. SPSS 20.0 statistical software (IBM, Corp) was used to analyze and process the data, and the data was expressed as mean ± standard deviation. If the data were normally distributed and the variance was uniform, one-way ANOVA was used, and LSD test was used for comparison between groups. If the data does not conform to the normal distribution or variance is not uniform, then non-parametric test is used. p < 0.05 was considered statistically significant, and all the graphs were drawn by GraphPad Prism 6.0 (GraphPad Software Inc.).
Result
Network pharmacology
Screening of active components and target genes of SCF
Quercetin, kaempferol, and isorhamnetin were collected through TCMSP and ETCM. Hyperin, astragalin, apigenin, rutin, luteolin, quercitrin and isoquercitrin were collected through literature review. The OB values of apigenin, astragalin, hyperin, quercitrin, isoquercitrin did not meet the screening criteria, but these compounds had high content or strong pharmacological activity, so they were also included for the following study. Finally, 10 flavonoids () and 359 target () genes were obtained.
Figure 1. Drug-compounds-target network. Red node represents SCF, yellow nodes represent compound components in SCF, blue nodes represent the targets of SCF, and edges represent interactions between them.
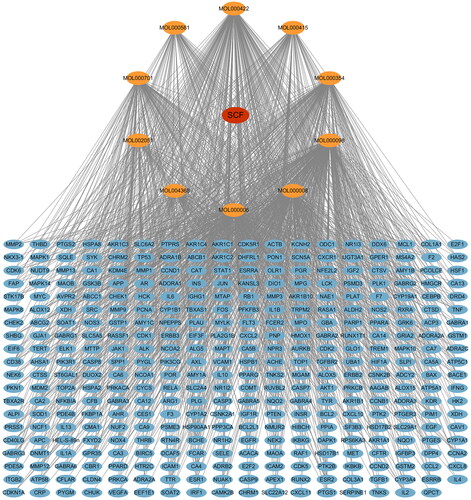
Table 1. The main component of SCF.
Collection of MI targets and screening of SCF-MI intersection targets
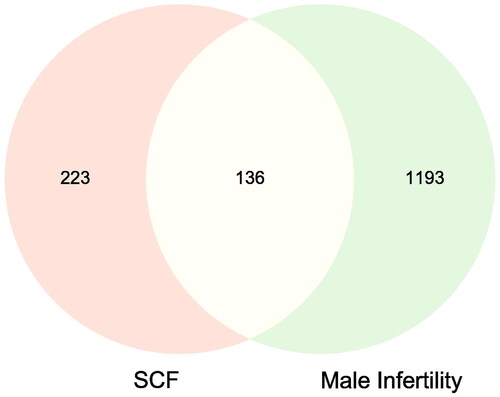
With ‘male infertility’, ‘man infertility’, ‘men infertility’ as the keywords, the genes associated with MI were collected from the GeneCard, OMIM and DisGeNet. Duplicate genes in the search results were discarded. Finally, a total of 1329 genes were obtained. As shown in , the intersection of targets of active components and MI-related targets was considered to be potential therapeutic targets of SCF against MI.
Construction of PPI network and screening of core targets
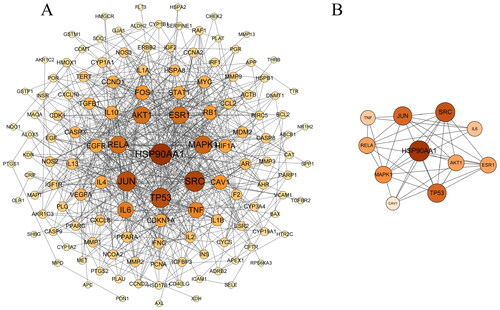
136 intersection targets were imported into STRING (https://string-db.org/, version 11.0) to obtain PPI information data table, TSV files were downloaded and uploaded to Cytoscape 3.8.2 for network visualization, and PPI network of potential targets of SCF for MI was constructed (). CytoNCA was used to calculate the Degree, Betweenness and Equivalent of every node in the network, and to screen nodes according to the median of these three values respectively. After repeated screening for two times, the node with high score was extracted, which was the core target (). The core targets include Heat shock protein HSP90-alpha (HPS90AA1), Proto-oncogene tyrosine-protein kinase Src (SRC), Transcription factor AP-1 (JUN), Cellular tumor antigen p53 (TP53), Mitogen-activated protein kinase 1 (MAPK1), RAC-alpha serine/threonine-protein kinase (AKT1), etc. These targets are strongly correlated with other targets in PPI network and also participate in multiple signaling pathways, indicating that these proteins play an important role in SCF treatment of MI.
Figure 3. (A) Network biology analysis for PPI constructed with potential targets of SCF against MI. Nodes represent targets and edges represent interactions between targets. The color intensity and size of a node is proportional to the value of a degree in the network. (B) The core targets of PPI network.
GO and KEGG pathway enrichment Analyses
GO analysis: The enrichment analysis of potential targets of SCF in the treatment of MI was performed using the DAVID platform to explore the BP, CC and MF in which these targets were involved. A total of 803 GO items were enriched, including 618 of BP, 57 of CC and 128 of MF. The top 10 enrichment terms were shown in . BP included ‘positive regulation of transcription from RNA polymerase II promoter’, ‘response to drug’, ‘Negative regulation of apoptotic process’, ‘positive regulation of transcription’, ‘DNA-templated’, ‘signal transduction’, ‘Positive regulation of cell proliferation’, etc., suggesting that the role of SCF may be closely related to information transcription, signal transduction, cell proliferation and apoptosis. MF included ‘protein binding’, ‘enzyme binding’, ‘identical protein binding’, ‘ATP binding’, ‘DNA binding’, ‘protein homodimerization activity’, etc., suggesting that the function of SCF may be realized by binding with proteins, enzymes and ATP in cytoplasm or nucleus.
Figure 4. (A) the gene Ontology (GO) enrichment analysis. Biological process (BP), cellular component (CC), and molecular functions (MF) are enriched here. The ordinate is the project description, and the abscissa is the p values and the number of targets enriched. (B) The KEGG enrichment analysis for the potential targets of SCF against MI. The ordinate is the type of pathway and the abscissa is the number of genes enriched in a pathway.
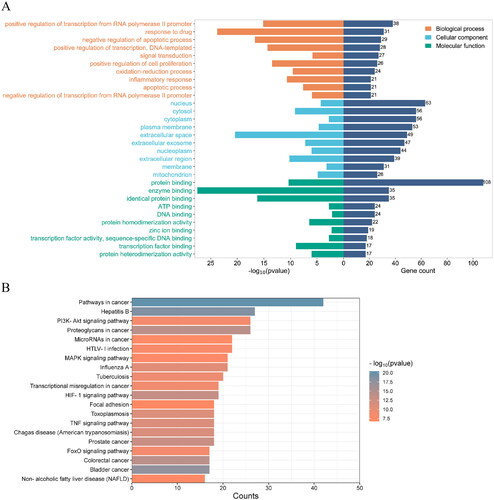
KEGG enrichment analysis: To further clarify the relationship between targets and pathways, David database was used to obtain KEGG signaling pathway enrichment information of potential therapeutic targets of SCF. A total of 114 pathways were enriched. The top 20 of the potential Pathways were shown in , included ‘Pathways in cancer’, ‘Hepatitis B’, ‘PI3K-AKT signaling Pathway’, ‘Proteoglycans in cancer’, ‘MicroRNAs in cancer’, ‘HTLV-I infection’, etc.
Molecular docking
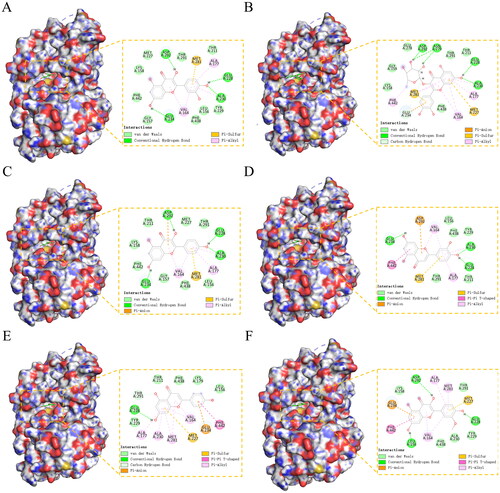
Based on the results of KEGG, PI3K-AKT signaling may be one of the most significant pathways, which could exert influence through interfering with cells proliferation and differentiation for male reproduction. To validate the possible biological interactions between SCF and PI3K-AKT signaling, molecular docking was used here. We selected AKT1 (PDBID: 7EPZ) in the core target for molecular docking with related compounds. AKT1 had strong docking activity with quercetin (-8.051 kcal/mol), quercitrin (-7.385 kcal/mol), kaempferol (-7.182 kcal/mol), luteolin (-6.951 kcal/mol), apigenin (-6.872 kcal/mol) and isorhamnetin (-6.674 kcal/mol), and the binding energies were less than −6 kcal/mol. The quercetin has the best docking score and binding mode with AKT1, and it could effectively bind to the active pocket of AKT1. The docking analysis showed that quercetin made hydrogen-bonding interactions with ASP-292, GLU-228, ALA-230, and GLU-234. In addition, the benzene ring part of quercetin also formed strong conjugate interaction with ALA-177, MET-281, and VAL-164. The other five compounds also had strong hydrogen-bonding interactions, hydrophobic interaction, and conjugated interactions with the active sites of AKT1 protein (). Therefore, based on the results of molecular docking, SCF could interfere with cells proliferation and differentiation through interacting with the AKT1 protein, exerting its efficacy in the treatment of MI.
In vitro experiments
Effects of SCF on the SCs viability, proliferation and dedifferentiation
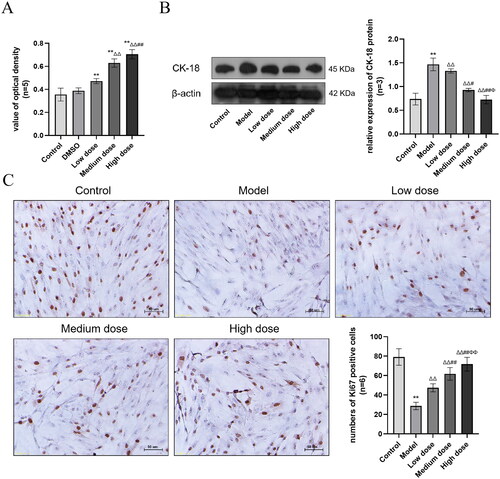
To investigate the mechanism via which SCF influences cell viability, proliferation and dedifferentiation, its OD value was measured by MTT assay, the number of Ki67 positive cells was assessed via immunohistochemistry, the expression of CK-18 was detected by Western blot. As shown in , the optical density of the DMSO group was not significantly different from that of the Control group (p > 0.05), indicating that DMSO at this dose did not affect the viability of SCs. The viability of the Low, Medium and High doses of SCF were significantly enhanced and dose-dependent (p < 0.01). As shown in , the expression of CK-18 in the Model group was increased significantly (p < 0.01), while decreased significantly in the three treatment groups, and the expression of CK-18 was inversely correlated with the dose of SCF (p < 0.01). As shown in , the number of Ki67 positive cells were significantly lower in the Model group than that in the Control group (p < 0.01), However, such trends were significantly reversed in the Low, Medium and High dose groups (p < 0.01). Furthermore, the number of Ki67 positive cells increased gradually with the dose.
Figure 6. (A) Results of cell viability based on the MTT assay. Compared with the Control group, *p < 0.05 and **p < 0.01. Compared with the Low dose group, Δp < 0.05 and ΔΔp < 0.01. Compared with the Medium dose group, #p < 0.05 and ##p < 0.01. (B) The expression of CK-18 protein. Compared with the Control group, *p < 0.05 and **p < 0.01. Compared with the Model group, Δp < 0.05 and ΔΔp < 0.01. Compared with the Low dose group, #p < 0.05 and ##p < 0.01. Compared with the Medium dose group, Φp < 0.05 and ΦΦp < 0.01. (C) The expression of Ki67 in SCs (Original magnification: ×200, Scale bar: 50 μm DAB staining). Ki67 is expressed in the nucleus, and the brown areas in the image are Ki67 positive expression areas. The data description is the same as CK-18.
Effects of SCF on the BTB structural proteins
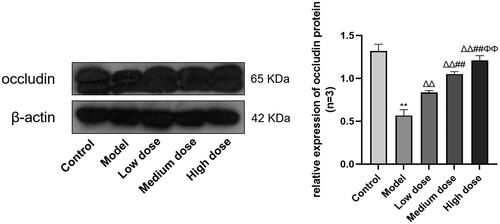
To illustrate the effect of SCF on BTB, we used Western blot to detect the expression of occludin. As shown in , the expression of occludin was significantly lower in the Model group than that in the Control group (p < 0.01), and the expression of occludin were increased significantly in the three treatments compared with the Model group and was dose-dependent (p < 0.01).
Effects of SCF on AR and PI3K-AKT signaling pathway
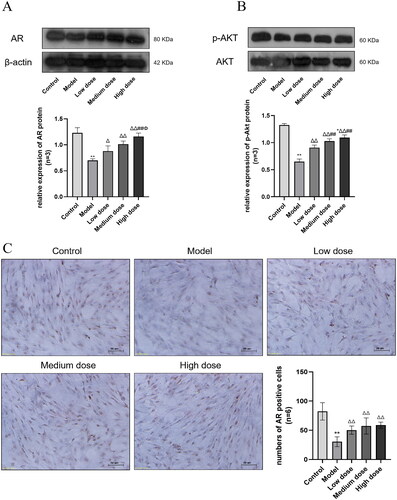
AKT is one of the most important downstream molecules in the PI3K-AKT signaling pathway and is biologically active after phosphorylation. AR is an upstream factor of the PI3K-AKT signaling pathway. To investigate the mechanism of SCF in improving heat stress damage and determine whether it is related to the PI3K-AKT signaling pathway, we examined the expression of AR and p-AKT. As shown in , the expression of AR (A, C) and p-AKT (B) was significantly lower in the Model group than that in the Control group (p < 0.01), and the expression of AR and p-AKT in the three treatment groups was increased significantly compared with the Model group and was dose-dependent (p < 0.01).
Figure 8. The expression of AR. (B) The expression of p-AKT. Compared with the Control group, *p < 0.05 and **p < 0.01. Compared with the Model group, Δp < 0.05 and ΔΔp < 0.01. Compared with the Low dose group, #p < 0.05 and ##p < 0.01. Compared with the Medium dose group, Φp < 0.05 and ΦΦp < 0.01. (C) The expression of AR in SCs (Original magnification: ×200, Scale bar: 50 μm DAB staining). AR is expressed in the nucleus, and the brown areas in the image are AR positive expression areas.
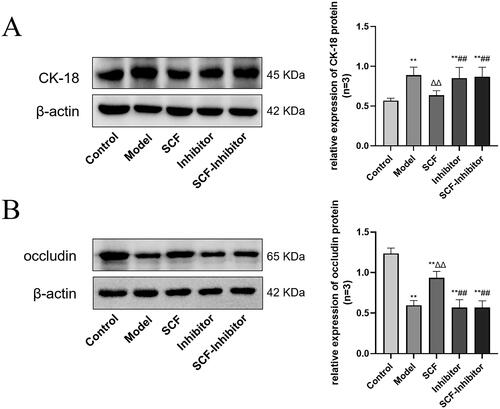
Effects of SCF on CK-18 and occludin expression after applying AKT inhibitor
We selected the Medium dose group as the SCF group, and set up the inhibitor group and SCF-inhibitor group. As shown in , the content of CK-18 (A) in the Model group, inhibitor group and SCF-inhibitor group increased significantly, while the content of occludin (B) decreased significantly. There were also significant differences between SCF group and SCF-inhibitor group. This indicated that SCF lost its regulatory effect on CK-18 and occludin after blocking PI3K-AKT signaling pathway with AKT inhibitor, which proved that SCF could regulate the expression of CK-18 and occludin through PI3K-AKT signaling pathway.
Figure 9. Effects of SCF on the expression of CK-18 and occludin after applying AKT inhibitors. Except the normal group and the inhibited group, the other groups were modeled with heat stress. (A) The expression of CK-18 protein. (B) The expression of occludin protein. Compared with the Control group, *p < 0.05 and **p < 0.01. Compared with the Model group, Δp < 0.05 and ΔΔp < 0.01. Compared with the SCF group, #p < 0.05 and ##p < 0.01.
Discussion
The important role of SCs and the BTB formed by SCs in male reproduction has been established. SCs and BTB may not be able to provide sufficient nutrients and a safe environment for spermatogenesis if their structure or function was destroyed, resulting in abnormal spermatogenesis and infertility. High temperature exposure is a common cause of structural and functional damage to SCs and BTB, and the method of 43 °C water bath is usually used to establish a testicular heat stress model (Aldahhan et al. Citation2019; Hu et al. Citation2021).
Semen cuscutae is one of the most common drugs used in the treatment of male reproductive disorders in TCM. It is a part of several famous prescriptions for the treatment of male reproductive disorders, such as Wuzi Yanzong pill, Zuogui pill, Yougui Pill, etc. Flavonoid is the main active component of Semen cuscutae. Studies have shown that SCF increased testicular weight, promoted testosterone secretion and androgen receptor expression (Qin et al. Citation2000; Yang et al. Citation2008), and alleviated reproductive injury. However, there is no report on the protective effect of SCF on SCs and BTB in heat stress related MI. Thus, the potential targets of SCF were predicted through network pharmacology, and the in vitro experiments were conducted to reveal the mechanism of SCF against MI.
The PPI network analysis revealed that the potential therapeutic targets of SCF included HPS90AA1, SRC, JUN, TP53, MAPK1, AKT1, etc. It is noteworthy that all these targets are related to the PI3K-AKT signaling pathway. HPS90AA1 is a protein produced under heat stress, is essential for the protection of cells, and plays an important role in cell growth, development, and differentiation. This is also the reason why we used the method of heat stress to prepare the MI model in this experiment. In addition, it is a downstream protein in the PI3K-AKT signaling pathway and can upregulate the expression of HSP90 (Zhang X et al. Citation2020). SRC, a tumor-associated non-receptor tyrosine kinase, is a key upstream regulator of the PI3K-AKT signaling pathway (Lovren et al. Citation2009). The JUN signaling pathway is an important branch of the MAPK signaling pathway and plays an important regulatory role in various physiological and pathological processes, such as cell cycle, reproduction, apoptosis, and stress. Also, c-Jun can inhibit the activation of the PI3K-AKT signaling pathway (Sun et al. Citation2021). AKT1 is a key protein in the PI3K-AKT signaling pathway, mainly regulating cell proliferation, apoptosis, stress, and other physiological and pathological processes. TP53 affects cell cycle progression, apoptosis, and aging by regulating the AKT pathway (Deng et al. Citation2020), and it also encodes the P53 protein, which promotes apoptosis (Vogelstein et al. Citation2000) and regulates cell cycle progression and redox equilibrium (Budanov Citation2014). It can be concluded that these core molecules participate in the PI3K-AKT signaling pathway, and regulate cell differentiation, proliferation, apoptosis, and other processes.
Enrichment analysis was performed to identify the biological processes and signaling pathways of potential targets of SCF. Several classical biological processes that are closely related to MI, such as positive regulation of transcription from RNA polymerase II promoter, response to drug, negative regulation of apoptotic process, positive regulation of transcription, DNA-templated, signal transduction, positive regulation of cell proliferation, etc., were identified using the GO analysis, suggesting that the mechanism of SCF is closely related to the processes of information transcription, signal transduction, cell proliferation and apoptosis. This result was consistent with that of the PPI network analysis. ‘PI3K-AKT Signaling Pathway’ also appeared in the KEGG enrichment analysis. Based on these results of the PPI network and enrichment analyses, we suggest that the PI3K-AKT signaling pathway might be one of the key signaling pathways underlying the alleviation of MI by SCF. Therefore, we chose the PI3K-AKT signaling pathway to confirm whether it plays a role in the mechanism of action of SCF. The results of molecular docking also verify the reliability of target prediction results.
In vitro experiments showed that heat stress influenced the viability, proliferation, differentiation, and maturity status of SCs, and destroyed the tight junctions between SCs and the integrity of BTB. However, SCF enhanced the viability, proliferation, and differentiation of SCs and strengthened the tight junction between SCs, thereby reversing the damage of heat stress. More specifically, Ki67 reflects the ability of cell proliferation. In the current study, the expression of Ki67 decreased in the Model group and increased in three treatment groups, indicating that SCF promoted the expression of Ki67 under heat stress. Combined with the results of the MTT assay, it is suggested that SCF reversed the heat stress related damage and improved the viability and proliferation of SCs. CK-18 reflects cell differentiation and maturation. In the current study, the expression of CK-18 in the Model group increased, indicating that heat stress caused cell dedifferentiation, leading to an immature and undifferentiated state; while the expression of CK-18 in the three treatment groups decreased, indicating that SCF ameliorated the heat stress related damage, maintained the normal state of differentiation and maturation. Occludin determines the tight junctions between the SCs and the integrity of the BTB. And high temperature would damage the tight junction proteins, such as occludin (Yang et al. Citation2018). In the current study, the expression of occludin decreased in the Model group, indicating that heat stress destroys the tight junction proteins between SCs, and the integrality of BTB; while the expression of occludin in the three treatment groups increased, suggesting that SCF promoted the occludin expression and maintained the normal structure and function of BTB, and spermatogenesis.
By analyzing the expression levels of Ki67, CK-18, and occludin, the therapeutic effect of SCF was confirmed using four parameters - SCs vitality, proliferation, differentiation, and BTB integrity. So, how does SCF regulate the above processes to interfere with SCs vitality, differentiation, proliferation, and BTB function?
Firstly, AR is an important protein and cannot be ignored, and AR is recognized as one of the most mature drug targets (Dalal et al. Citation2018). In the current network pharmacological study, the potential targets of SCF included AR. The in vitro experiment, the expression of AR decreased in the Model group and increased in the three treatment groups in a dose-dependent manner, which also proved that SCF promotes AR expression. Abnormal proliferation and maturation of SCs and increased permeability of BTB are common causes of spermatogenesis disorders and MI, and AR is the key upstream factor to regulate proliferation, maturation, and permeability of SCs (Sharpe et al. Citation2003). Combined with the in vitro experiments results, it is suggested that SCF regulates the above factors (Ki67, CK-18, and occludin) through AR to show the therapeutic effect.
Secondly, the PI3K-AKT signaling pathway is closely related to the processes of cell differentiation, proliferation, and apoptosis and widely participates in the biological processes that we have mentioned, such as viability, differentiation and proliferation of SCs. The expression of Ki67, CK-18, and occludin is regulated by the PI3K-AKT signaling pathway. For example, activating the PI3K-AKT signaling pathway promoted the expression of occludin and Ki67 (Fan et al. Citation2015; Zhao et al. Citation2022), while blocking it significantly increased the expression of CK-18 (Zhou et al. Citation2014). In addition, AR is also an upstream factor of the PI3K-AKT signaling pathway (Choi et al. Citation2022). AR could activate the PI3K-AKT signaling pathway and regulate downstream factors by activating Ser473 of AKT to form p-AKT through non-genomic pathway. If the phosphorylation of AKT is blocked, AR signaling is also inhibited (Deng et al. Citation2017). In the current study, the expression of AKT in the Model group decreased significantly, while it increased in the three treatment groups, indicating that SCF significantly promoted the expression of AKT under heat stress. In addition, AR is upstream of the PI3K-AKT signaling pathway, and Ki67, CK-18, and occludin are the downstream factors. Therefore, we speculated that SCF activates the PI3K-AKT signaling pathway by promoting the expression of AR, and then promotes the expression of occludin and Ki67, and inhibits that of CK-18. After the addition of the AKT inhibitor, the expression of CK-18 increased significantly, while that of occludin decreased significantly. However, SCF did not reverse the results, indicating that AKT inhibitors did block the PI3K-AKT signaling pathway and SCF could not regulate the downstream factors via AR, resulting in a poor therapeutic effect. This conclusion confirmed the prediction of the network pharmacology analysis - SCF can regulate the PI3K-AKT signaling pathway and downstream factors through AR, and then ameliorate the vitality, proliferation, and differentiation of SCs and BTB function, to maintain normal spermatogenesis and reproductive ability.
Conclusions
Using network pharmacology, we identified and enriched the active components, potential targets, and signaling pathways of SCF involved in the treatment of MI. Using in vitro experiments, we verified that the active components of SCF regulated AR and the PI3K-AKT signaling pathway and improved the vitality, proliferation, and differentiation of SCs and BTB permeability, to maintain normal spermatogenesis and reproductive capacity. The current study revealed the potential mechanism underlying the treatment of MI by SCF at the molecular level. The preliminary results clarified the role of semen cuscutae in treating MI and provided insights into the drug research and development for MI.
Author contributions
Su-qin Hu developed hypotheses and conceived the project; Chen-xiao Liu and Su-qin Hu designed the experiments and performed animal experiments; Chen-xiao Liu wrote the manuscript; Dian-long Liu, Ya-hui Xu and Ke Hu analyzed the data and validated it; Jian Guo was involved in the administration of the project and in acquiring funding. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. 2015. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 13:37. doi: 10.1186/s12958-015-0032-1.
- Aldahhan RA, Stanton PG, Ludlow H, de Kretser DM, Hedger MP. 2019. Acute heat-treatment disrupts inhibin-related protein production and gene expression in the adult rat testis. Mol Cell Endocrinol. 498:110546. doi: 10.1016/j.mce.2019.110546.
- Al-Otaibi ST. 2018. Male infertility among bakers associated with exposure to high environmental temperature at the workplace. J Taibah Univ Med Sci. 13(2):103–107. doi: 10.1016/j.jtumed.2017.12.003.
- Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. 2015. OMIM.org: online mendelian inheritance in man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 43(Database issue):D789–D798. doi: 10.1093/nar/gku1205.
- Baptista R, Bhowmick S, Shen J, Mur LAJ. 2021. Molecular docking suggests the targets of anti-mycobacterial natural products. Molecules. 26(2):475. doi: 10.3390/molecules26020475.
- Barazani Y, Katz BF, Nagler HM, Stember DS. 2014. Lifestyle, environment, and male reproductive health. Urol Clin North Am. 41(1):55–66. doi: 10.1016/j.ucl.2013.08.017.
- Budanov AV. 2014. The role of tumor suppressor p53 in the antioxidant defense and metabolism. Subcell Biochem. 85:337–358.
- Choi YH, Kim J, Shin JY, Kang NG, Lee S. 2022. Antiandrogenic activity of riboflavin 5′-phosphate (FMN) in 22Rv1 and LNCaP human prostate cancer cell lines. Eur J Pharmacol. 917:174743. doi: 10.1016/j.ejphar.2022.174743.
- Dalal K, Morin H, Ban F, Shepherd A, Fernandez M, Tam KJ, Li H, LeBlanc E, Lack N, Prinz H, et al. 2018. Small molecule-induced degradation of the full length and V7 truncated variant forms of human androgen receptor. Eur J Med Chem. 157:1164–1173. doi: 10.1016/j.ejmech.2018.08.059.
- Deng XY, Li Y, Gu S, Chen YY, Yu BB, Su J, Sun LK, Liu YN. 2020. p53 affects PGC1α stability through AKT/GSK-3β to enhance cisplatin sensitivity in non-small cell lung cancer. Front Oncol. 10:1252. doi: 10.3389/fonc.2020.01252.
- Deng Q, Zhang Z, Wu Y, Yu WY, Zhang J, Jiang ZM, Zhang Y, Liang H, Gui YT. 2017. Non-genomic action of androgens is mediated by rapid phosphorylation and regulation of androgen receptor trafficking. Cell Physiol Biochem. 43(1):223–236. doi: 10.1159/000480343.
- Erkanli Şentürk G, Ersoy Canillioĝlu Y, Umay C, Demiralp-Eksioglu E, Ercan F. 2012. Distribution of zonula occludens-1 and occludin and alterations of testicular morphology after in utero radiation and postnatal hyperthermia in rats. Int J Exp Pathol. 93(6):438–449. doi: 10.1111/j.1365-2613.2012.00844.x.
- Fan Y, Liu Y, Xue K, Gu GB, Fan WM, Xu YL, Ding ZD. 2015. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 10(4):e0120775. doi: 10.1371/journal.pone.0120775.
- Fazi R, Tintori C, Brai A, Botta L, Selvaraj M, Garbelli A, Maga G, Botta M. 2015. Homology model-based virtual screening for the identification of human helicase DDX3 inhibitors. J Chem Inf Model. 55(11):2443–2454. doi: 10.1021/acs.jcim.5b00419.
- Guo J, Tao SX, Chen M, Shi YQ, Zhang ZQ, Li YC, Zhang XS, Hu ZY, Liu YX. 2007. Heat treatment induces liver receptor homolog-1 expression in monkey and rat Sertoli cells. Endocrinology. 148(3):1255–1265. doi: 10.1210/en.2006-1004.
- Hu SQ, Liu DL, Liu SJ, Li CR, Guo J. 2021. Lycium barbarum polysaccharide ameliorates heat-stress-induced impairment of primary Sertoli cells and the blood-testis barrier in rat via androgen receptor and Akt phosphorylation. Evid Based Complement Alternat Med. 2021:5574202. doi: 10.1155/2021/5574202.
- Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 4(1):44–57. doi: 10.1038/nprot.2008.211.
- Huang YH, Zheng GY. 2022. Circ_UBE2D2 attenuates the progression of septic acute kidney injury in rats by targeting miR-370-3p/NR4A3 axis. J Microbiol Biotechnol. 32(6):740–748. doi: 10.4014/jmb.2112.12038.
- Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, Dohle G, Krausz C, European Association of Urology Working Group on Male Infertility. 2012. European association of urology guidelines on male infertility: the 2012 update. Eur Urol. 62(2):324–332. doi: 10.1016/j.eururo.2012.04.048.
- Lovren F, Pan Y, Shukla PC, Quan A, Teoh H, Szmitko PE, Peterson MD, Gupta M, Al-Omran M, Verma S. 2009. Visfatin activates eNOS via Akt and MAP kinases and improves endothelial cell function and angiogenesis in vitro and in vivo: translational implications for atherosclerosis. Am J Physiol Endocrinol Metab. 296(6):E1440–E1449. doi: 10.1152/ajpendo.90780.2008.
- Min KB, Min JY. 2017. Exposure to environmental noise and risk for male infertility: a population-based cohort study. Environ Pollut. 226:118–124. doi: 10.1016/j.envpol.2017.03.069.
- Möller ML, Bulldan A, Scheiner-Bobis G. 2021. Tetrapeptides modelled to the androgen binding site of ZIP9 stimulate expression of tight junction proteins and tight junction formation in Sertoli cells. Biology (Basel. 11(1):55. doi: 10.3390/biology11010055.
- Mruk DD, Cheng CY. 2015. The mammalian blood-testis barrier: its biology and regulation. Endocr Rev. 36(5):564–591. doi: 10.1210/er.2014-1101.
- Piñero J, Bravo À, Queralt-Rosinach N, Gutiérrez-Sacristán A, Deu-Pons J, Centeno E, García-García J, Sanz F, Furlong LI. 2017. DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 45(D1):D833–D839. doi: 10.1093/nar/gkw943.
- Qin DN, She BR, She YC, Wang JH. 2000. Effects of flavonoids from semen cuscutae on the reproductive system in male rats. Asian J Androl. 2:99–102.
- Rajeswari M, Santhi N, Bhuvaneswari V. 2014. Pharmacophore and virtual screening of JAK3 inhibitors. Bioinformation. 10(3):157–163. doi: 10.6026/97320630010157.
- Rangel-Gamboa L, Reyes-Castro M, Dominguez-Cherit J, Vega-Memije E. 2013. Proliferating trichilemmal cyst: the value of ki67 immunostaining. Int J Trichology. 5(3):115–117. doi: 10.4103/0974-7753.125599.
- Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, et al. 2014. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 6:13. doi: 10.1186/1758-2946-6-13.
- Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, Nativ N, Bahir I, Doniger T, Krug H, et al. 2010. GeneCards Version 3: the human gene integrator. Database. 2010:baq020. doi: 10.1093/database/baq020.
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. 2003. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 125(6):769–784. doi: 10.1530/rep.0.1250769.
- Sun Y, Chen K, Lin G, Wan F, Chen L, Zhu X. 2021. Silencing c-Jun inhibits autophagy and abrogates radioresistance in nasopharyngeal carcinoma by activating the PI3K/AKT/mTOR pathway. Ann Transl Med. 9(13):1085. doi: 10.21037/atm-21-2563.
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. 2019. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47(D1):D607–D613. doi: 10.1093/nar/gky1131.
- Turczyn A, Pańczyk-Tomaszewska M, Krzemień G, Górska E, Demkow U. 2021. The usefulness of urinary periostin, cytokeratin-18, and endoglin for diagnosing renal fibrosis in children with congenital obstructive nephropathy. JCM. 10(21):4899. doi: 10.3390/jcm10214899.
- Vogelstein B, Lane D, Levine AJ. 2000. Surfing the p53 network. Nature. 408(6810):307–310. doi: 10.1038/35042675.
- Wang H, Li T, Zhao L, Sun M, Jian Y, Liu J, Zhang Y, Li Y, Dang M, Zhang G. 2019. Dynamic effects of ioversol on the permeability of the blood-brain barrier and the expression of ZO-1/occludin in rats. J Mol Neurosci. 68(2):295–303. doi: 10.1007/s12031-019-01305-z.
- Wang X, Shen Y, Wang S, Li S, Zhang W, Liu X, Lai L, Pei J, Li H. 2017. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45(W1):W356–W360. doi: 10.1093/nar/gkx374.
- Xu HY, Zhang YQ, Liu ZM, Chen T, Lv CY, Tang SH, Zhang XB, Zhang W, Li ZY, Zhou RR, et al. 2019. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 47(D1):D976–D982. doi: 10.1093/nar/gky987.
- Yang Y, Li Q, Huang R, Xia H, Tang Y, Mai W, Liang J, Ma S, Chen D, Feng Y, et al. 2022. Small-molecule-driven direct reprogramming of fibroblasts into functional sertoli-like cells as a model for male reproductive toxicology. Adv Biol (Weinh). 6(5):e2101184. doi: 10.1002/adbi.202101184.
- Yang WR, Liao TT, Bao ZQ, Zhou CQ, Luo HY, Lu C, Pan MH, Wang XZ. 2018. Role of AMPK in the expression of tight junction proteins in heat-treated porcine Sertoli cells. Theriogenology. 121:42–52. doi: 10.1016/j.theriogenology.2018.08.005.
- Yang J, Wang Y, Bao Y, Guo J. 2008. The total flavones from semen cuscutae reverse the reduction of testosterone level and the expression of androgen receptor gene in kidney-yang deficient mice. J Ethnopharmacol. 119(1):166–171. doi: 10.1016/j.jep.2008.06.027.
- Zhang X, Chen B, Wu J, Sha J, Yang B, Zhu J, Sun J, Hartung J, Bao E. 2020. Aspirin enhances the protection of Hsp90 from heat-stressed injury in cardiac microvascular endothelial cells through PI3K-Akt and PKM2 pathways. Cells. 9(1):243. doi: 10.3390/cells9010243.
- Zhang W, Fu ZT, Xie Y, Duan ZW, Wang Y, Fan RH. 2020. High resolution UPLC-MS/MS method for simultaneous separation and determination of six flavonoids from semen cuscutae extract in rat plasma: application to comparative pharmacokinetic studies in normal and kidney-deficient rats. Nat Prod Res. 34(10):1446–1451. doi: 10.1080/14786419.2018.1511556.
- Zhang Y, Gong XG, Wang ZZ, Sun HM, Guo ZY, Hu JH, Ma L, Li P, Chen NH. 2016. Overexpression of DJ-1/PARK7, the Parkinson’s disease-related protein, improves mitochondrial function via Akt phosphorylation on threonine 308 in dopaminergic neuron-like cells. Eur J Neurosci. 43(10):1379–1388. doi: 10.1111/ejn.13216.
- Zhang B, Su H, Ren XQ, Li WX, Ding Y, Zhang X, Zhai WS, Song CD. 2019. [Study on mechanism of cuscutae semen flavonoids in improving reproductive damage of tripterygium glycosides tablets in rats based on high-throughput transcriptome sequencing]. Zhongguo Zhong Yao Za Zhi. 44(16):3478–3485. Chinese. doi: 10.19540/j.cnki.cjcmm.20190527.402.
- Zhang Y, Sun HM, He X, Wang YY, Gao YS, Wu HX, Xu H, Gong XG, Guo ZY. 2013. Da-Bu-Yin-Wan and Qian-Zheng-San, two traditional Chinese herbal formulas, up-regulate the expression of mitochondrial subunit NADH dehydrogenase 1 synergistically in the mice model of Parkinson’s disease. J Ethnopharmacol. 146(1):363–371. doi: 10.1016/j.jep.2013.01.005.
- Zhang XS, Zhang ZH, Guo SH, Yang W, Zhang ZQ, Yuan JX, Jin X, Hu ZY, Liu YX. 2006. Activation of extracellular signal-related kinases 1 and 2 in Sertoli cells in experimentally cryptorchid rhesus monkeys. Asian J Androl. 8(3):265–272. doi: 10.1111/j.1745-7262.2006.00142.x.
- Zhang W, Zhang L, Wang WJ, Ma S, Wang M, Yao M, Li R, Li WW, Zhao X, Hu D, et al. 2022. Network pharmacology and in vitro experimental verification to explore the mechanism of sanhua decoction in the treatment of ischemic stroke. Pharm Biol. 60(1):119–130. doi: 10.1080/13880209.2021.2019281.
- Zhao R, Wu X, Bi XY, Yang H, Zhang Q. 2022. Baicalin attenuates blood-spinal cord barrier disruption and apoptosis through PI3K/Akt signaling pathway after spinal cord injury. Neural Regen Res. 17(5):1080–1087. doi: 10.4103/1673-5374.324857.
- Zhou X, Hu Y, Dai L, Wang Y, Zhou J, Wang W, Di W, Qiu L. 2014. MicroRNA-7 inhibits tumor metastasis and reverses epithelial-mesenchymal transition through AKT/ERK1/2 inactivation by targeting EGFR in epithelial ovarian cancer. PLoS One. 9(5):e96718. doi: 10.1371/journal.pone.0096718.
- Zhu B, Zhang W, Lu Y, Hu S, Gao R, Sun Z, Chen X, Ma J, Guo S, Du S, et al. 2018. Network pharmacology-based identification of protective mechanism of Panax notoginseng saponins on aspirin induced gastrointestinal injury. Biomed Pharmacother. 105:159–166. doi: 10.1016/j.biopha.2018.04.054.