Abstract
Context
Atopic dermatitis (AD) is a common inflammatory skin disease characterized with hyperactivation of type 2 T helper (Th2) immune responses. Icariin is a flavonoid glucoside with anti-inflammatory activities, which has been used to treat multiple diseases.
Objective
The present study investigates the underlying mechanisms by which icariin regulates Th2 responses and AD development.
Materials and methods
BALB/c mice were induced by DNFB to establish AD models, and injected with or without 10 mg/kg icariin for 2 weeks (i.p., daily). CD4+T cells were induced by Th2 condition to simulate AD in vitro, and also treated with or without 100 µM icariin.
Results
Icariin ameliorated AD-like skin lesion, manifested as a significant decrease in dermatitis scores (from 8.00 ± 1.00 to 3.67 ± 0.58), serum IgE levels (from 3119.15 ± 241.81 to 948.55 ± 182.51 ng/mL), epidermal thickness (from 93.86 ± 4.61 to 42.67 ± 2.48 µm) and infiltration of mast cells (from 60.67 ± 3.21 cells to 36.00 ± 2.65 cells). Also, icariin inactivated NLRP3 inflammasome, inhibited Th2 skewing, reduced lncRNA MALAT1 expression, but elevated miR-124-3p expression in vivo and in vitro. MALAT1 increased NLRP3 expression through targeting miR-124-3p. Knockdown of MALAT1 repressed NLRP3 inflammasome activation and mitigated Th1/Th2 imbalance in Th2-conditioned CD4+T cells, whereas both MALAT1 overexpression and miR-124-3p inhibition ablated the inhibitory effects of icariin on Th2 immune responses.
Discussion and conclusions
The findings further improve our understanding of the mechanism by which icariin affects AD progression, and highlights the potential of icariin in the treatment of AD.
Introduction
Atopic dermatitis (AD) is an immune-related chronic and recurrent inflammatory skin disease. Its main clinical manifestations are dry skin, erythema on the flexor side of the body, papules, severe erosion, exudation and severe pruritus. In addition to damaging the skin, AD usually complicates asthma and allergic rhinitis, and increases the incidence of cataract, infection and hypoalbuminaemia (Li H et al. Citation2021). It not only causes great harm to the physical and mental health of patients, but also imposes a serious burden on the society and economy (Shi et al. Citation2017). At present, the treatments of AD only improve symptoms, reduce recurrence and improve the quality of life of patients, but the disease is almost incurable (Li JX et al. Citation2021). Many researchers in related fields tend to believe that AD is an immune inflammatory reaction that is self-sensitized to the external environment based on genetic factors, and the continuous activation of type 2 T helper (Th2) cells leads to continuous inflammatory state of the skin microenvironment and impairment of the skin barrier function (Bieber Citation2020; He et al. Citation2020; Möbus et al. Citation2021). Therefore, overcoming the immune inflammatory microenvironment of type 1 T helper (Th1)/Th2 imbalance might be a potential therapeutic strategy for AD.
Traditional Chinese medicine has shown good efficacy and fewer side effects in the treatment of various chronic diseases, including AD (Lee et al. Citation2019; Zhao et al. Citation2021; Zheng et al. Citation2021). Icariin, the main active ingredient of the plant Epimedium brevicornum Maxim (Berberidaceae) species, is an 8-prenyl flavonoid glycoside compound. Modern pharmacological studies have shown that icariin has the activities of immunomodulation, anti-inflammation, anti-aging and antitumour (El-Shitany and Eid Citation2019; Song et al. Citation2020; Sun et al. Citation2021), and may have improvement effects on diseases related to nervous system, immune, endocrine, cardiovascular system and bone (Jin J et al. Citation2019; Zhang et al. Citation2021; Wang G et al. Citation2022). Notably, icariin is proved to have immunoadjuvant activity that can enhance Th1-immune response, and is suggested to be beneficial for the treatment of Th1/Th2-disordered diseases (Rhew and Han Citation2012). A few studies have confirmed that icariin can modulate balance of Th1/Th2 cytokines to mitigate airway inflammation in asthmatic rats (Xu et al. Citation2011; Wang J et al. Citation2014). Additionally, an animal study showed that icariin inhibits the inflammation of AD by regulating the skin hypothalamus pituitary adrenal axis (Kong et al. Citation2019). This evidence prompted us that icariin might improve Th2 response to restrain AD development.
Among the molecular mechanisms contributing to the pathogenesis of AD, epigenetic factors such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and other non-coding RNAs (ncRNAs) play an increasingly prominent role in inducing Th cell differentiation and regulating cytokine secretion (Gibbons et al. Citation2018; Wu et al. Citation2018; Zhou et al. Citation2018; Iaccarino et al. Citation2022). These ncRNAs can regulate gene expression through epigenetic modification, transcription and post-transcription, and participate in various biological processes such as cell differentiation, proliferation and apoptosis (Zhong et al. Citation2019; Tang et al. Citation2021). Nevertheless, it is unclear whether these ncRNAs participate in Th2 polarization during AD and how icariin affects some of these ncRNAs to alleviate AD progression. In this study, we aimed to identify the involving ncRNAs and their interaction mechanism in icariin regulating Th1/Th2 disorder during AD development, and we believed that this exploration would provide more reliable evidences for the application of icariin in AD management.
Materials and methods
Animals
BALB/c mice without specific pathogen (6–8 weeks old, 18–22 g) were provided by Henan Experimental Animal Center. They were raised at an environment at 22 ± 2 °C, with relative humidity of 40–70% and a 12 h light/dark cycle. These mice were adaptively reared for 1 w before experiment, and divided into three groups according to the random number table, with three mice in each group: sham group, model group and icariin group. The study protocol was established according to the ethical guidelines of the Helsinki Declaration and approved by the Ethics Committee of the Second Affiliated Hospital of Henan University of traditional Chinese medicine (PZ-HNSZYY-2021-025).
Model establishment
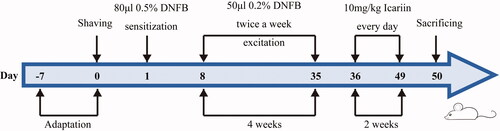
One day before modelling, the back hair of all mice was shaved off about an area of 2 cm × 3 cm. To establish the AD model, 80 μL of 2,4-dinitrofluorobenzene (DNFB, 0.5%, dissolved in a 4:1 mixed solution of acetone and olive oil, Qianyan Chemical Technology, Wuhan, China, CAS:70-34-8) was evenly applied to the exposed skin for sensitization. After 1 w, 50 μL of DNFB (0.2%) was applied to the exposed back for excitation twice a week for 4 w. The mice in sham group was coated with the same volume of matrix solution (acetone:olive oil = 4:1). Erythema, scale and scab in the mice were regarded as successful modelling. Then, the modelling mice in icariin group were administered with icariin via intraperitoneal injection (10 mg/kg, purity ≥98%, dissolved in normal saline, Ruiding, Shanghai, China, CAS: 489-32-7) every day for 2 w, and mice in the other two groups were injected with normal saline. The procedure for AD model establishment and icariin treatment is shown in .
Serum immunoglobulin E (IgE)
Blood was collected from the above mice at 50 d before sacrifice and centrifuged at 3500 rpm for 10 min at room temperature to obtain serum. The concentration of serum IgE was detected by using mouse IgE enzyme-linked immunosorbent assay (ELISA) kits (Thermo Fisher, Waltham, MA) according to the manufacturer’s instructions.
Histological analysis
Histological analysis was performed by staining with haematoxylin and eosin (H&E) or toluidine blue (TB). The dorsal skin tissues of mice were fixed in 10% neutral buffered formalin for 24 h at 4 °C and made into 5 µm-thick sections after paraffin embedding. Sections were stained with H&E solution, and five visual fields were randomly selected under the microscope for observation. The thickness of epidermis was measured by Image J software (Bethesda, MD). The dermatitis scores were evaluated according to four main characteristics of diseased skin: dryness/crusting, bleeding/erythema, erosion/peeling and oedema (Wang X et al. Citation2020). Subsequently, these sections were stained with TB solution to measure the degree of mast cell infiltration, and the number of mast cells in five fields was counted under the microscope. Three sections were examined for each mouse, and all measurements were performed in a blinded manner.
CD4+T cells preparation
Healthy BALB/c mouse was used for preparation of CD4+T cells. Briefly, mouse was euthanized and soaked in 75% ethanol for 5 min. The spleen was taken out under sterile conditions, put into a culture dish containing pre-cooled medium, ground to chylous liquid and filtered with 70 µm nylon membrane. The mouse spleen single cell suspension was centrifuged at 1500 rpm for 10 min at 4 °C and the supernatant was discarded. The cell precipitate was treated with 2 mL red blood cell lysate for 10 min, and the reaction was terminated by using 6 mL complete culture medium. After washing with 10 mL phosphate buffer, the cell pellet was re-suspended with 5 mL complete medium and counted. Subsequently, naive CD4+T cells were isolated by using a mouse naive CD4+T cell isolation kit (Miltenyi, Bergisch Gladbach, Germany, CAS: 130-104-453) according to the manufacturer’s instructions. Cells were cultured in RPMI 1640 medium supplemented with 10% foetal calf serum, and placed in a humidified incubator containing 5% CO2 at 37 °C.
Th2-cell polarization
Naive CD4+T cells were cultured in Th2-cell conditions with 5 μg/mL immobilized anti-mouse CD3 antibody (eBioscience, San Diego, CA, CAS:16-0031-85) and 1 μg/mL anti-CD28 antibody (eBioscience, San Diego, CA, 16-0281-85) for 2 d. Th2-cell conditions contained 10 ng/mL recombinant murine IL-4 (Peprotech, Cranbury, NJ, CAS: 214-14) and 5 µg/mL anti-IFN-γ (Bioss, Beijing, China). Then, these cells were re-cultured in Th2 condition without anti-CD3 or anti-CD28 antibody for another 3 d.
Cell transfection and treatment
NC mimic, miR-124-3p mimic, NC inhibitor and miR-124-3p inhibitor, lentivirus vector (LV) – MALAT1 and shRNA-MALAT1 were purchased from Ribobio Co., Ltd. (Guangzhou, China). To investigate the role of NLRP3 inflammasome activation in AD, CD4+T cells were co-treated with NLRP3 activator MSU (0.5 mg/mL) and icariin (100 µM) or treated with NLRP3 inhibitor MCC950 (10 µM) alone, and all cells were cultured in Th2-condition. To investigate the molecular regulatory mechanism of icariin in Th2 skewed cells, CD4+T cells were transfected with the indicated transfectants for 24 h to obtain stably transfected cells, then treated with or without 100 µM icariin and cultured in Th2-cell condition.
Measurement of cytokines
The Th1-mediated cytokines (IFN‐γ and IL-12) and Th2-mediated cytokines (IL-4 and IL-13) in the homogenate of lesion skin tissue or cell supernatant were measured by corresponding ELISA kit (Enzyme-linked Biotechnology, Shanghai, China) according to the instructions. The OD value at 450 nm was measured on a microplate reader, and the corresponding concentration was converted according to the standard curve of concentration–OD value.
Evaluation of Th1/Th2 balance
The blood sample of mice was collected before sacrifice, and peripheral blood mononuclear cells (PBMC) were separated by using a mouse PBMC separation kit (Procell, Wuhan, China, cat. no. CP-M190). PBMC was counted and incubated with brefeldin-A (Beyotime, Beijing, China) for 5 h. The cells were stained by 5 µL anti-CD3-APC (Invitrogen, Carlsbad, CA, cat. no. 17-0032-82, 1/4000 dilution), anti-CD4-FITC (Invitrogen, Carlsbad, CA, cat. no. 11-0042-82, 1/2000 dilution) at 4 °C in dark for 10 min. They were fixed/permeabilized using BD-Cytofix/Cytoperm™ and BD-Perm/Wash™ (BD Biosciences, Franklin Lakes, NJ) following the instructions, and then stained with anti-IFN-γ-PerCP-cy5.5 (Invitrogen, Carlsbad, CA, cat. no. 45-7311-82, 1/800 dilution) and anti-IL-4-PE (Invitrogen, Carlsbad, CA, cat. no. 12-7041-82, 1/160 dilution) for 30 min at 4 °C in the dark. After washing, they were re-suspended in 500 µL phosphate buffer and then analysed quantitatively by flow cytometry. For detection of Th1/Th2 polarization in vitro, the CD4+T cells were only stained with anti-IFN-γ-PerCP-cy5.5 and anti-IL-4-PE. Th1/Th2 balance was evaluated by calculating IFN-γ/IL-4 CD4+ T cell ratio.
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from lesion skin tissues and CD4+T cells using TRIzol reagent (Sangon Biotech, Shanghai, China) according to the manufacture’s instruction, and quantified by measuring absorbance at 260 nm. Total RNA (1 µg) was reversely transcribed to cDNA using Prime ScriptTMRT reagent kit (TaKaRa, Dalian, China) or Mir-X™ miRNA First-Strand Synthesis Kit (TaKaRa, Dalian, China) following its protocols. Then, cDNA was used as template for RT-PCR analysis using SYBR®Premix Ex Taq™ II (TaKaRa, Dalian, China). The primers were designed as shown in . Relative expression was normalized to the internal references (GAPDH or U6).
Table 1. Primers used for RT-PCR.
Western blot
Total protein was isolated from lesion skin tissues and CD4+T cells using RIPA lysis buffer (Beyotime, Beijing, China) on ice, and then quantified using bicinchoninic acid protein assay kit (Beyotime, Beijing, China). Protein samples were denatured in SDS-PAGE loading buffer for 10 min at 100 °C, and separated in 10% SDS-PAGE. The proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA) through electrophoresis. Then, the membranes were blocked with 5% nonfat milk for 1 h at room temperature, and incubated with primary antibodies against mouse NLRP3 (Invitrogen, Carlsbad, CA, cat. no. MA5-23919, 1/250 dilution), cleaved caspase-1 (Invitrogen, Carlsbad, CA, cat. no. PA5-38099, 1/500 dilution), IL-1β (Invitrogen, Carlsbad, CA, cat. no. P420B, 1/1000 dilution) and GAPDH (Invitrogen, Carlsbad, CA, cat. no. PA1-988, 1/500 dilution) at 4 °C overnight. Membranes were incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody IgG (Invitrogen, Carlsbad, CA, cat. no. 31402, 1/5000 dilution) for 2 h at room temperature. The blots were visualized with an enhanced chemiluminescence reagent (Beyotime, Beijing, China), and quantified by a gel imaging system.
Luciferase reporter assay
The wild type or mutant untranslated region (UTR) of MALAT1 and NLRP3 was connected to the luciferase plasmids to construct the luc-UTR vectors. Then, the recombinant plasmids were co-transfected with Renilla luciferase plasmid, NC mimic or miR-124-3p mimic into 293T cells for 48 h. The luciferase activities in different cells were detected by using the dual luciferase reporter gene detection kit (Yeasen, Shanghai, China, CAS: 11402ES60) according to its manufacturer’s instructions and using a microplate reader (Promega GloMax Multi Plus, Madison, WI).
Statistical analysis
Statistical analysis was performed by using Prism 7.0 software (GraphPad, La Jolla, CA). Data were expressed as the form of mean ± standard deviation. Data between two groups were analysed by independent sample t-test. The differences with p value less than 0.05 were considered statistically significant.
Results
Icariin ameliorated AD-like symptoms in DNFB-stimulated mice in vivo
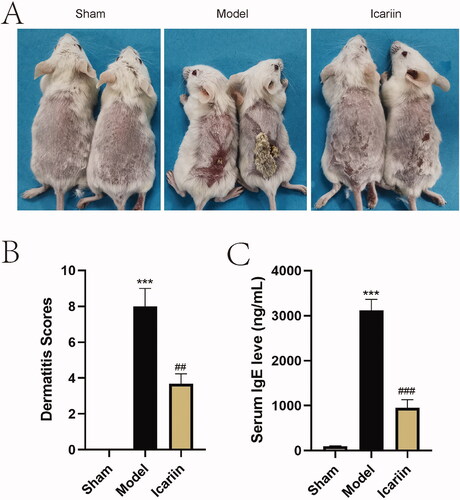
To investigate the effect of icariin on AD, AD-like skin lesion was induced by applying DNFB on BALB/c mice, and then these mice were treated with icariin. The establishment procedures of AD mice are shown in . As shown in , topically applied DNFB triggered obvious skin lesions, characterized as dryness, crusting, erythema, peeling, etc. Based on these epidermal changes, dermatitis scores were assessed, and a significant increased score was displayed in the model mice in contrast to sham mice (). Interestingly, the administration of icariin for 14 d significantly improved AD-like epidermal symptoms. In addition, the serum concentrations of IgE were remarkably elevated in AD model mice induced by DNFB, and reduced by icariin treatment ().
Figure 2. Icariin ameliorated AD-like symptoms in DNFB-stimulated mice. The AD mouse model was established by DNFB, and 5 weeks later, mice were administered with icariin for 2 weeks. (A) The change of skin was observed. (B) The dermatitis scores were evaluated according to four main characteristics of diseased skin: dryness/crusting, bleeding/erythema and erosion/peeling and oedema. (C) Serum IgE levels were determined by ELISA. ***p < 0.001 vs. sham; ##p < 0.01, ###p < 0.001 vs. model. n = 6.
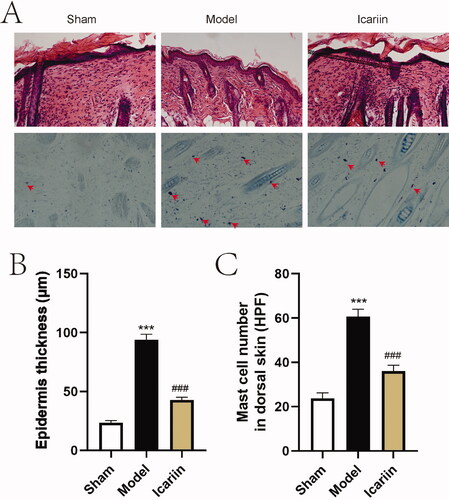
To evaluate histological changes of AD model mice treated by icariin, H&E staining and TB staining were then performed in lesion tissue slides (). Results showed that DNFB induced epidermal hyperplasia and increased infiltration of mast cells in model mouse skin, while icariin alleviated epidermal thickening and mast cell infiltration (). The above data suggested that icariin ameliorated AD-like symptoms in vivo.
Figure 3. Icariin ameliorated DNFB-stimulated histological change in AD model mice. (A) After treatment with icariin, all mice were euthanized. Skin tissues at lesion site were stained with H&E solution or TB solution, and observed under a microscope (magnification: ×200, mast cells indicated by red arrows). (B) The epidermal thickness was measured after modelling. (C) The infiltrated mast cells in lesion skin tissues were counted under a microscope. ***p < 0.001 vs. sham; ###p < 0.001 vs. model. n = 6.
Icariin inhibited activation of NLRP3 inflammasome and restored Th1/Th2 balance in AD model mice
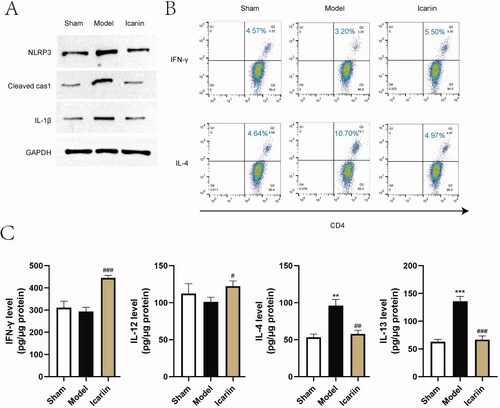
To evaluate the effects of icariin on regulating NLRP3 inflammasome activation and Th1/Th2 imbalance, the two main pathological changes during AD, the expression of NLRP3 inflammasome related markers detected by western blot, and the differentiation of Th1 and Th2 was measured by flow cytometry and ELISA. Results showed that the expression of NLRP3, cleaved caspase-1 and IL-1β was significantly enhanced in DNFB-induced AD model mice, suggesting an activation of NLRP3 inflammasome, which were obviously inhibited by icariin treatment (). Meanwhile, notable increases of IL-4 positive cells and type II cytokines (IL-4 and IL-13) were observed in AD model mice, while icariin elevated IFN-γ+ CD4+/IL-4+CD4+ T cell ratio and type I cytokines (IFN-γ and IL-12) levels to antagonize Th2 skewing induced by DNFB (). The above data suggested that icariin inhibited the activation of NLRP3 inflammasome and improved Th1/Th2 imbalance in DNFB-induced AD model mice.
Figure 4. Icariin inhibited activation of NLRP3 and restored Th1/Th2 balance in AD model mice. (A) Activation markers of NLRP3 inflammasome (NLRP3, cleaved caspase-1, IL-1β) in the lesion skin tissue were detected by western blot. (B) Th1/Th2 (IFN-γ/IL-4) ratio in the homogenate of lesion skin tissue was evaluated by flow cytometry. (C) The levels of Th1-mediated cytokines (IFN‐γ and IL-12) and Th2-mediated cytokines (IL-4 and IL-13) in the homogenate of skin tissue were measured by ELISA. **p < 0.01, ***p < 0.001 vs. sham; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. model. n = 6.
Icariin ameliorated Th2 response by inhibiting NLRP3 inflammasome activation in vitro
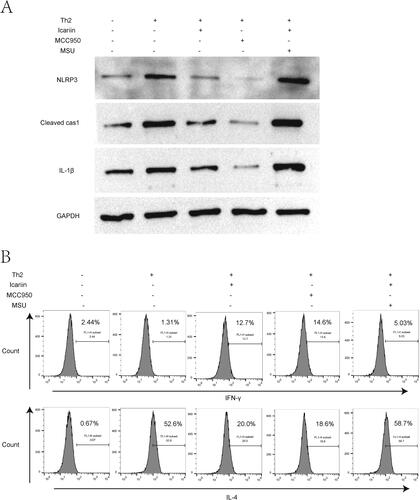
Previous studies have demonstrated that activation of NLRP3 inflammasome contributes to Th2 immune response (Gurung et al. Citation2015; Jin X et al. Citation2020; Huanosta-Murillo et al. Citation2021). To clarify the role of NLRP3 on DNFB-induced Th2 polarization in AD, Th2-conditioned CD4+T cells were co-treated with NLRP3 activator MSU and icariin, or treated with NLRP3 inhibitor MCC950 alone. The application of MCC950 significantly decreased the protein expressions of NLRP3, cleaved caspase-1 and IL-1β in Th2-cultured CD4+T cells, showing a strong inhibitory effect on NLRP3 inflammasome, while MSU distinctly recovered the activation of NLRP3 inflammasome in icariin-treated and Th2-condioned CD4+T cells (). Data from flow cytometry showed that MCC950 elevated Th1 differentiation, mitigated Th2 skewing in Th2-conditioned CD4+T cells as icariin did, while MSU exacerbated the Th2 response, counteracting the improving effect of icariin on Th1/Th2 imbalance (). These results indicated that icariin ameliorated Th2 immune response by inhibiting NLRP3 inflammasome activation in vitro.
Figure 5. Icariin ameliorated Th2 response by inhibiting NLRP3 inflammasome activation in vitro. Naive CD4+T cells were extracted and purified from normal mice, and induced for Th2 skewing. Next, cells were treated with NLRP3 activator MSU, icariin or NLRP3 inhibitor MCC950 for 24 h. (A) Activation markers of NLRP3 inflammasome (NLRP3, cleaved caspase-1, IL-1β) in the cells were detected by western blot. (B) Th1/Th2 (IFN-γ/IL-4) ratio in CD4+T cells was evaluated by flow cytometry. n = 3.
NLRP3 was upregulated by a MALAT1/miR-124-3p ceRNA regulatory network
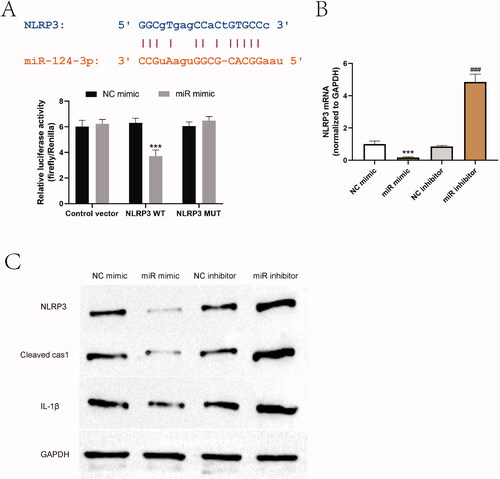
To investigate the regulatory molecular mechanism of NLRP3 inflammasome activation in AD, we searched the upstream targets of NLRP3, and the function of miR-124-3p and MALAT1 in inflammation and innate immune had attracted our attention. Of note, miR-124 has been proved to play an immunosuppressive role in AD (Yang et al. Citation2017), and it exerts its anti-inflammatory effect by repressing the activation of NLRP3 (Fang and Hong Citation2021; Wang B et al. Citation2021). Through online bioinformatics analysis tools, miR-124-3p was found to target 5′UTR of NLRP3, and luciferase reporter assay data demonstrated that co-transfection with miR-124-3p mimic and reporter vectors containing wild-type NLRP3 resulted in a decrease of the luciferase activity, compared to the control vectors or vectors containing mutant NLRP3, suggesting that NLRP3 was directly targeted by miR-124-3p (). Additionally, miR-124-3p mimic and inhibitor were transfected to CD4+T cells to achieve ectopic expression or inhibition of miR-124-3p. As verified by RT-PCR and western blot, the expression levels of NLRP3 and the protein levels of cleaved caspase-1 and IL-1β were suppressed by miR-124-3p mimic, but elevated by miR-124-3p inhibitor in CD4+T cells ().
Figure 6. NLRP3 was targeted by miR-124-3p. (A) Bioinformatics analysis was used to predict the target site between miR-124-3p and NLRP3, and the targeting relationship was verified by dual luciferase reporter gene system. ***p < 0.001 vs. NC mimic. n = 3. (B, C) The effect of miR-124-3p mimic and inhibitor on NLRP3 activation was examined in CD4+T cells by western blot. ***p < 0.001 vs. NC mimic; ###p < 0.001 vs. NC inhibitor. n = 3.
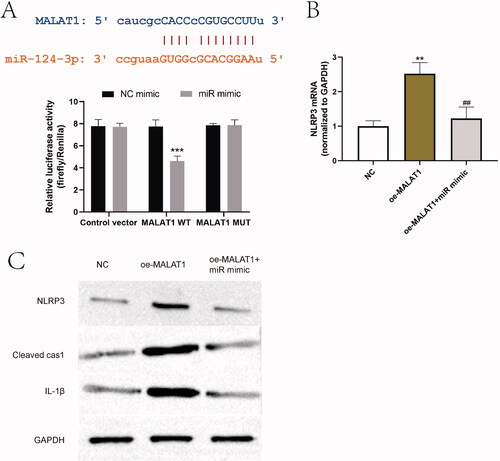
MALAT1 is a well-known inflammatory factor and proved to be an activator of NLRP3 inflammasome (Yu et al. Citation2018; Che et al. Citation2020; Du et al. Citation2020). Through bioinformatic analysis and luciferase reporter assay, we also demonstrated that miR-124-3p could bind to 3′UTR of MALAT1 (). To verify the regulatory relationship between MALAT1, miR-124-3p and NLRP3, LV-MALAT1 vectors and miR-124-3p mimic were then co-transfected into CD4+T cells. Exogenous MALAT1 increased the expression of NLRP3 and its activation markers, cleaved caspase-1 and IL-1β, which was partially reversed by overexpression of miR-124-3p (). These results suggested that NLRP3 was upregulated by a MALAT1/miR-124-3p ceRNA regulatory network in AD model in vivo and in vitro.
Figure 7. miR-124-3p was targeted by MALAT1. (A) Bioinformatic analysis was used to predict the target site between MALAT1 and miR-124-3p, and the targeting relationship was verified by dual luciferase reporter gene system. ***p < 0.001 vs. NC mimic. n = 3. (B, C) The effect of overexpressing MALAT1 and miR-124-3p on NLRP3 axis was examined by RT-PCR and western blot. **p < 0.01, ***p < 0.001 vs. NC; ##p < 0.01 vs. oe-MALAT1. n = 3.
Icariin ameliorated Th2 response in CD4+T cells by repressing the MALAT1/miR-124-3p axis-modulated NLRP3 inflammasome activation
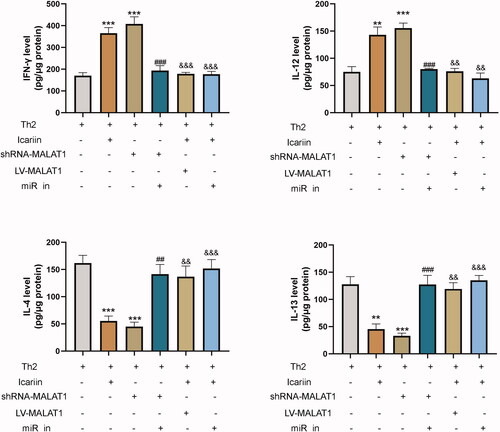
To clarify the molecular regulatory mechanism of icariin affecting Th2 response during AD, we transfected shRNA-MALAT1, LV-MALAT1 or miR-124-3p inhibitor into CD4+T cells, and treated cells with or without icariin. As delineated by western blot (), knockdown of MALAT1 achieved a similar effect with icariin in restraining the activation of NLRP3 inflammasome in Th2-conditioned CD4+T cells, but simultaneous knockdown of miR-124-3p removed the restriction. Both MALAT1 overproduction and miR-124-3p inhibition reversed the inhibitory effect of icariin on NLRP3 activation. Next, flow cytometry () and ELISA () were performed to evaluate Th2 response in CD4+T cells. Similar to icariin, deleting MALAT1 increased IFN-γ/IL-4 ratio and the secretion of type I cytokines in Th2-cultured CD4+T cells, decreased the production of type II cytokines, suggesting a recovery of Th1/Th2 balance. Nevertheless, overexpression of MALAT1 or deletion of miR-124-3p destroyed this balance through enhancing Th2 polarization and blocking Th1 differentiation, abolishing the effect of icariin treatment in Th2-conditioned CD4+T cells. The data suggested that icariin ameliorated Th2 response in CD4+T cells by repressing the MALAT1/miR-124-3p axis and the activation of NLRP3 inflammasome.
Figure 8. Icariin ameliorated Th2 response in CD4+T cells by the MALAT1/miR-124-3p axis. CD4+T cells were transfected with miR-124-3p inhibitor, shRNA-MALAT1 plasmid or LV-MALAT1 plasmid, treated with or without icariin, and then cultured in Th2-cell condition. (A) Activation markers of NLRP3 inflammasome (NLRP3, cleaved caspase-1, IL-1β) in the cells were detected by western blot. (B) Th1/Th2 (IFN-γ/IL-4) ratio in CD4+T cells was evaluated by flow cytometry. n = 3.
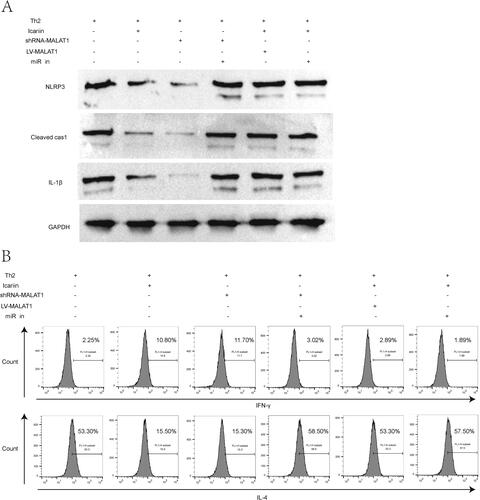
Figure 9. Icariin modulated Th1/Th2 cytokines in CD4+T cells by the MALAT1/miR-124-3p axis. CD4+T cells were transfected with miR-124-3p inhibitor, shRNA-MALAT1 plasmid or LV-MALAT1 plasmid, treated with or without icariin, and then cultured in Th2-cell condition. The levels of Th1-mediated cytokines (IFN‐γ and IL-12) and Th2-mediated cytokines (IL-4 and IL-13) in the cell supernatant were measured by ELISA. **p < 0.01, ***p < 0.001 vs. Th2 induced group; ##p < 0.01, ###p < 0.001 vs. Th2 and shRNA-MALAT1 induced group; &&p < 0.01, &&&p < 0.001 vs. Th2 and icariin induced group. n = 3.
Discussion
Th2-mediated immune response plays a prominent role in the pathological mechanism of allergic disease, such as bronchial asthma, allergic rhinitis and AD (Ji et al. Citation2021; Kim et al. Citation2021; Yánez et al. Citation2021). Targeting Th1/Th2 differentiation of CD4+T cells is regarded as a potential strategy for AD management (Wang J et al. Citation2020). In our present study, icariin was verified to inhibit the hyperactivated Th2 response in AD model mice and Th2-induced CD4+T cells through regulating a novel MALAT1/miR-124-3p interaction network and its downstream NLRP3 inflammasome.
According to the theory of western medicine, AD is considered as an abnormal immune regulation caused by environmental factors acting on genetically susceptible individuals (Tsoi et al. Citation2019). Icariin has been well-known for its effect on immune regulation, anti-inflammation, anti-aging, anti-tumour, etc. (Liu XJ et al. Citation2021; Luo and Zhang Citation2021). On the other side, traditional Chinese medicine believes that the repeated attack of AD is due to the Yang Qi of kidney being insufficient and unable to resist the exodus of Evil Qi (Liu Y et al. Citation2018; Yan et al. Citation2020). The traditional Chinese medicine for reinforcing the Yang Qi and tonifying the kidney can improve the clinical symptoms of AD. Icariin is extracted from Epimedium plant species, and indeed a key medicine for tonifying kidney and strengthening Yang Qi in clinic. Therefore, our study showed that treatment of icariin decreased dermatitis scores, serum IgE levels, epidermal thickness and infiltration of mast cells in AD-like model mice. However, our previous knowledge of icariin remains at the level of pharmacological efficacy. This study further investigated the detailed molecular regulatory mechanism of icariin, and found that the activation of NLRP3 inflammasome was a key regulatory target of icariin on Th2 differentiation of CD4+T cells. Previous research demonstrated that NLRP3 inflammasome activation is a trigger of AD, and its inhibition reduces Th1/Th2-mediated cytokines in AD-like skin lesions (Park et al. Citation2021). Other studies also reported that the activation of NLRP3 induces a Th2 cell-mediated response in other inflammatory diseases (Braga et al. Citation2019; Sendler et al. Citation2020). Our results revealed that icariin inactivated NLRP3 inflammasome to restrain Th2 skewing, which were supported by these evidences.
The interaction between lncRNA and miRNA plays an important role in the pathogenesis of inflammatory diseases (Wang X et al. Citation2018; Fan et al. Citation2021). In our study, we proved that MALAT1 sequestered miR-124-3p to elevate NLRP3 expression in CD4+T cells in vitro, and the lncRNA MALAT1/miR-124-3p interaction network was identified as the target of icariin to inhibit NLRP3 expression. LncRNA MALAT1 is one of the most studied lncRNAs, and its function in tumours and endocrine diseases has been widely reported in the previous publications (Liu W et al. Citation2020). It has been demonstrated that MALAT1 is involved in the immune imbalance of Th1/Th2 cells via inhibiting the inflammation of Th1 cells and promoting the inflammatory response of Th2 cells (Liang and Tang Citation2020; Azari et al. Citation2021). miRNAs also have been identified to play diagnostic and therapeutic roles in allergic diseases (Specjalski and Jassem Citation2019). miR-124-3p is reported to be upregulated after treatment in allergic rhinitis (Hou et al. Citation2017), and it can alleviate Th2-mediated inflammatory response (Liu Q et al. Citation2022). Our findings proved that miR-124-3p was indirectly elevated by icariin administration, and exerted remarkable effects on anti-inflammatory and anti-Th2 hyperactivation in Th2-conditioned CD4+T cells. According to the above evidence, the lncRNA MALAT1/miR-124-3p/NLRP3 signaling cascade might be a novel regulatory mechanism in allergic diseases, which still needs further experimental verification.
Conclusions
Our work illustrates that icariin restrains the activation of the NLRP3 inflammasome to repress Th2 response in CD4+T cells during AD development by regulating a novel MALAT1/miR-124-3p competing endogenous RNA network. The findings further improve our understanding of the mechanism by which icariin affects AD progression, and provide new ideas for the development of management strategies for AD disease.
Author contributions
Wei Zhao, Huan-Huan Yu and Yu-Fu Fang conceived and designed the research. Huan-Huan Yu, Wei-Wei Meng and Bu-Xin Zhang performed the experiments. Wei-Wei Meng, Ying Wang and Jie Li analysed, interpretated the data and drafted the manuscript. Ai-Min Liu, Li Wang and Yu-Fu Fang revised the manuscript. Wei Zhao and Huan-Huan Yu contributed to the whole manuscript. All authors read and approved the final manuscript.
Disclosure statement
The authors declared no conflict of interest in this study.
Data availability statement
The data were all included in this manuscript. The original data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Azari H, Karimi E, Shekari M, Tahmasebi A, Nikpoor AR, Negahi AA, Sanadgol N, Mousavi P. 2021. Construction of a lncRNA–miRNA–mRNA network to determine the key regulators of the Th1/Th2 imbalance in multiple sclerosis. Epigenomics. 13(22):1797–1815. doi: 10.2217/epi-2021-0296.
- Bieber T. 2020. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. 75(1):54–62. doi: 10.1111/all.13954.
- Braga TT, Brandao WN, Azevedo H, Terra FF, Melo ACL, Pereira FV, Andrade-Oliveira V, Hiyane MI, Peron JPS, Camara NOS. 2019. NLRP3 gain-of-function in CD4+ T lymphocytes ameliorates experimental autoimmune encephalomyelitis. Clin Sci. 133(17):1901–1916. doi: 10.1042/CS20190506.
- Che H, Wang Y, Li H, Li Y, Sahil A, Lv J, Liu Y, Yang Z, Dong R, Xue H, et al. 2020. Melatonin alleviates cardiac fibrosis via inhibiting lncRNA MALAT1/miR-141-mediated NLRP3 inflammasome and TGF-β1/Smads signaling in diabetic cardiomyopathy. FASEB J. 34(4):5282–5298. doi: 10.1096/fj.201902692R.
- Du P, Wang J, Han Y, Feng J. 2020. Blocking the LncRNA MALAT1/miR-224-5p/NLRP3 axis inhibits the hippocampal inflammatory response in T2DM with OSA. Front Cell Neurosci. 14:97. doi: 10.3389/fncel.2020.00097.
- El-Shitany NA, Eid BG. 2019. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed Pharmacother. 120:109567. doi: 10.1016/j.biopha.2019.109567.
- Fan K, Shen Y, Xu X, Tao L, Bao T, Li J. 2021. LncRNA-WAS and lncRNA-C8807 interact with miR-142a-3p to regulate the inflammatory response in grass carp. Fish Shellfish Immunol. 111:201–207. doi: 10.1016/j.fsi.2021.02.003.
- Fang Y, Hong X. 2021. miR-124-3p inhibits microglial secondary inflammation after basal ganglia hemorrhage by targeting TRAF6 and repressing the activation of NLRP3 inflammasome. Front Neurol. 12:653321. doi: 10.3389/fneur.2021.653321.
- Gibbons HR, Shaginurova G, Kim LC, Chapman N, Spurlock CF, Aune TM. 2018. Divergent lncRNA GATA3-AS1 regulates GATA3 transcription in T-helper 2 cells. Front Immunol. 9:2512. doi: 10.3389/fimmu.2018.02512.
- Gurung P, Karki R, Vogel P, Watanabe M, Bix M, Lamkanfi M, Kanneganti TD. 2015. An NLRP3 inflammasome-triggered Th2-biased adaptive immune response promotes leishmaniasis. J Clin Invest. 125(3):1329–1338. doi: 10.1172/JCI79526.
- He H, Suryawanshi H, Morozov P, Gay-Mimbrera J, Del Duca E, Kim HJ, Kameyama N, Estrada Y, Der E, Krueger JG, et al. 2020. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol. 145(6):1615–1628. doi: 10.1016/j.jaci.2020.01.042.
- Hou M, Li W, Xie Z, Ai J, Sun B, Tan G. 2017. Effects of anticholinergic agent on miRNA profiles and transcriptomes in a murine model of allergic rhinitis. Mol Med Rep. 16(5):6558–6569. doi: 10.3892/mmr.2017.7411.
- Huanosta-Murillo E, Alcántara-Hernández M, Hernández-Rico B, Victoria-Acosta G, Miranda-Cruz P, Domínguez-Gómez MA, Jurado-Santacruz F, Patiño-López G, Pérez-Koldenkova V, Palma-Guzmán A, et al. 2021. NLRP3 regulates IL-4 expression in TOX+ CD4+ T cells of cutaneous T cell lymphoma to potentially promote disease progression. Front Immunol. 12:668369.
- Iaccarino I, Mourtada F, Reinke S, Patil P, Doose G, Monaco G, Hoffmann S, Siebert R, Klapper W. 2022. LINC00892 is an lncRNA induced by T cell activation and expressed by follicular lymphoma-resident T helper cells. Noncoding RNA. 8(3):40.
- Ji KY, Jung DH, Pyun BJ, Kim YJ, Lee JY, Choi S, Jung MA, Song KH, Kim T. 2021. Angelica gigas extract ameliorates allergic rhinitis in an ovalbumin-induced mouse model by inhibiting Th2 cell activation. Phytomedicine. 93:153789. doi: 10.1016/j.phymed.2021.153789.
- Jin J, Wang H, Hua X, Chen D, Huang C, Chen Z. 2019. An outline for the pharmacological effect of icariin in the nervous system. Eur J Pharmacol. 842:20–32. doi: 10.1016/j.ejphar.2018.10.006.
- Jin X, Bai X, Yang Y, Ding J, Shi H, Fu B, Boireau P, Liu M, Liu X. 2020. NLRP3 played a role in Trichinella spiralis-triggered Th2 and regulatory T cells response. Vet Res. 51(1):107. doi: 10.1186/s13567-020-00829-2.
- Kim YK, Lee JY, Hwang JH, Suh HN. 2021. A pilot study to establish an ovalbumin-induced atopic dermatitis minipig model. J Vet Res. 65(3):307–313. doi: 10.2478/jvetres-2021-0045.
- Kong L, Liang X, Liu A, Yang X, Luo Q, Lv Y, Dong J. 2019. Icariin inhibits inflammation via immunomodulation of the cutaneous hypothalamus–pituitary–adrenal axis in vitro. Clin Exp Dermatol. 44(2):144–152. doi: 10.1111/ced.13735.
- Lee JH, Jo EH, Lee B, Noh HM, Park S, Lee YM, Kim DK, Park MC. 2019. Soshiho-Tang, a traditional herbal medicine, alleviates atopic dermatitis symptoms via regulation of inflammatory mediators. Front Pharmacol. 10:742. doi: 10.3389/fphar.2019.00742.
- Li H, Zhang Z, Zhang H, Guo Y, Yao Z. 2021. Update on the pathogenesis and therapy of atopic dermatitis. Clin Rev Allergy Immunol. 61(3):324–338. doi: 10.1007/s12016-021-08880-3.
- Li JX, Dong RJ, Zeng YP. 2021. Characteristics, mechanism, and management of pain in atopic dermatitis: a literature review. Clin Transl Allergy. 11(10):e12079.
- Liang Z, Tang F. 2020. The potency of lncRNA MALAT1/miR-155/CTLA4 axis in altering Th1/Th2 balance of asthma. Biosci Rep. 40(2):BSR20190397.
- Liu Q, Shen Y, Xiao Y, Xiang H, Chu L, Wang T, Liu H, Tan G. 2022. Increased miR-124-3p alleviates type 2 inflammatory response in allergic rhinitis via IL-4Rα. Inflamm Res. 71(10–11):1271–1282. doi: 10.1007/s00011-022-01614-x.
- Liu W, Wang Z, Liu L, Yang Z, Liu S, Ma Z, Liu Y, Ma Y, Zhang L, Zhang X, et al. 2020. LncRNA Malat1 inhibition of TDP43 cleavage suppresses IRF3-initiated antiviral innate immunity. Proc Natl Acad Sci U S A. 117(38):23695–23706. doi: 10.1073/pnas.2003932117.
- Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, Dong F. 2021. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio. 11(11):2966–2976. doi: 10.1002/2211-5463.13276.
- Liu Y, Zhao YD, Yan XN. 2018. Meta-analysis of traditional Chinese medicine Jianpi therapy in treatment of atopic dermatitis. Zhongguo Zhong Yao Za Zhi. 43:1922–1933.
- Luo H, Zhang R. 2021. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp Ther Med. 21(1):72. doi: 10.3892/etm.2020.9504.
- Möbus L, Rodriguez E, Harder I, Stölzl D, Boraczynski N, Gerdes S, Kleinheinz A, Abraham S, Heratizadeh A, Handrick C, et al. 2021. Atopic dermatitis displays stable and dynamic skin transcriptome signatures. J Allergy Clin Immunol. 147(1):213–223. doi: 10.1016/j.jaci.2020.06.012.
- Park G, Moon BC, Ryu SM, Kim WJ, Lim HS. 2021. Cicadidae Periostracum attenuates atopic dermatitis symptoms and pathology via the regulation of NLRP3 inflammasome activation. Oxid Med Cell Longev. 2021:8878153. doi: 10.1155/2021/8878153.
- Rhew KY, Han Y. 2012. Immunoadjuvant activity of icariin that induces Th1-type antibody in mice. Arch Pharm Res. 35(9):1685–1691. doi: 10.1007/s12272-012-0920-2.
- Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL, Mishra N, Mahajan UM, et al. 2020. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 158(1):253–269.e214. doi: 10.1053/j.gastro.2019.09.040.
- Shi ZF, Song TB, Xie J, Yan YQ, Du YP. 2017. The traditional Chinese medicine and relevant treatment for the efficacy and safety of atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2017:6026434. doi: 10.1155/2017/6026434.
- Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, Luo X, Zhang Q, Lu H, Liang X. 2020. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 111(11):4242–4256. doi: 10.1111/cas.14648.
- Specjalski K, Jassem E. 2019. MicroRNAs: potential biomarkers and targets of therapy in allergic diseases? Arch Immunol Ther Exp. 67(4):213–223. doi: 10.1007/s00005-019-00547-4.
- Sun JB, Wang Z, An WJ. 2021. Protection of icariin against hydrogen peroxide-induced MC3T3-E1 cell oxidative damage. Orthop Surg. 13(2):632–640. doi: 10.1111/os.12891.
- Tang H, Yuan S, Chen T, Ji P. 2021. Development of an immune-related lncRNA–miRNA–mRNA network based on competing endogenous RNA in periodontitis. J Clin Periodontol. 48(11):1470–1479. doi: 10.1111/jcpe.13537.
- Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, Szymczak S, Swindell WR, Sarkar MK, Raja K, et al. 2019. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. 139(7):1480–1489. doi: 10.1016/j.jid.2018.12.018.
- Wang B, Li J, Tian F. 2021. Downregulation of lncRNA SNHG14 attenuates osteoarthritis by inhibiting FSTL-1 mediated NLRP3 and TLR4/NF-κB pathway through miR-124-3p. Life Sci. 270:119143. doi: 10.1016/j.lfs.2021.119143.
- Wang G, Li X, Li N, Wang X, He S, Li W, Fan W, Li R, Liu J, Hou S. 2022. Icariin alleviates uveitis by targeting peroxiredoxin 3 to modulate retinal microglia M1/M2 phenotypic polarization. Redox Biol. 52:102297. doi: 10.1016/j.redox.2022.102297.
- Wang J, Luo J, Xu Y, Yan Z, Qu X, Chen M, Tao Q. 2020. Wang-Bi tablet, a patented Chinese medicine, maintains the balance of Th1/Th2 in mice with collagen-induced arthritis. J Tradit Chin Med. 40:401–406.
- Wang J, Wu J, Kong L, Nurahmat M, Chen M, Luo Q, Li B, Wu X, Dong J. 2014. BuShenYiQi formula strengthens Th1 response and suppresses Th2–Th17 responses in RSV-induced asthma exacerbated mice. J Ethnopharmacol. 154(1):131–147. doi: 10.1016/j.jep.2014.03.041.
- Wang X, Bao K, Wu P, Yu X, Wang C, Ji L, Hong M. 2018. Integrative analysis of lncRNAs, miRNAs, and mRNA-associated ceRNA network in an atopic dermatitis recurrence model. Int J Mol Sci. 19:3263. doi: 10.3390/ijms19103263.
- Wang X, Li S, Liu J, Kong D, Han X, Lei P, Xu M, Guan H, Hou D. 2020. Ameliorative effects of sea buckthorn oil on DNCB induced atopic dermatitis model mice via regulation the balance of Th1/Th2. BMC Complement Med Ther. 20(1):263. doi: 10.1186/s12906-020-02997-2.
- Wu R, Zeng J, Yuan J, Deng X, Huang Y, Chen L, Zhang P, Feng H, Liu Z, Wang Z, et al. 2018. MicroRNA-210 overexpression promotes psoriasis-like inflammation by inducing Th1 and Th17 cell differentiation. J Clin Invest. 128(6):2551–2568. doi: 10.1172/JCI97426.
- Xu CQ, Le JJ, Duan XH, Du WJ, Liu BJ, Wu JF, Cao YX, Dong JC. 2011. Molecular mechanism of icariin on rat asthmatic model. Chin Med J. 124(18):2899–2906.
- Yan F, Li F, Liu J, Ye S, Zhang Y, Jia J, Li H, Chen D, Mo X. 2020. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomed Pharmacother. 127:110142. doi: 10.1016/j.biopha.2020.110142.
- Yánez DC, Papaioannou E, Chawda MM, Rowell J, Ross S, Lau CI, Crompton T. 2021. Systemic pharmacological smoothened inhibition reduces lung T-cell infiltration and ameliorates Th2 inflammation in a mouse model of allergic airway disease. Front Immunol. 12:737245. doi: 10.3389/fimmu.2021.737245.
- Yang Z, Zeng B, Wang C, Wang H, Huang P, Pan Y. 2017. MicroRNA-124 alleviates chronic skin inflammation in atopic eczema via suppressing innate immune responses in keratinocytes. Cell Immunol. 319:53–60. doi: 10.1016/j.cellimm.2017.08.003.
- Yu SY, Dong B, Tang L, Zhou SH. 2018. LncRNA MALAT1 sponges miR-133 to promote NLRP3 inflammasome expression in ischemia-reperfusion injured heart. Int J Cardiol. 254:50. doi: 10.1016/j.ijcard.2017.10.071.
- Zhang X, Chen Y, Zhang C, Zhang X, Xia T, Han J, Song S, Xu C, Chen F. 2021. Effects of icariin on the fracture healing in young and old rats and its mechanism. Pharm Biol. 59:1245–1255.
- Zhao Y, Zhang L, Ding Y, Tao X, Ji C, Dong X, Lu J, Wu L, Wang R, Lu Q, et al. 2021. Efficacy and safety of SHR0302, a highly selective janus kinase 1 inhibitor, in patients with moderate to severe atopic dermatitis: a phase II randomized clinical trial. Am J Clin Dermatol. 22(6):877–889. doi: 10.1007/s40257-021-00627-2.
- Zheng T, Fan M, Wei Y, Feng J, Zhou P, Sun X, Xue A, Qin CX, Yu D. 2021. Huangbai liniment ameliorates skin inflammation in atopic dermatitis. Front Pharmacol. 12:726035. doi: 10.3389/fphar.2021.726035.
- Zhong YX, Li WS, Liao LS, Liang L. 2019. LncRNA CCAT1 promotes cell proliferation and differentiation via negative modulation of miRNA-218 in human DPSCs. Eur Rev Med Pharmacol Sci. 23(9):3575–3583. doi: 10.26355/eurrev_201905_17779.
- Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y, Wang X. 2018. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 6(12):1578–1592. doi: 10.1158/2326-6066.CIR-17-0479.