Abstract
Context
Levodopa combined with traditional Chinese medicine has a synergistic effect on Parkinson’s disease (PD). Recently, we demonstrated that Nardostachys jatamansi (D. Don) DC. [syn. Patrinia jatamansi D.Don, N. grandiflora DC.] (Valerianaceae) (NJ) can alleviate PD.
Objective
To explore the synergistic effect of NJ combined with levodopa against PD.
Materials and methods
The PD model was established by injecting rotenone. Eighty-four Sprague-Dawley rats were randomly divided into seven groups: sham, model, different doses of NJ (0.31, 0.62, or 1.24 g/kg) combined with levodopa (25 mg/kg), and levodopa alone (25 and 50 mg/kg) groups. The synergistic effect of the combination was investigated by pharmacodynamic investigation and detection of expression of nuclear factor erythro2-related factor 2 (Nrf2) and NLR family proteins containing Pyrin-related domain 3 (NLRP3) pathways.
Results
Compared with the model group, NJ + levodopa (1.24 g/kg + 25 mg/kg) increased the moving distance of PD rats in the open field (2395.34 ± 668.73 vs. 1501.41 ± 870.23, p < 0.01), enhanced the stay time on the rotating rod (84.86 ± 18.15 vs. 71.36 ± 17.53, p < 0.01) and the combination was superior to other treatments. The synergistic effects were related to NJ + levodopa (1.24 g/kg + 25 mg/kg) increasing the neurotransmitter levels by 38.80%-88.67% in PD rats, and inhibiting oxidative stress and NLRP3 pathway by activating Nrf2 pathway.
Discussion and conclusions
NJ combined with levodopa is a promising therapeutic candidate for PD, which provides a scientific basis for the subsequent clinical combination therapy of levodopa to enhance the anti-PD effect.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder which is characterized by a loss of dopaminergic neurons expressing tyrosine hydroxylase (TH) in the substantia nigra (SN) and striatum (ST). The clinical manifestations of PD are motor disorders such as static tremor and muscle tonic, as well as non-motor symptoms such as anxiety and depression, which seriously affect the health and quality of life of patients. Levodopa is the drug of choice for PD treatment and relieves motor dysfunction. In the earlier stages of PD, there are spared dopamine neurons that are able to store the exogenous dopamine and regulate its release and maintain normal physiological dopamine receptor stimulation within the ST (Bezard Citation2013). However, levodopa has a short half-life of 1.5 h and low blood-brain barrier permeability of less than 5% (Astradsson et al. Citation2009) and long-term administration of levodopa can lead to overactivation of dopamine D1 receptors, resulting in dyskinesia (Nevalainen et al. Citation2013). Around 80% of PD patients develop levodopa-induced dyskinesia (LID), with 30% developing it after just 3 years of levodopa treatments (Kwon et al. Citation2022). These features limit the effectiveness of levodopa alone in PD patients.
Oxidative stress is considered to be the key factor inducing PD. It occurs when the antioxidant substances in the body, such as superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), are reduced due to external stimuli or other factors, or when reactive oxygen species (ROS) cannot be removed in time. Studies have found that the SN of the brain is highly vulnerable to oxidative damage, and oxidative damage can cause lipid peroxidation of the cell membrane structure and changes of intracellular protein structure (Wijeyekoon et al. Citation2020). Meanwhile, oxidative stress also causes DNA damage, which leads to degeneration and apoptosis of dopaminergic neurons in the SN of the midbrain, resulting in PD (McCoy et al. Citation2006). Studies on rotenone (ROT)-induced PD rats have also verified the occurrence of lipid peroxidation in rat brain tissues (Sayed et al. Citation2022). In addition, it has been found that sustained inflammatory response plays a key role in the loss of dopaminergic neurons and the development of PD (de Araújo et al. Citation2018), suggesting that neuroinflammation response is another important factor inducing PD.
There is evidence that PD neuroinflammation depends on the activation of the NLR family pyrin domain-containing 3 (NLRP3) inflammasome (Nuvolone et al. Citation2015). Once NLRP3 is activated, caspase-1 cleaves pro-IL-1β and pre-IL-18 precursors into mature interleukin 1β (IL-1β) and interleukin 18 (IL-18) and promotes the release of tumor necrosis factor-α (TNF-α), resulting in neuroinflammatory response and dopaminergic neurons injury (Zhang et al. Citation2021). Therefore, inhibition of NLRP3 inflammasome activation might be an important breakthrough in PD therapy. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key endogenous cell regulator (Yan et al. Citation2018), which can effectively inhibit oxidative stress and neuroinflammatory response. Under oxidative stress, Nrf2 separates from Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm and enters the nucleus where it binds to antioxidant response elements, initiating the expression of phase II antioxidant enzymes (Ma Citation2013). In addition, Nrf2 negatively regulates NLRP3 activation and inhibits downstream inflammatory cytokines by inhibiting the ROS-NLRP3-IL-1β signaling axis (Nam et al. Citation2018). Therefore, drugs that activate the Nrf2 signaling pathway and inhibit the NLRP3 inflammasome would advance PD treatment.
It has been found that the combination of traditional Chinese medicine and levodopa can improve the anti-PD effect of levodopa and inhibit LID, which has the effect of ‘enhancing the effect and reducing the toxicity’ (Huang et al. Citation2017). Nardostachys jatamansi (D. Don) DC. [syn. Patrinia jatamansi D.Don, N. grandiflora DC.](Valerianaceae) (NJ) is a traditional Chinese Medicine which has the effects of regulating Qi and relieving pain, opening depression, and waking up the spleen. Studies have suggested that it also has anti-inflammatory and antioxidant effects (Lyle et al. Citation2009; Shin et al. Citation2015). Previous study from our group found that 80% ethanol extract of NJ can effectively alleviate PD symptoms in rats and protect dopaminergic neurons, and the effect is better than other extracts (Wan et al. Citation2022). Moreover, the combination of NJ and levodopa can reduce the side effects of excessive levodopa induced dyskinesia (Li et al. Citation2022). However, whether the combination of 80% ethanol extract of NJ and levodopa could bring a synergistic effect in the treatment of PD has not been tested. Therefore, the goal of the current study was to investigate whether an 80% ethanol extract of NJ combined with levodopa had synergistic effects in the ROT-induced PD rat model and any involvement of the Nrf2/NLRP3 pathway in the underlying synergy.
Material and methods
Plant material
Intact NJ plants were purchased from Ruoergai County, Aba, Sichuan and specimens of these materials (batch number:190302) were deposited at the Department of Traditional Chinese Medicine Identification Laboratory, Beijing University of Chinese Medicine. The plant material was authenticated by Prof. Jinli Shi, and NJ roots and rhizomes were used for experiment.
Chemicals and reagents
Nardosinone was purchased from the Shanghai Yuanye Biotechnology Co., Ltd. (P19A10L86397, HPLC ≥98%, Shanghai, China). Anti-Nrf2 (12721S) antibody was purchased from CST (Boston, MA, USA). NLRP3 (ab263899), and anti-β-actin (ab227387) antibodies from Abcam (Cambridge, UK). Anti-HO-1 (10701-1-AP) and anti-caspase-1 (22915-1-AP) antibodies from Proteintech (Chicago, IL, USA). Test kits for ROS, superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), (batch number: 20210617K); DA, 3, 4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytryptamine (5-HT), 5-hydroxyindolacetic acid (5-HIAA), (batch number: 20210618AS1); IL-18, IL-1β and TNF-α (batch number: 20210617C3) detection kits were purchased from Jiangsu Enzyme Industry Co., Ltd. (Jiangsu, China). Levodopa was purchased from Beijing Dawning Pharmaceutical Co., Ltd. (H11021055, Beijing, China). Urethane was purchased from Shanghai Maclin Biochemical Technology Co., Ltd. (C10821103, Shanghai, China). Rotenone was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (R105076, Shanghai, China).
Preparation of 80% ethanol extract from NJ
The medicinal materials were crushed into a coarse powder and 100 g was extracted three times with 80% ethanol by ultrasonication (below 35 °C). Successive volumes of extraction solvent were 10/8/8 (w/v) and corresponding extraction times were 60, 45 and 45 min. The solution was allowed to settle and filtered before filtrates were combined. The brown extract obtained after rotary evaporation was freeze-dried, with the final extraction rate being 10.7%.
HPLC analysis of NJ 80% ethanol extract
Lyophilized powder of 80% ethanol extract of NJ (24.3 mg) was redissolved in 10 mL methanol, filtered with a 0.22 µm membrane and analyzed using the Shimadzu DGU-20A3R system (Shimadzu, Japan). Separation was performed on an Agilent Zorbax SB-C18 column (4.6 mm × 250 mm × 5 μm) at 35 °C with a flow rate of 1 mL/min. The mobile phase was water (A) and acetonitrile (B). The gradient elution program was: 0–5 min, 40% B-40% B; 5–15 min, 40% B-50% B; 15–20 min, 50% B-50% B; 20–25 min, 50% B-70% B; 25–35 min, 70% B-70% B; 35–36 min, 70% B-40% B; 36 to 45 min, 40% B-40% B. The injection volume was 20 μL. The detector was set at 254 nm for chromatogram collection.
Animals
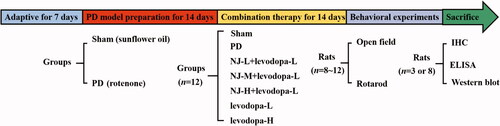
Eight four male Sprague-Dawley rats, weighing 200–220 g were purchased from Beijing Weitong Lihua Laboratory Animal Technology Co., Ltd., with animal certificate number SCXK (Beijing) 2016–0006. Rats were acclimatized for 1 week at 20–22 °C and 40–60% humidity, 12 h light/dark cycle and free access to food and water. The animal experiment protocol complied with the national requirements of laboratory animal welfare ethics and has been approved by the Animal Ethics Committee of Beijing University of Chinese Medicine (approval number 2020091406-3054).
Experimental design and drug treatments
After acclimatization, rats were randomly divided into sham (n = 12) and PD model (n = 80) groups. The model group was injected subcutaneously with ROT sunflower oil solvent (1.5 mg/kg) in the nape and the sham group was injected with the same amount of sunflower oil solvent once a day for 14 days. When rats showed symptoms such as yellow hair, lethargy, hunched back and weakened limbs, the PD model was successfully established (Bian et al. Citation2022) and a total of 72 PD rats were obtained. Model rats were randomly divided into 6 groups (n = 12 each) for 2 weeks. The groups were:
Sham rats subjected to intragastric physiological saline administration;
PD rats subjected to intragastric physiological saline administration;
PD rats with NJ low dose [NJ-L, 0.31 g/kg (crude drugs) and levodopa low dose (levodopa-L, 25 mg/kg)];
PD rats with NJ medium dose (NJ-M, 0.62 g/kg) and levodopa-L;
PD rats with NJ high dose (NJ-H, 1.24 g/kg) and levodopa-L;
PD rats with levodopa-L only;
PD rats with levodopa high dose (levodopa-H, 50 mg/kg).
The open field and rotarod tests were performed on days 15 and 16 of intragastric administration. shows the experimental flow chart.
Open field test
The open field test was used to assess spontaneous movements and exploratory behavior (Arab et al. Citation2021; Liu et al. Citation2021). The open field device (length 100 cm, width 100 cm, height 50 cm) was placed in a quiet environment under low-level illumination. The bottom of the open field was black and evenly divided into 25 (5 × 5) squares. The 9 (3 × 3) squares in the middle were regarded as the central area of the open field and the remaining 16 fields were represented the marginal areas. During the experiment, rats were placed one at a time in an open environment to adapt for 5 min and then in the center of the open field. Video tracking technology (EthoVision XT 9, Noldus, Netherlands) was used to record the distance the animal traveled during the subsequent 5 min period in the open field and the number of times it entered the central area.
Rotarod test
The rotarod test was used to assess the motor coordination and balance of the animal (Zhang et al. Citation2019; Lin et al. Citation2022). Rats were trained for 3 days prior to the test. During training, rats were placed on the rotating rod with the tail facing the outside. After 20 s of adaptation to the environment, the rotating speed was gradually increased from 4 to 20 rpm during a 180 s period with 6 rpm/min acceleration. During the actual test, the rotation speed and acceleration were the same as during the training sessions and the time it took the rat to fall off the rotary rod for the first time in the trial was recorded. Each animal was tested at 2 h intervals for a total of 3 tests. The final measure was obtained by averaging the three fall-off times.
Immunohistochemistry (IHC)
After the end of the behavioral experiments, 3 rats from each group were subjected to cardiac perfusion and brains were taken for immunohistochemical (IHC) staining. Briefly, a 5 μm paraffin section of the brain was deparaffinized and rehydrated with xylene and alcohol. After washing with PBS, the sections were incubated overnight with anti-TH primary antibodies (1:1000 dilution; GB111B1, Servicebio, Wuhan, China) followed by HRP labeled goat anti-rabbit secondary antibody (1:200 dilution; 5220–0336, SeraCare, USA) for 50 min. Sections were mounted, cover slipped and observed using a light microscope (CX21; Olympus Japan Co., Tokyo, Japan). Image J software was used to analyze the average optical density value of TH.
Enzyme-linked immunosorbent assay (ELISA)
Rats were anesthetized with urethane and the ST was removed onto ice, homogenized and the supernatant collected. ELISA kits were used to detect levels of selected neurotransmitters and their metabolites (DA, DOPAC, HVA, 5-HT, 5-HIAA), ROS, antioxidant enzymes (SOD, GSH-PX) and inflammatory factors (IL-18, IL-1β, TNF-α) in the ST. All experimental procedures were carried out in accordance with the manufacturer’s instructions for the corresponding kit.
Western blot analysis
The ST was extracted and homogenized with RIPA buffer on ice, centrifuged at 4 °C for 5 min at 13000 g and supernatants were collected. The appropriate amount of 5× protein loading buffer was added to the supernatant which was incubated in a 95 °C boiling water bath for 5 min. Proteins were electrophoresed by 10% SDS-PAGE and transferred to PVDF membranes which were blocked in TBST containing 5% skimmed milk powder and incubated in a shaking flask for 2 h at room temperature to block non-specific antibody binding. Membranes were incubated overnight at 4 °C with the following primary antibodies: anti-Nrf2 (1:1000), anti-HO-1 (1:5000), anti-NLRP3 (1:1000) and anti-caspase-1 (1:1000), washed 3 times with 0.1% TBST and incubated in a solution containing HRP-conjugated secondary antibodies. Western blots were visualized using an enhanced chemiluminescence system and analyzed using Image J software. The β-actin band was used as the reference.
Molecular docking
Nardosinone is a main component of NJ and has been shown to promote the proliferation of mouse embryonic neural stem cells and to have neuroprotective effects (Li et al. Citation2014). Analysis of brain tissue from PD rats treated with NJ has shown that nardosinone penetrates the blood-brain barrier (Bian et al. Citation2021). These researches showed that nardosinone may be an effective component of NJ against PD.
AutoDock software was used to conduct molecular docking to clarify the dynamic interaction between nardosinone and selected key proteins Keap1-Nrf2 and NLRP3. Protein structures of Keap1-Nrf2 (PDB ID: 2FLU) (Bhakkiyalakshmi et al. Citation2016) and NLRP3 (PDB ID: 6NPY) (Zhang et al. Citation2021) were downloaded from the PDB database (https://www.rcsb.org/). The structure of the small molecule compound was downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and saved in SDF format. Chem 3 D software was used to convert the SDF file to a PDB file. The docking procedure was conducted using AutoDock software and a binding energy ≤ −5.0 kcal/mol was considered to indicate stronger binding affinity. Results were visualized using PyMol 2.5 software.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (ver. 6; GraphPad Software, Inc., CA, USA) and data are presented as the mean ± standard deviation (mean ± SD). Data from different groups were compared using one-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons, and p < 0.05 was considered to be statistically significant.
Results
HPLC analysis of NJ 80% ethanol extract
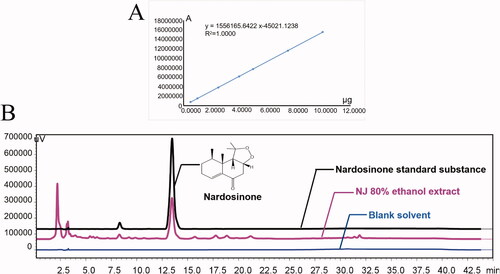
HPLC was used to determine whether the NJ extract met the requirements of the Chinese Pharmacopoeia 2020 edition (dry product must contain not less than 0.10% of nardosinone). The linear regression equation for the standard substance of nardosinone was Y = 1556165.6422X-45021.1238 (). 24.3 mg of the 80% ethanol NJ extract was dissolved in 10 mL methanol and with an injection volume of 20 μL, the peak area (A) of nardosinone was 6667996 (). According to the linear regression equation, the 80% ethanol extract of NJ contained 8.88% nardosinone, and the extraction rate of NJ was 10.7%. This indicated that the crude drug contained 0.95% of nardosinone, which would meet the Chinese Pharmacopoeia standard. An evaluation of our methodology showed that its specificity met the requirements. The repeatability, precision and relative standard deviation (RSD) of the sample recovery rate were all less than 3%.
Combination of NJ and levodopa had a synergistic alleviating effect on PD symptoms in rats
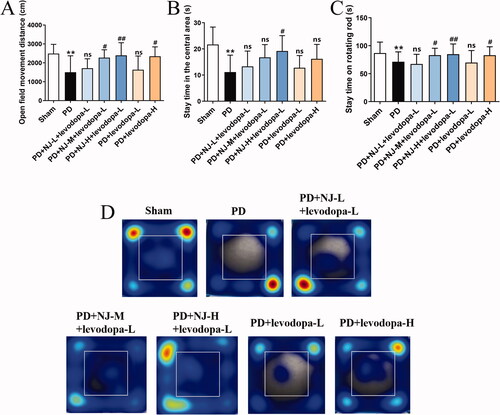
Open field test and the rotarod test after 14 days of the combined treatment were conducted to test for synergistic effects on alleviating PD symptoms. Compared with the sham group, the moving distance of PD rats in the open field and the stay time in the central area were significantly reduced (p < 0.01, ) and the stay time on the rotating rod was also significantly reduced (p < 0.01, ). This indicated that the PD rats showed dyskinesia and anxiety. Compared with the PD group, the combination of NJ-M and NJ-H with levodopa-L significantly ameliorated the dyskinesia of PD rats in the open field and rotarod tests (PD + NJ-M + levodopa-L, p < 0.05; PD + NJ-H + levodopa-L, p < 0.01; ). The combination of NJ-H and levodopa-L also relieved anxiety in the open field (p < 0.05, ). Moreover, NJ-H combined with levodopa-L had a stronger effect than levodopa-L alone and levodopa-H alone. This indicated that the combination of NJ-H and levodopa-L still had a good synergistic effect while reducing the dosage of levodopa. This laid the foundation for the subsequent mechanism research.
Figure 3. NJ combined with levodopa ameliorated ROT-induced PD symptoms in rats. (A) the Movement distance of rats in the open field. (B) The stay time of rats in the central area. (C) The stay time of rats on rotating rods. (D) The heat map of rat tracks in the open field test. Data are expressed as the mean ± SD (n = 8 ∼ 12/group). **p < 0.01 compared with sham group; #p < 0.05, ##p < 0.01 compared with PD group; ns, p > 0.05, compared with PD group.
NJ combined with levodopa prevented the loss of dopaminergic neurons
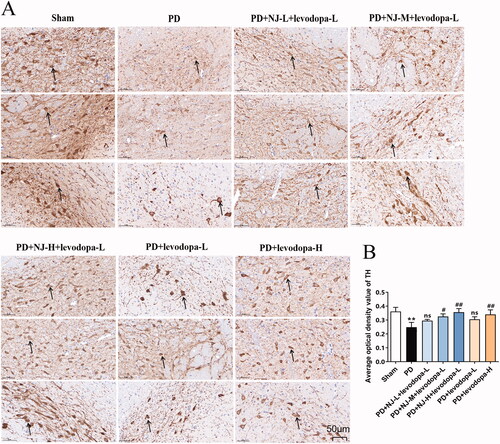
To test whether the combination of NJ and levodopa has a protective effect on dopaminergic neurons, IHC staining was used to investigate the expression of TH in the SN of rats. Compared with the sham group, the density of TH-positive neurons in the SN of PD rats was significantly reduced, the neurons had a necrotic appearance and their contours were diffuse (p < 0.01, ). However, after 14 days of treatment with NJ-M and NJ-H combined with levodopa-L, the density of TH-positive neurons in the SN of rats in the treatment group was significantly increased compared with that in the untreated PD group with individual neurons having uniform staining and sharp contours (PD + NJ-M + levodopa-L, p < 0.05; PD + NJ-H + levodopa-L, p < 0.01; ). In addition, levodopa-H alone promoted TH expression (p < 0.01, ) but the therapeutic effect was not as prominent as for the combination of NJ-H and levodopa-L. These results showed that the combination of NJ-H and levodopa-L had a beneficial synergistic effect on the protection of dopaminergic neurons and acted as a reference for subsequent mechanistic research.
Figure 4. NJ combined with levodopa prevented the loss of dopaminergic neurons. (A) the IHC staining of TH in SN (the Arrow points to TH positive neurons; magnification: ×30). (B) The average optical density values of TH. Data are expressed as the mean ± SD (n = 3 per group). **p < 0.01 compared with sham group; #p < 0.05, ##p < 0.01 compared with PD group; ns, p > 0.05, compared with PD group.
NJ combined with levodopa increased the levels of neurotransmitters
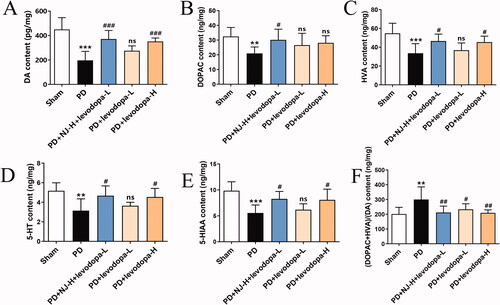
Neurotransmitter levels in the ST of rats were measured by ELISA. Compared with the sham group, the levels of DA, DOPAC, HVA, 5-HT, 5-HIAA in the ST of PD rats were significantly reduced (DOPAC, 5-HT, p < 0.01; DA, HVA, 5-HIAA, p < 0.001; ) and DA metabolism was increased (p < 0.01, ). However, the combination of NJ-H and levodopa-L significantly restored neurotransmitters levels in the ST of PD rats (DOPAC, HVA, 5-HT, 5-HIAA, p < 0.05; DA, p < 0.001; ) and reduced the DA metabolic rate (p < 0.01, ).
Figure 5. NJ combined with levodopa increased the Neurotransmitter levels. (A) DA content. (B) DOPAC content. (C) HVA content. (D) 5-HT content. (E) 5-HIAA content. (F) The metabolic rate of DA. Data are expressed as the mean ± SD (n = 6 ∼ 8 per group). **p < 0.01, ***p < 0.001 compared with sham group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with PD group; ns, p > 0.05, compared with PD group.
NJ combined with levodopa reduced the ROT-induced oxidative stress response
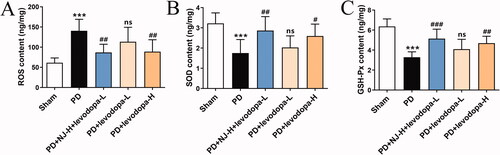
ELISA was used to investigate the effect of NJ combined with levodopa on ROT-induced oxidative stress in PD rats. Compared with the sham group, the content of ROS in the ST of PD rats was significantly increased (p < 0.001, ) while SOD and GSH-Px were significantly reduced (p < 0.001, ). Compared with the PD group, NJ-H combined with levodopa-L reduced the content of ROS in the ST of PD rats (p < 0.01, ) and increased SOD and GSH-Px (SOD, p < 0.01; GSH-Px, p < 0.001; ). This result showed that the combination of NJ-H and levodopa-L alleviated the oxidative stress response in ROT-induced PD rats.
Figure 6. NJ combined with levodopa reduced ROT-induced oxidative stress response. (A) ROS content. (B) SOD content. (C) GSH-Px content. Data are expressed as the mean ± SD (n = 8 per group). ***p < 0.001 compared with sham group; #p < 0.05, ##p < 0.01, ###p < 0.001 compared with PD group; ns, p > 0.05, compared with PD group.
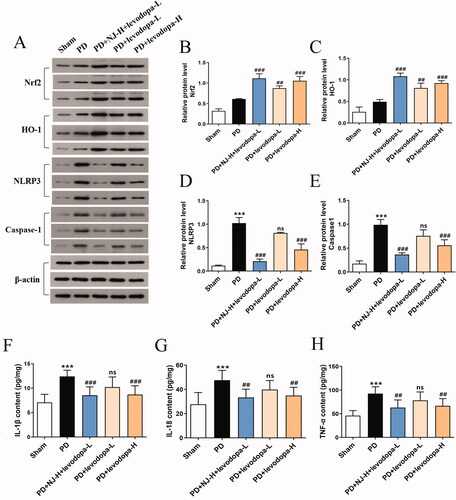
NJ combined with levodopa inhibited the NLRP3 signaling pathway by activating the Nrf2 signaling pathway
Many studies have shown that the Nrf2 signaling pathway and its targeted antioxidant enzymes, such as HO-1, play an important role in alleviating oxidative stress and inhibiting neuroinflammatory response caused by the NLRP3 signaling pathway (Bian et al. Citation2020). Therefore, Western blot and ELISA methods were used to test whether the combination of NJ and levodopa exerted its anti-PD effect by regulating Nrf2 and NLRP3 signaling pathways. Levels of NLRP3, caspase-1 and related inflammatory factors (IL-1β, IL-18, TNF-α) were significantly increased in PD rats compared with sham rats (p < 0.001, ). However, the combination of NJ-H and levodopa-L reversed this effect (IL-18, TNF-α, p < 0.01; NLRP3, caspase-1, IL-1β, p < 0.001; ). In addition, compared with the PD group, NJ-H combined with levodopa-L significantly increased the expression of Nrf2 and HO-1 (p < 0.001, ). These results suggested that the Nrf2 signaling pathway played an important role in the combined action of NJ and levodopa to inhibit oxidative stress and reduce the neuroinflammatory response caused by the NLRP3 signaling pathway.
Figure 7. NJ combined with levodopa alleviated oxidative stress and neuroinflammation by activating the Nrf2 signaling pathway and inhibiting the NLRP3 signaling pathway. (A) the contents of Nrf2, HO-1, NLRP3 and caspase-1 in the ST of rats were detected by Western blot analysis. (B-E) Densitometry of the Western blot results (n = 3 per group). (F-H) quantitative analysis of IL-1β, IL-18, TNF-α levels in the ST of rats by ELISA (n = 8 per group). Data are expressed as the mean ± SD. ***p < 0.001 compared with sham group; ##p < 0.01, ###p < 0.001 compared with PD group; ns, p > 0.05, compared with PD group.
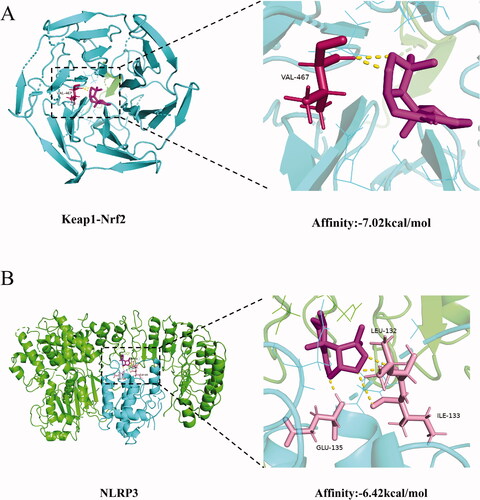
Nardosinone had a significant binding affinity to Keap1-Nrf2 and NLRP3 proteins
Molecular docking results revealed that the binding energy of nardosinone and Keap1-Nrf2 protein was −7.02 kcal/mol and that binding mainly depended on a hydrogen bond at residue VAL-467 (). Nardosinone and NLRP3 protein had a binding energy of −6.42 kcal/mol and binding mainly depended on hydrogen bonds at the residues GLU-135, LEU-132 and ILE-133 (). Thus, nardosinone blocked the Nrf2-binding site of Keap1, activated Nrf2 protein and inhibited NLRP3 expression. These results supported our conclusion that the combination of NJ and levodopa alleviated PD symptoms in rats by activating the Nrf2 signaling pathway and inhibiting the NLRP3 signaling pathway.
Discussion
PD is the second most common chronic progressive neurodegenerative disease. The primary neuropathological characteristics of PD are degeneration of dopaminergic neurons in the SN, resulting in reduction of dopamine content in the ST. Clinical symptoms occur when approximately 50% of dopaminergic neurons are lost in the SN, and dopamine concentration in the ST is reduced by 80% (Toulouse and Sullivan Citation2008). This study was the first to find that NJ combined with levodopa had a synergistic anti-PD effect, which provided new ideas for the clinical application of levodopa. At present, the pathogenesis of PD is mostly explored from Nrf2 or NLRP3 single pathway. Interestingly, our study focused on the whole NLRP3-Nrf2 axis and found that the combination of NJ and levodopa can exert anti-PD effect by activating Nrf2 and inhibiting NLP3 pathway, which may help to improve the understanding of the clinical application of NJ and the treatment mechanism of PD.
ROT is a commonly used neurotoxin in the study of PD according to its pathological features. Studies have shown that ROT-induced animal models of PD are similar to clinical PD patients in both behavioral symptoms and pathological features (Klein et al. Citation2011). There is evidence showing that ROT can induce mitochondrial dysfunction, increase ROS production, and lead to the degeneration of dopaminergic neurons, and finally resulting in PD (Maturana et al. Citation2015). Moreover, ROT can induce activation of microglia by inhibiting mitochondrial complex I, and activated microglia can indirectly damage dopaminergic neurons by producing inflammatory factors (Ye et al. Citation2016). In this study, it was found that compared with the sham group, the rats in the PD group showed obvious dyskinesia, and dopaminergic neurons in the SN and ST were damaged, which was consistent with previous studies. NJ-M and NJ-H combined with levodopa-L had a synergistic anti-PD effect, which reversed behavioral PD symptoms and protected dopaminergic neurons, and the therapeutic effect was better than that of levodopa alone.
Levodopa, as a precursor of dopamine, can relieve PD symptoms by supplementing the content of exogenous dopamine. Whilst due to the short half-life and low blood-brain barrier permeability of levodopa, when levodopa in rats accumulated overdose, the behavior of rats shows neurotoxic effects, which may be related to the oxidative metabolic decomposition products of levodopa causing a large amount of DNA damage. This, in turn, will cause a large number of neurons damaged and dead during PD treatment (Stansley and Yamamoto Citation2013). Therefore, in this study, the combination of NJ and levodopa was used to treat PD, in order to achieve better therapeutic effect under the condition of lower dosage of levodopa. NJ combined with levodopa or levodopa alone improved the expression of dopaminergic neurons in PD rats, which may be related to the lower dose of levodopa (Olanow Citation2015) and the inhibitory effect of NJ on the side effects of levodopa (Li et al. Citation2022).
In order to elucidate the anti-PD mechanism of the combination of NJ and levodopa, NLRP3 and Nrf2 signaling pathway related proteins in the ST of PD rats were detected. Previous studies have shown that NLRP3 inflammasome is closely related to the development of PD neuroinflammation (Fan et al. Citation2017), and involved in the entire process of PD. NLRP3 acts as a platform for caspase 1 to induce IL-1β maturation, leading to neuronal pyroptosis (Yan et al. Citation2018)—this is a specific pathway of caspase-1-induced programmed cell death that is dependent on a GSDMD-generated pore (Shin et al. Citation2015). During pyroptosis, cells divide and release more pro-inflammatory cytokines. These inflammatory cytokines are released from microglia and have toxic effects on dopaminergic neurons resulting in neuroinflammation, leading to injury and death of adjacent dopaminergic neurons (de Farias et al. Citation2016). There is evidence showing that NLRP3 inflammasome in PD animal models can be activated by ROT, which may be related to the failure of ROS timely clearance caused by ROT (Martinez et al. Citation2017). Activated NLRP3 inflammasome causes motor dysfunction and degeneration of dopaminergic neurons in PD models (Cuevas et al. Citation2015). Consistent with the above studies, this study found that compared with the sham group, the rats in the PD group showed a significant neuroinflammatory response, and NJ-H + levodopa-L could reduce the expression of NLRP3 pathway related proteins (caspase 1, IL-1β and IL-18) in the ST of PD rats, and alleviate the symptoms of PD rats.
Nrf2 is a major transcription factor that plays antioxidant and anti-inflammatory roles in cells. Under physiological conditions, Nrf2 is sequestered in the cytoplasm by Keap1 that promotes its ubiquitination and degradation. Under oxidative stress, Nrf2 is released from oxidized Keap1 and translocates to the nucleus to activate the transcription of cytoprotective genes (HO-1, SOD, GSH-PX) (Kobayashi and Yamamoto Citation2006). According to the activation mechanism of Nrf2, when it is activated, the content of Nrf2 in cytoplasm decreases, while that in nucleus increases significantly. It has been reported that Nrf2 in normal control subjects is mainly present in the cytoplasm of dopaminergic neurons in the SN, while the content of Nrf2 in PD patients is significantly increased in the nucleus (Bu et al. Citation2015). The activated Nrf2 signaling pathway exerts neuroprotective function in PD animal models and cell toxicity models in vitro (Todorovic et al. Citation2016). The low expression of Nrf2 and HO-1 promotes the up-regulation of NLRP3, caspase-1 and IL-1β in PD rats (Bian et al. Citation2020). Consistent with these earlier results, the combination of NJ-H and levodopa-L effectively improved the expression of Nrf2 pathway-related proteins in the PD rats of the current study and inhibited the oxidative stress response and NLRP3-mediated neuroinflammation. However, there was a limitation since the total protein content of Nrf2 in cytoplasm and nucleus was detected in this study, compared with the sham group, the PD group only showed an increasing trend. In future experiments, the contents of Nrf2 and HO-1 after treatment in nucleus and cytoplasm will be respectively detected.
Conclusions
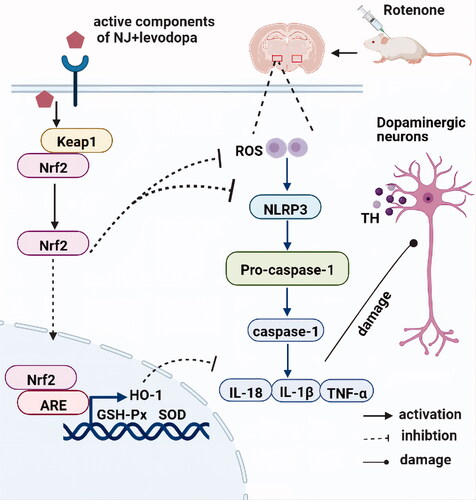
The 80% ethanol extracts of NJ-M and NJ-H combined with levodopa-L have beneficial synergistic anti-PD effects. ROT-induced PD symptoms in rats were reversed and the damage to dopaminergic neurons was alleviated. The therapeutic effect of NJ-H combined with levodopa-L was better than that of levodopa-H alone. Moreover, the synergistic effect was associated with an increase of the levels of relevant striatal neurotransmitters, activation of Nrf2 signaling pathway and inhibition of NLRP3 signaling pathway (see our findings illustrated in which was created using BioRender: https://biorender.com/). Therefore, NJ combined with levodopa may offer a promising treatment for PD.
Authors’ contributions
Jinli Shi acquired funding for the research. Jiayuan Li, Jiahe Yu and Jinli Shi were responsible for the concept and design of the study. Jiayuan Li, Jiahe Yu and Guohui Wan participated in the generation of animal models and behavioral testing. Jiayuan Li and Jinfeng Liu were responsible for HPLC analysis. Xiaojia Wei and Xue Yang participated in data collection and analysis. Jiayuan Li and Jiahe Yu were responsible for drafting the manuscript. Jinli Shi and Jianyou Guo reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
All data generated or analyzed in the course of this study are included in the article.
Additional information
Funding
References
- Arab HH, Safar MM, Shahin NN. 2021. Targeting ROS-dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced Parkinson’s disease rat model. ACS Chem Neurosci. 12(4):689–703. doi:10.1021/acschemneuro.0c00722.
- Astradsson A, Jenkins BG, Choi JK, Hallett PJ, Levesque MA, McDowell JS, Brownell AL, Spealman RD, Isacson O. 2009. The blood-brain barrier is intact after levodopa-induced dyskinesias in Parkinsonian primates–evidence from in vivo neuroimaging studies. Neurobiol Dis. 35(3):348–351. doi:10.1016/j.nbd.2009.05.018.
- Bezard E. 2013. Experimental reappraisal of continuous dopaminergic stimulation against L-dopa-induced dyskinesia. Mov Disord. 28(8):1021–1022. doi:10.1002/mds.25251.
- Bhakkiyalakshmi E, Dineshkumar K, Karthik S, Sireesh D, Hopper W, Paulmurugan R, Ramkumar KM. 2016. Pterostilbene-mediated Nrf2 activation: mechanistic insights on Keap1: nrf2 interface. Bioorg Med Chem. 24(16):3378–3386. doi:10.1016/j.bmc.2016.05.011.
- Bian H, Wang G, Huang J, Liang L, Zheng Y, Wei Y, Wang H, Xiao L, Wang H. 2020. Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. J Neuroinflammation. 17(1):166–178. doi:10.1186/s12974-020-01836-y.
- Bian LH, Yao ZW, Wang ZY, Wang XM, Li QY, Yang X, Li JY, Wei XJ, Wan GH, Wang YQ, et al. 2022. Nardosinone regulates the slc38a2 gene to alleviate Parkinson’s symptoms in rats through the GABAergic synaptic and cAMP pathways. Biomed Pharmacother. 153:113269. doi:10.1016/j.biopha.2022.113269.
- Bian LH, Yao ZW, Zhao CB, Li QY, Shi JL, Guo JY. 2021. Nardosinone alleviates Parkinson’s disease symptoms in mice by regulating dopamine D2 receptor. Evid Based Complement Alternat Med. 2021:6686965. doi:10.1155/2021/6686965.
- Bu XL, Wang X, Xiang Y, Shen LL, Wang QH, Liu YH, Jiao SS, Wang YR, Cao HY, Yi X, et al. 2015. The association between infectious burden and Parkinson’s disease: a case-control study. Parkinsonism Relat Disord. 21(8):877–881. doi:10.1016/j.parkreldis.2015.05.015.
- Cuevas C, Huenchuguala S, Muñoz P, Villa M, Paris I, Mannervik B, Segura-Aguilar J. 2015. Glutathione transferase-M2-2 secreted from glioblastoma cell protects SH-SY5Y cells from aminochrome neurotoxicity. Neurotox Res. 27(3):217–228. doi:10.1007/s12640-014-9500-1.
- de Araújo DP, Nogueira PCN, Santos ADC, Costa RO, de Lucena JD, Jataí Gadelha-Filho CV, Lima FAV, Neves KRT, Leal L, Silveira ER, et al. 2018. Aspidosperma pyrifolium Mart: neuroprotective, antioxidant and anti-inflammatory effects in a Parkinson’s disease model in rats. J Pharm Pharmacol. 70(6):787–796. doi:10.1111/jphp.12866.
- de Farias CC, Maes M, Bonifácio KL, Bortolasci CC, de Souza Nogueira A, Brinholi FF, Matsumoto AK, do Nascimento MA, de Melo LB, Nixdorf SL, et al. 2016. Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson’s disease and its progression: disease and staging biomarkers and new drug targets. Neurosci Lett. 617:66–71. doi:10.1016/j.neulet.2016.02.011.
- Fan Z, Liang Z, Yang H, Pan Y, Zheng Y, Wang X. 2017. Tenuigenin protects dopaminergic neurons from inflammation via suppressing NLRP3 inflammasome activation in microglia. J Neuroinflammation. 14(1):256–267. doi:10.1186/s12974-017-1036-x.
- Huang L, Deng M, Zhang S, Lu S, Gui X, Fang Y. 2017. β-Asarone and levodopa coadministration increases striatal levels of dopamine and levodopa and improves behavioral competence in Parkinson’s rat by enhancing dopa decarboxylase activity. Biomed Pharmacother. 94:666–678. doi:10.1016/j.biopha.2017.07.125.
- Klein A, Gidyk DC, Shriner AM, Colwell KL, Tatton NA, Tatton WG, Metz GA. 2011. Dose-dependent loss of motor function after unilateral medial forebrain bundle rotenone lesion in rats: a cautionary note. Behav Brain Res. 222(1):33–42. doi:10.1016/j.bbr.2011.03.018.
- Kobayashi M, Yamamoto M. 2006. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regul. 46:113–140. doi:10.1016/j.advenzreg.2006.01.007.
- Kwon DK, Kwatra M, Wang J, Ko HS. 2022. Levodopa-induced dyskinesia in Parkinson’s disease: pathogenesis and emerging treatment strategies. Cells. 11:3736. doi:10.3390/cells11233736.
- Li ZH, Li W, Shi JL, Tang MK. 2014. Nardosinone improves the proliferation, migration and selective differentiation of mouse embryonic neural stem cells. PLoS One. 9(3):e91260. doi:10.1371/journal.pone.0091260.
- Lin ZH, Liu Y, Xue NJ, Zheng R, Yan YQ, Wang ZX, Li YL, Ying CZ, Song Z, Tian J, et al. 2022. Quercetin protects against MPP+/MPTP-induced dopaminergic neuron death in Parkinson’s disease by inhibiting ferroptosis. Oxid Med Cell Longev. 2022:7769355.
- Liu X, Wei F, Liu H, Zhao S, Du G, Qin X. 2021. Integrating hippocampal metabolomics and network pharmacology deciphers the antidepressant mechanisms of Xiaoyaosan. J Ethnopharmacol. 268:113549. doi:10.1016/j.jep.2020.113549.
- Li JY, Wei XJ, Wan GH, Yang X, Yu JH, Liu JF, Jin ZX, Wang YQ, Lyu Y, Shi JL. 2022. [Mechanism of Nardostachys jatamansi on levodopa-induced dyskinesia in rats based on Nrf2/D1R-ERK signaling pathway]. Chinese Trad Herbal Drugs. 53:134–142.
- Lyle N, Gomes A, Sur T, Munshi S, Paul S, Chatterjee S, Bhattacharyya D. 2009. The role of antioxidant properties of Nardostachys jatamansi in alleviation of the symptoms of the chronic fatigue syndrome. Behav Brain Res. 202(2):285–290. doi:10.1016/j.bbr.2009.04.005.
- Ma Q. 2013. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. doi:10.1146/annurev-pharmtox-011112-140320.
- Martinez EM, Young AL, Patankar YR, Berwin BL, Wang L, von Herrmann KM, Weier JM, Havrda MC. 2017. Editor’s highlight: nlrp3 is required for inflammatory changes and nigral cell loss resulting from chronic intragastric rotenone exposure in mice. Toxicol Sci. 159(1):64–75. doi:10.1093/toxsci/kfx117.
- Maturana MG, Pinheiro AS, de Souza TL, Follmer C. 2015. Unveiling the role of the pesticides paraquat and rotenone on α-synuclein fibrillation in vitro. Neurotoxicology. 46:35–43. doi:10.1016/j.neuro.2014.11.006.
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. 2006. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. J Neurosci. 26(37):9365–9375. doi:10.1523/JNEUROSCI.1504-06.2006.
- Nam HY, Nam JH, Yoon G, Lee JY, Nam Y, Kang HJ, Cho HJ, Kim J, Hoe HS. 2018. Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J Neuroinflammation. 15(1):271. doi:10.1186/s12974-018-1308-0.
- Nevalainen N, Lundblad M, Gerhardt GA, Strömberg I. 2013. Striatal glutamate release in L-DOPA-induced dyskinetic animals. PLoS One. 8(2):e55706. doi:10.1371/journal.pone.0055706.
- Nuvolone M, Sorce S, Schwarz P, Aguzzi A. 2015. Prion pathogenesis in the absence of NLRP3/ASC inflammasomes. PLoS One. 10(2):e0117208. doi:10.1371/journal.pone.0117208.
- Olanow CW. 2015. Levodopa: effect on cell death and the natural history of Parkinson’s disease. Mov Disord. 30(1):37–44. doi:10.1002/mds.26119.
- Sayed AS, El Sayed NS, Budzyńska B, Skalicka-Woźniak K, Ahmed MK, Kandil EA. 2022. Xanthotoxin modulates oxidative stress, inflammation, and MAPK signaling in a rotenone-induced Parkinson’s disease model. Life Sci. 310:121129. doi:10.1016/j.lfs.2022.121129.
- Shin JY, Bae GS, Choi SB, Jo IJ, Kim DG, Lee DS, An RB, Oh H, Kim YC, Shin YK, et al. 2015. Anti-inflammatory effect of desoxo-narchinol-A isolated from Nardostachys jatamansi against lipopolysaccharide. Int Immunopharmacol. 29(2):730–738. doi:10.1016/j.intimp.2015.09.002.
- Stansley BJ, Yamamoto BK. 2013. L-DOPA-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology. 67:243–251. doi:10.1016/j.neuropharm.2012.11.010.
- Todorovic M, Wood SA, Mellick GD. 2016. Nrf2: a modulator of Parkinson’s disease? J Neural Transm (Vienna). 123(6):611–619. doi:10.1007/s00702-016-1563-0.
- Toulouse A, Sullivan AM. 2008. Progress in Parkinson’s disease-where do we stand? Prog Neurobiol. 85(4):376–392. doi:10.1016/j.pneurobio.2008.05.003.
- Wan GH, Wei XJ, Li JY, Yang X, Yu JH, Liu JF, Wang YQ, Lyu Y, Jin ZX, Shi JL. 2022. Effects of Nardostachys jatamansi on gut microbiota of rats with Parkinson’s disease. China J Chinese Materia Med. 47:499–510.
- Wijeyekoon RS, Moore SF, Farrell K, Breen DP, Barker RA, Williams-Gray CH. 2020. Cerebrospinal fluid cytokines and neurodegeneration-associated proteins in Parkinson’s disease. Mov Disord. 35(6):1062–1066. doi:10.1002/mds.28015.
- Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y, Xu H, Jiang H, Hu R. 2018. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med. 121:78–85. doi:10.1016/j.freeradbiomed.2018.04.557.
- Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N. 2016. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp Cell Res. 340(2):315–326. doi:10.1016/j.yexcr.2015.10.026.
- Zhang X, Liu Y, Deng G, Huang B, Kai G, Chen K, Li J. 2021. A purified biflavonoid extract from Selaginella moellendorffii alleviates gout arthritis via NLRP3/ASC/Caspase-1 axis suppression. Front Pharmacol. 12:676297. doi:10.3389/fphar.2021.676297.
- Zhang W, Tao WW, Zhou J, Wu CY, Long F, Shen H, Zhu H, Mao Q, Xu J, Li SL, et al. 2021. Structural analogues in herbal medicine ginseng hit a shared target to achieve cumulative bioactivity. Commun Biol. 4(1):549–562. doi:10.1038/s42003-021-02084-3.
- Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, Feng L. 2019. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res. 150:104538. doi:10.1016/j.phrs.2019.104538.