Abstract
Context
Panax japonicus is the dried rhizome of Panax japonicus C.A. Mey. (Araliaceae). Saponins from Panax japonicus (SPJ) exhibit anti-oxidative and anti-aging effects.
Objective
We evaluated the neuroprotective effects of SPJ on aging rats.
Materials and methods
Sprague-Dawley rats (18-months-old) were randomly divided into aging and SPJ groups (n = 8). Five-month-old rats were taken as the adult control (n = 8). The rats were fed a normal chow diet or the SPJ-containing diet (10 or 30 mg/kg) for 4 months. An in vitro model was established by d-galactose (d-Gal) in the SH-SY5Y cell line and pretreated with SPJ (25 and 50 µg/mL). The neuroprotection of SPJ was evaluated via Nissl staining, flow cytometry, transmission electron microscopy and Western blotting in vivo and in vitro.
Results
SPJ improved the neuronal degeneration and mitochondrial morphology that are associated with aging. Meanwhile, SPJ up-regulated the protein levels of mitofusin 2 (Mfn2) and optic atrophy 1 (Opa1) and down-regulated the protein level of dynamin-like protein 1 (Drp1) in the hippocampus of aging rats (p < 0.05 or p < 0.01 vs. 22 M). The in vitro studies also demonstrated that SPJ attenuated d-Gal-induced cell senescence concomitant with the improvement in mitochondrial function; SPJ, also up-regulated the Mfn2 and Opa1 protein levels, whereas the Drp1 protein level (p < 0.05 or p < 0.01 vs. d-Gal group) was down-regulated.
Discussion and conclusions
Further research on the elderly population will contribute to the development and utilization of SPJ for the treatment of neurodegenerative disorders.
Introduction
In recent years, population aging has increased the risk of related neurodegenerative diseases (NDs) (Marcos-Rabal et al. Citation2021). The most common NDs include Alzheimer’s disease, Huntington’s disease, motor neuron disease, and Parkinson’s disease, which exact a heavy burden on a person’s life and on society (Li et al. Citation2022). NDs are caused by the progressive loss of function or abnormal structure of neuronal cells in the central nervous system (Juan and Adlard Citation2019). However, the pathogenesis of NDs associated with brain aging is still not fully elucidated; thus, effective clinical treatments are lacking. Previous studies have confirmed that brain aging is associated with dysregulated energy metabolism, neuroinflammation, mitochondrial dysfunction and other pathological mechanisms, among which neuronal mitochondrial dysfunction is one of the main pathological manifestations of NDs; this is receiving increased attention by researchers (Mattson and Arumugam Citation2018). Therefore, it is important to delay aging by improving neuronal mitochondrial dysfunction.
Numerous studies have shown that mitochondrial dysfunction is the initiating link in a variety of diseases such as aging-related neurodegenerative disease (Hou et al. Citation2019); mitochondrial reactive oxygen species (ROS) production and accumulation, mitochondrial dynamics imbalance, and mitochondrial DNA mutations can lead to neurodegeneration (Balog et al. Citation2016). Furthermore, mitochondrial dysfunction is accompanied by synapses and neuronal death, resulting in decreased learning memory capacity and consequently Alzheimer’s disease (AD) (Du et al. Citation2017), while significant ultrastructural changes in mitochondria, such as cristae breakage and loss of cristae membranes, are also present in NDs (Wang et al. Citation2019). In addition, the number of mitochondria, the mitochondrial membrane potential (MMP), and ATP levels were decreased in the ovarian and brain tissues of naturally aging rats (Wu et al. Citation2018; Wan et al. Citation2020). All of these studies suggest the presence of mitochondrial dysfunction during aging.
Mitochondrial dynamics (fission and fusion) is the basis of mitochondrial function. For example, the efficiency of ATP production is increased, and the exchange of matrix content is favored in fused organelles. By contrast, fragmented organelles produce more ROS (Giacomello et al. Citation2020). A growing number of studies have shown that mitochondrial dysfunction due to imbalances in mitochondrial dynamics is strongly associated with the development of many age-related NDs (Panchal and Tiwari Citation2019), with reduced mitochondrial fusion and increased fission (Reddy and Reddy Citation2011). In AD, mitochondrial breakage is caused by the increased expressions of Drp1 and Fis1, while the levels of Mfn1, Mfn2, and Opa1 decrease (Manczak et al. Citation2011). However, a blockade of Drp1 phosphorylation restored mitochondrial length, and led to memory improvements in a transgenic APP/PS1 mouse model (Yan et al. Citation2015). In Parkinson’s disease, the inhibition of Drp1 attenuates neurotoxicity and restores striatal dopamine release (Rappold et al. Citation2014); therefore, whether Drp1 inhibition can restore mitochondrial function and exert neuroprotection by adjusting the balance between mitochondrial dynamics is scientifically significant.
Panax japonicus (PJ), the dried rhizome of Panax japonicus C. A. Mey. (Araliaceae), can disperse blood stasis and hemostasis, reduce swelling, relieve pain, nourish deficiencies, and strengthen the body (OuYang et al. Citation2010). Studies from our group and others showed that Chikusetsusaponin V could attenuate MPP+ or H2O2-induced SH-SY5Y neurocytotoxicity by inhibiting ROS accumulation, increasing MMP (Yuan et al. Citation2014; Wan et al. Citation2016). Moreover, saponins from Panax japonicus (SPJ) could improve H2O2- or d-galactose (d-Gal)-induced SH-SY5Y cell damage (Deng et al. Citation2015; Wan et al. Citation2018), attenuate mitochondrial damage, reduce neuronal apoptosis, and thereby improve d-Gal- and Aβ-induced cognitive dysfunction in aging rats (Wang et al. Citation2015; Deng et al. Citation2017). However, whether SPJ regulates mitochondrial dynamics remains unclear. Thus, the present study evaluated the neuroprotective effect of SPJ on aging-induced neuronal degeneration in cell and animal models. Furthermore, we investigated the underlying molecular mechanism of mitochondrial fission fusion homeostasis. Our in vivo study showed that SPJ ameliorated the age-related decline in brain function by improving the pathological structure and morphology of neurons. Meanwhile, SPJ partially recovered the imbalance of mitochondrial dynamics due to its up-regulation of Mfn2 and Opa1 protein levels and its down-regulation of the Drp1 protein level in the hippocampus of aging rats, and in d-Gal-induced cell senescence. In conclusion, SPJ ameliorated neuronal mitochondrial injury through regulating mitochondrial fission fusion homeostasis, which could be used to treat neurodegenerative disorders.
Materials and methods
Drug sources and extraction
Panax japonicus was harvested from the planting base of Chunmuying in Xuanen County, Enshi City, Hubei Province in October 2016. It was identified by Dr. He Yumin in the Key Laboratory of Natural Products Research and Utilization of Three Gorges University. SPJ were extracted according to the method of our research team (He et al. Citation2017). Briefly, the roots of dried Panax japonicus (1000 g) were cut into small pieces and refluxed with a 3-fold volume of 70% ethanol three times, with 2 h for each time. The extract was filtered and concentrated to 5 L, purified by M-5 macroporous resin, eluted with double distilled water, and eluted with 70% ethanol when the Molish reaction was negative. The collected eluent was then decompressed to concentrate, followed by freeze-drying. The dried sample was added with water to make a 20 mg/mL solution, and the pH value was adjusted to 10. The extraction rate was 18.4% and the purity was 83.5%.
Animals and treatment
The specific pathogen-free male Sprague Dawley rats (n = 32) used were purchased and fed in the Experimental Animal Center of China Three Gorges University in Yichang, China. This study was approved by the China Three Gorges University Council on Animal Care Committee (2018080B). The handling, experimental procedures, and care of the animals were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were divided into four groups, and treated with various regimens: (a) the adult control group (n = 8, normal feed, 1-month-old rats were purchased and raised to 5 months old, 5 M); (b) the aging control group (n = 8, normal feed, 18-month-old rats were purchased and raised to 22 months old, 22 M); (c) the SPJ 10 mg/kg-treated group (n = 8, contained SPJ feed, 18-month-old rats were purchased and administered the food treatment for 4 consecutive months until they were 22 months old); (d) the SPJ 30 mg/kg-treated group (n = 8, contains SPJ feed, 18-month-old rats were purchased and administered the food treatment for 4 consecutive months until they were 22 months old). Briefly, we measured the daily food intake of each rat, and calculated the SPJ content per kilogram of feed based on the average weight and food intake. Rat feed containing SPJ was processed by Beijing Huafukang Biotechnology Co., Ltd. After the treatment with SPJ or normal feed for 4 months, the rats were anesthetized with intraperitoneally injected urethanes. The cortex and hippocampus were snap frozen for further experiments.
Nissl staining analysis
The perfused brain tissues were soaked in 4% neutral paraformaldehyde for 24 h, dehydrated in an ascending ethanol series, and equilibrated with xylene. Then, the brain tissues were embedded in paraffin and cut into 5 μm thick slices. First, the slices were immersed in xylene for 30 min (twice), 100% ethanol for approximately 10 min (twice), 95% ethanol for 10 min (twice), 80% ethanol for 10 min, 75% ethanol for 10 min, and in double-distilled water for 5 min. Next, the slices were incubated with Nissl staining solution for 10 min at 37 °C, then washed quickly in double distilled water, and hydrated in 95% ethanol for 10 sec. Finally, the samples were sealed with neutral gum and observed under a microscope according to a double-blind method. Then, the number of Nissl bodies was calculated by randomly selecting six images per group (two images per rat).
Cell culture and viability assay
SH-SY5Y cells were kindly provided by the Stem Cell Bank, Chinese Academy of Sciences. The cells were sub-cultured and maintained in minimum (MEM)-F12 essential medium supplemented with nonessential amino acids and 10% fetal bovine serum in an incubator containing 5% CO2 at 37 °C. The cell viability was evaluated with an MTT colorimetric assay. Briefly, the cells were seeded in 96-well sterile plates at a density of 104 cells per well, then pre-incubated with different concentrations of SPJ (3.125, 6.25, 12.5, 25, and 50 μg/mL) for 12 h before being treated with 200 mM d-Gal for an additional 48 h. Then, a 10 μL aliquot of MTT solution was added to the medium to yield a final MTT concentration of 0.5 mg/mL; the medium was incubated at 37 °C for 4 h. The MTT solution was removed gently and 150 μL of DMSO added to each well for 15 min of incubation. The absorbance of each sample was measured at 490 nm with a spectrophotometer. The survival ratio of the control group was defined as 100%, and those of the other groups were expressed as percentages of this control group.
Senescence-associated β-galactosidase (SA-β-gal) staining analysis
SA-β-gal staining was applied to detect cellular senescence using an SA-β-gal staining kit (C0602, Beyotime, China) according to the manufacturer’s instructions. Briefly, SH-SY5Y cells in the different groups were separately seeded in 6-well plates with 5 × 104/well. After the designated exposure, the medium was discarded and the cells were rinsed with PBS. Fixative (1 mL) was then added to each well for 15 min of fixing. The washed cells were subsequently reacted with the working solution of β-gal with X-Gal for incubation at 37 °C overnight. The senescent cells with positive staining were observed with an optical microscope and counted from ten random fields of vision to calculate the positive rate.
Transmission electron microscope analysis
The perfused brain tissues or collected SH-SY5Y cells were immersed in 2.5% glutaraldehyde for 2 h and post-fixed in 1% osmium tetroxide for 1 h. Then, the tissue was dehydrated once with 50% ethanol, 70% ethanol, and 90% ethanol, 90% ethanol: 90% acetone = 1:1, 15–20 min each time, and then dehydrated once with 90% acetone, each time for 30 min. Finally, the tissues were dehydrated with anhydrous acetone 3 times, for 15–20 min each time. The dehydrated tissue was first treated with anhydrous acetone: embedding agent = 2:1 for 3–4 h, and then treated overnight with anhydrous acetone: embedding agent = 1:2. The infiltrated tissue was placed in an embedded plate, first polymerized overnight in a 37 °C incubator, then heated to 45 °C for 12 h, and finally to 60 °C for 24 h. The tissues and cells were chipped with a ultramicrotome in 50 nm sections, and stained with uranyl acetate and aluminum citrate for 15–30 min. We recorded the images of neurons, synapses and mitochondrial ultrastructure under a Hitachi H-7500 transmission electron microscope (Hitachi Ltd., Japan). We used Image J software to analyze the mitochondrial ultrastructure and measure the mitochondria aspect ratio and area. The numbers of synapses, mitochondria aspect ratios and areas were counted by randomly selecting six images from each group (two images per rat, or one plate). The number of synapses was obtained by counting the total number of synapses in each image.
ROS level measurement
The ROS were measured on the basis of the intracellular peroxide-dependent oxidation of DCFH-DA (C1300-1, Applygen, China) to form the fluorescent compound 2′,7′-dichlorofluorescein (DCF). The cells were seeded in 6-well plates at a density of 5 × 104 cells per well and cultured for 24 h. After the series of indicated experimental treatments, the cells were incubated with 10 μM DCFH-DA for 30 min at 37 °C. The cells were washed thrice with PBS, trypsinized and resuspended in 300 μL of PBS. The fluorescence intensity was determined with a FACSVerse flow cytometer (BD Biosciences, USA).
Mitochondrial membrane potential (MMP) assay
The MMP was measured with the MMP assay kit with JC-1 (C2006, Beyotime, China). The cells were seeded in 6-well plates at a density of 5 × 104 cells per well and cultured for 24 h. After the designated exposure, the cells were incubated at 37 °C for 30 min with 0.5 mL of JC-1 working solution. After that, the staining solution was removed by centrifugation at 600 g for 3–4 min, and the cells were washed twice with JC-1 staining 1 × buffer. Finally, cells were resuspended in 300 μL of buffer. If the MMP level was low, the monomeric form of JC-1 fluoresced green (emission ∼529 nm); if within the mitochondrial matrix, the MMP level was high, the JC-1 is able to form aggregates, which fluoresce red (emission ∼590 nm). The MMP was quantified with a FACSVerse flow cytometer (BD Biosciences, USA) to determine the cells with green and red fluorescence.
Adenosine triphosphate (ATP) levels measurement
The intracellular ATP levels were measured using an ATP assay kit (A095-1-1, Nanjing Jiancheng Bioengineering Institute, China). In brief, the cells were washed once with PBS and transferred to a lysis buffer. The SH-SY5Y cells were centrifuged at 12,000 g for 5 min. In 96-well plates, 100 mL of each supernatant was mixed with 100 mL of ATP detection working dilution. The absorbance was measured by a microplate reader. The ATP concentration was calculated according to the standard curve.
Western blot analysis
All of the tissues or cells were lysed in RIPA buffer, and their total protein concentrations were determined with a BCA protein assay kit. The total protein was loaded into precast 10% SDS-PAGE gels, and then the target protein was transferred to PVDF membranes. The membrane was blocked in 5% milk for 1 h and washed once with Tris-buffered saline with Tween-20 (TBST). After that, the membranes were incubated at 4 °C overnight with the following primary antibodies: anti-β-actin (Servicebio, GB11001, 1:5000), anti-Drp1 (Abcam, ab184247, 1:2000), anti-Mfn2 (Abcam, ab124773, 1:2000), and anti-Opa1 (Proteintech, 27733-1-AP, 1:2000). The primary antibodies were recycled and the membranes were washed 3 times in TBST for 6 min each. Goat anti-Rabbit IgG secondary antibody (Jackson ImmunoResearch, 103069, 1:5000) was incubated for 1 h at room temperature. After removing the secondary antibody, the membranes were washed 3 times with TBST for 6 min each. Finally, the levels of the relative proteins were detected using an enhanced chemiluminescence reagent. The signals were confirmed with a band intensity analysis using Image J software.
Statistical analysis
The obtained data are presented as mean ± SEM of at least three independent experiments. The groups were compared using one-way ANOVA with post hoc Tukey’s multiple comparisons test. All of the data were analyzed with GraphPad Prism 6.0 software. The statistical significance was set at p < 0.05 for all of the analyses.
Results
SPJ alleviated neurodegeneration in aging rats
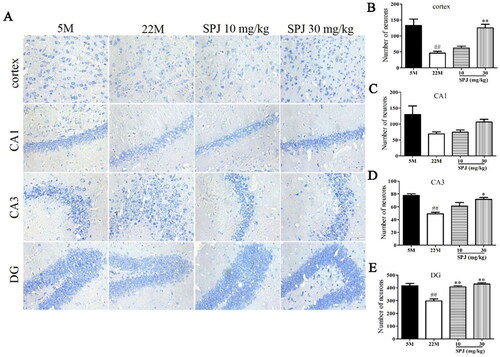
Nissl staining was used to analyze the neurodegeneration, and its degree of staining was a crucial indicator in evaluating the number and function of neurons (Ali and Kim Citation2015). As shown in , the number of survival neurons in the cortical, CA3 and DG regions was reduced in the 22 M group compared to the 5 M group (p < 0.01). However, SPJ treatment significantly increased the number of survival neurons in the cortex and hippocampus regions compared to the 22 M group (p < 0.05 or p < 0.01, except the CA1 region), indicating that SPJ effectively inhibited neurodegeneration in the aging rats.
Figure 1. SPJ improved cortical and hippocampal neurodegeneration in aging rats. (A) Representative images of Nissl staining in the cortex and CA1, CA3, and DG regions of the hippocampus (400×, scale bar = 100 μm). (B, C, D, E) Analysis of Nissl staining. The data are from six photos per group (two photos per rat) and expressed as mean ± SEM. ##p < 0.01 vs. 5 M; *p < 0.05; **p < 0.01 vs. 22 M.
SPJ improved the neuronal morphology and elevated the number of synapses in aging rats
Electron microscopy analysis showed that the nuclei of cortical neurons in the 5 M group were large and round with clear and intact nuclear membranes as well as obvious nucleoli; in contrast, the cortical neurons in the 22 M group were solidly shrunken, with wrinkled nuclear membranes and shifted nucleoli. Nonetheless, these neuronal pathological changes in ultrastructure can be attenuated by the SPJ intervention, as evidenced with the nuclear membranes of cortical neurons in the aging rats that gradually became intact ().
Figure 2. SPJ improved the neuronal morphology and elevated the numbers of synapses in the cortex of aging rats. (A) Representative images of cortical neuronal morphology from electron microscopy observations in aging rats (10,000×, scale bar = 1 μm). (B) Representative images of cortical synaptic numbers in aging rats (20,000×, scale bar = 800 nm). (C) Analysis of numbers of synapses. The data are from six photos per group (two photos per rat) and expressed as mean ± SEM. Red arrows indicate synapse, and the red box indicates enlarged synapse. ##p < 0.01 vs. 5 M; *p < 0.05 vs. 22 M.
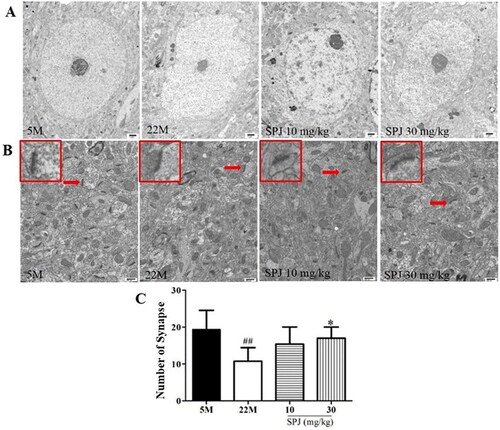
Additionally, the number of synapses decreased in the 22 M group, compared with the 5 M group (10.778 ± 3.667 vs. 19.4 ± 5.168, p < 0.01). However, SPJ intervention increased the number of synapses in the 22 M group at the dose of 30 mg/kg (17 ± 3.033, p < 0.05) ().
SPJ improved the mitochondrial morphology in aging rats
As shown in , the transmission electron microscope observations showed that the neuronal mitochondrial cristae in the 5 M group were densely arranged. In contrast, the neuronal mitochondrial cristae of aging rats in the 22 M group were broken or disappeared, which can be improved by SPJ administration.
Figure 3. SPJ improved the neuronal mitochondrial morphology in the cortex of aging rats. (A) Representative images of neuronal mitochondrial morphology in the cortex of aging rats (20,000×, scale bar = 800 nm). (B) Analysis of mitochondrial aspect ratio. (C) Analysis of mitochondrial area. The data are from six photos per group (two photos per rat) and expressed as mean ± SEM. Red arrows indicate mitochondria, and the red box indicates enlarged mitochondria. ##p < 0.01 vs. 5 M; *p < 0.05 vs. 22 M.
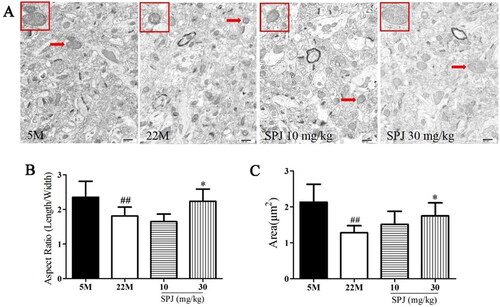
Meanwhile, two morphological metrics were used to determine the balance between mitochondrial fission and fusion. The aspect ratio is defined as the ratio of the major to minor axes, which indicates the mitochondrial shape. The area was used as the measurement of mitochondrial size. Low values of the aspect ratio and area indicated circular mitochondria, whereas high values indicated elongated and highly interconnected mitochondria (Durand et al. Citation2019; Esencan et al. Citation2020; Sisalli et al. Citation2020; Delmotte et al. Citation2021). Our results showed that the mitochondrial aspect ratio and area decreased in the 22 M group, compared with the 5 M group (1.808 ± 0.258 vs. 2.352 ± 0.46, 1.28 ± 0.2 vs. 2.13 ± 0.498, p < 0.01). However, SPJ intervention increased the mitochondrial aspect ratio and area in the 22 M group at the dose of 30 mg/kg (2.234 ± 0.354 and 1.748 ± 0.364, p < 0.05) ().
SPJ improved the impaired balance of mitochondrial dynamics in aging rats
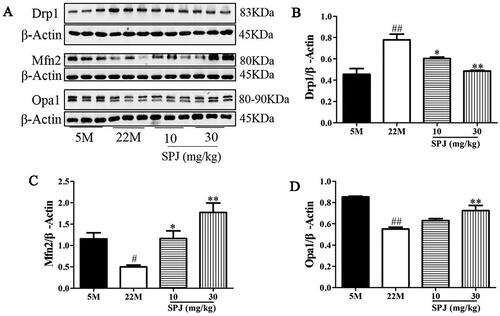
To further explore the effects of SPJ on the impaired balance of mitochondrial dynamics in the hippocampus of aging rats, we determined the levels of fission protein (Drp1) and fusion proteins (Mfn2 and Opa1). Western blot analysis revealed that the Drp1 protein level significantly increased, but the protein levels of Mfn2 and Opa1 decreased in the 22 M group when compared to the 5 M group (p < 0.05 or p < 0.01). However, the above changes in protein expression were obviously reversed by SPJ intervention, indicating that SPJ improved the impaired balance of the mitochondrial dynamics of aging rats (p < 0.05 or p < 0.01, ).
Figure 4. SPJ improved impaired mitochondrial dynamics in aging rats. (A) Representative immunoblot bands of Drp1, Mfn2, and Opa1. (B, C, D) Quantification of Drp1/β-Actin ratio, Mfn2/β-Actin ratio, and Opa1/β-Actin ratio. Values are expressed as mean ± SEM, n = 4. #p < 0.05; ##p < 0.01 vs. 5 M; *p < 0.05; **p < 0.01 vs. 22 M.
SPJ promoted the cell viability and mitigated the cell senescence in d-Gal-treated SH-SY5Y cells
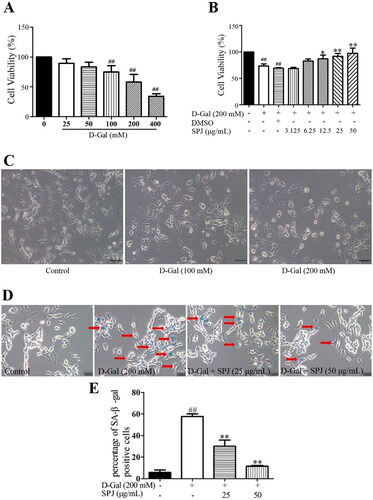
As shown in , with the increased concentration of d-Gal, the cell viability determined by the MTT assay gradually decreased (); as well, there were significant changes in cell morphology such as cytosolic wrinkling, reduced protrusions, and intracellular granular material deposition. Based on the cell viability and morphological observations, we selected a 200 mM d-Gal for subsequent experiments in this study. To determine the effect of SPJ on d-Gal-induced cell viability, the SH-SY5Y cells were pre-incubated with different concentrations of SPJ (3.125, 6.25, 12.5, 25, and 50 μg/mL) for 12 h, and then treated with 200 mM d-Gal for an additional 48 h. As shown in , the cell viability of the 200 mM d-Gal-induced group decreased by 42% when compared with the control group (p < 0.01). After the dose-dependent administration of SPJ, the cell viability increased by 26 and 34%, respectively (p < 0.05 or p < 0.01, ). We used the SA-β-gal staining assay (Liu et al. Citation2012) to detect any senescence of the SH-SY5Y cells induced by d-Gal. As shown in , the number of SA-β-gal-positive cells in the d-Gal-treated group increased compared to the control group (p < 0.01). The number of SA-β-gal-positive cells under the SPJ treatment were significantly reduced compared to the d-Gal-treated group (p < 0.01).
Figure 5. SPJ promoted cell viability and mitigated cell senescence in d-Gal-treated SH-SY5Y cells. Cellular viability was detected using MTT assay. (A) SH-SY5Y cells were cultured for 24 h and then incubated with different concentrations of d-Gal (0–400 mM) for 48 h. (B) SH-SY5Y cells were pretreated with SPJ (3.125–50 μg/mL) or DMSO (0.1%) for 12 h, and then incubated with 200 mM d-Gal for 48 h. (C) Representative images of morphological changes in d-Gal-treated SH-SY5Y cells through light microscope (200×, scale bar = 200 μm), n = 4. SH-SY5Y cells were pretreated with SPJ (25, 50 μg/mL) for 12 h, and then incubated with 200 mM d-Gal for 48 h. (D) Representative images of stained SA-β-gal cells (200×, scale bar = 200 μm). (E) Analysis of positive SA-β-gal cells. Red arrows indicate positive SA-β-gal cells. Values are expressed as mean ± SEM, n = 3. ##p < 0.01 vs. control; *p < 0.05; **p < 0.01 vs. d-Gal-treated group.
SPJ improved mitochondrial morphology in d-Gal-treated SH-SY5Y cells
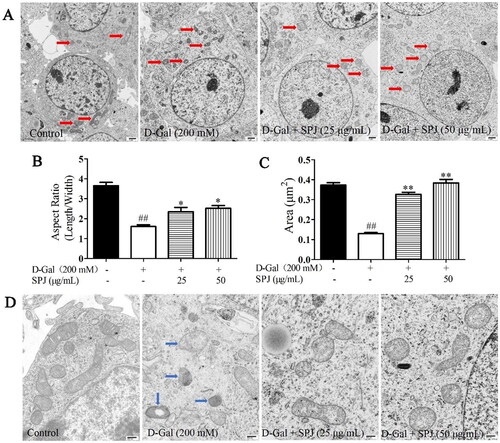
The transmission electron microscope results showed that compared with the control group, the d-Gal treatment changed the mitochondrial morphology of the SH-SY5Y cells. There was an increase in small spherical mitochondria (). The mitochondrial aspect ratio and mitochondrial area of the control group were 3.653 ± 0.419 and 0.373 ± 0.03, respectively, while in the d-Gal-stimulated group they decreased to 1.605 ± 0.22 and 0.13 ± 0.013 (p < 0 .01), respectively. However, 25 and 50 μg/mL SPJ interventions increased the mitochondrial aspect ratio (2.349 ± 0.712 and 2.512 ± 0.421, respectively) and area (0.327 ± 0.034 and 0.384 ± 0.05, respectively) (p < 0.05 or p < 0.01, ). Meanwhile, the mitochondrial cristae in the control group were neatly arranged and dense, with a clear structure; in contrast, those in the d-Gal group were disordered or had disappeared, and their mitochondrial cristae morphology was improved with SPJ intervention ().
Figure 6. SPJ improved mitochondrial morphology in d-Gal-treated SH-SY5Y cells. (A, D) Representative images of mitochondrial morphology in d-Gal-induced senescent SH-SY5Y cells (A, 12,000×, scale bar = 1 μm; D, 40,000×, scale bar = 400 nm). (B) Analysis of mitochondrial aspect ratio. (C) Analysis of mitochondrial area. The data are from six photos per group (two photos per one plate) and expressed as mean ± SEM. Red arrows indicate mitochondria, blue arrow indicates damaged mitochondria. ##p < 0.01 vs. control; *p < 0.05; **p < 0.01 vs. d-Gal-treated group.
SPJ promoted mitochondrial function in d-Gal-treated SH-SY5Y cells
In the presence of ROS, DCFH is oxidized to the strong green fluorescent substance DCF, and the fluorescence level is proportional to the intracellular ROS level. A DCFH-DA fluorescent probe was used to detect ROS levels through flow cytometry. As shown in , compared with the control group (2179.667 ± 68.5), the green fluorescence level in the d-Gal group significantly increased to 4873 ± 757.416 (p < 0.01). However, SPJ interventions of 25 and 50 μg/mL reduced the fluorescence levels to 3185.667 ± 173.324 and 2453.333 ± 338.522, respectively (p < 0.01).
Figure 7. SPJ improved mitochondrial function in d-Gal-treated SH-SY5Y cells. SH-SY5Y cells at 5 × 105/well in 6-well plates were pretreated with SPJ (25 and 50 μg/mL) for 12 h, and then incubated with 200 mM d-Gal for 48 h. (A, C) Intracellular ROS level was evaluated with DCFH-DA fluorescence probe via flow cytometry. (B, D) The MMP level was measured using JC-1 fluorescent probe via flow cytometry. (E) Intracellular ATP content was measured using ATP assay kit. Values are expressed as mean ± SEM, n = 3. ##p < 0.01 vs. control; *p < 0.05; **p < 0.01 vs. d-Gal-treated group.
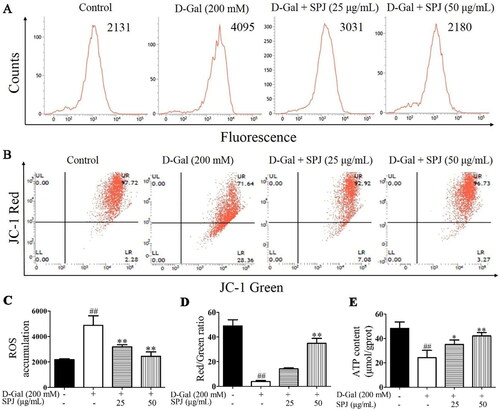
When the MMP was high, the JC-1 aggregates in the mitochondrial matrix formed a polymer and emitted a red fluorescence. When the MMP decreased or was lost, the JC-1 could only exist in the cytoplasm as a monomer and produced a green fluorescence; thus, the MMP level could be judged by the ratio of red to green fluorescence. The JC-1 fluorescent probe was used to detect the MMP level through flow cytometry. As shown in , compared with the control group, the red fluorescence weakened, but the green fluorescence increased in the d-Gal group, indicating that the MMP level decreased after d-Gal stimulation (p < 0.01). However, the 25 and 50 μg/mL SPJ interventions increased the red fluorescence but decreased the green fluorescence, indicating that SPJ could increase the MMP level (p < 0.01).
Additionally, the intracellular ATP content was measured with the ATP assay kit, and the results are shown in . Compared with the control group (48.38 ± 5.142), the ATP level in the d-Gal group significantly decreased (24.286 ± 5.197, p < 0.01), which could be reversed by treatments with SPJ at doses of 25 and 50 μg/mL (35.111 ± 3.644 and 42.098 ± 2.89, respectively; p < 0.05 or p < 0.01).
SPJ ameliorated the impaired balance in mitochondrial dynamics in d-Gal-treated SH-SY5Y cells
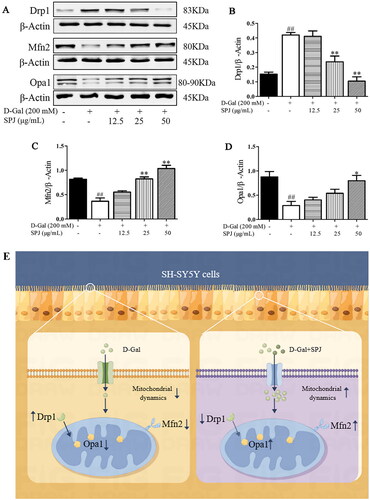
As shown in , compared with the control group, the d-Gal group had an increased Drp1 protein level (0.153 ± 0.024 vs. 0.421 ± 0.029, respectively, p < 0.01), but decreased protein levels of Mfn2 (0.815 ± 0.041 vs. 0.365 ± 0.117, p < 0.01) and Opa1 (0.815 ± 0.041 vs. 0.287 ± 0.147, respectively, p < 0.01). After SPJ treatment with 25 and 50 μg/mL doses, the Drp1 protein level was significantly down-regulated to 0.237 ± 0.067 and 0.105 ± 0.051, respectively (p < 0.01), while the Mfn2 protein level was significantly up-regulated to 0.823 ± 0.071 and 1.037 ± 0.116, respectively (p < 0.01), and the Opa1 protein level was also up-regulated to 0.797 ± 0.191 (p < 0.05) in the SPJ 50 μg/mL group.
Figure 8. SPJ ameliorated the impaired balance of the mitochondrial dynamics in d-Gal-treated SH-SY5Y cells. (A) Representative immunoblot bands of Drp1, Mfn2 and Opa1 in d-Gal-treated SH-SY5Y cells. (B, C, D) Quantification of Drp1/β-actin ratio, Mfn2/β-actin ratio and Opa1/β-actin ratio. (E) A schematic diagram. Values are expressed as mean ± SEM, n = 3. ##p < 0.01 vs. control; *p < 0.05; **p < 0.01 vs. d-Gal-treated group.
Discussion
Our previous studies demonstrated that SPJ can improve brain function and cognitive impairment in d-Gal-induced aging rats and naturally aging rats (Wang et al. Citation2015; Deng et al. Citation2017). Meanwhile, many aging-related NDs exhibit mitochondrial dysfunction, such as reduced mitochondrial enzyme activity and respiratory chain complex activity, in addition to ROS accumulation and reduced ATP production (Friedland-Leuner et al. Citation2014). Studies have illustrated that the Panax notoginseng Rg1 can improve neuronal apoptosis by regulating mitochondrial function (Meng et al. Citation2014). Therefore, our study further elucidates the protective effect of SPJ on aging neurons which is associated with improved mitochondrial function.
Numerous studies have proven that mitochondrial structure is more heterogeneous and fragmented in older animals (Rana et al. Citation2017), and manifested by disruptions in the mitochondrial membranes and their cristae structure, and consequent reductions in mitochondrial function (Sharma et al. Citation2019). It was found that in the SAMP8 aging mouse model, neuronal mitochondria showed severe structural damage with significant swelling and blurred contours; treatment with Panax notoginseng saponins improved the mitochondrial structure with neatly aligned cristae, which eventually improved the learning memory ability of the dementia model mice (Wang et al. Citation2021). In this study, we found that the mitochondrial cristae of aging rats were broken or had disappeared; meanwhile, the mitochondrial aspect ratio and area were reduced. However, SPJ reversed the structure of the mitochondrial cristae of aging rats, and increased the mitochondrial aspect ratio and area, indicating that SPJ could improve the mitochondrial structure of aging rats. Numerous studies have indicated that d-Gal could be used to construct in vivo and in vitro aging models (Maharajan and Cho Citation2021). Al-Baqami and Hamza (Citation2021) demonstrated that d-Gal treatment led to nuclear division in hepatocytes and the degeneration of most liver structures in male Wistar rats. d-Gal could also cause nuclear membrane infolding and swollen mitochondria with vacuolation in vascular smooth muscle cells, while ginsenoside Rg3 could improve mitochondrial structure to inhibit d-Gal-induced senescence of vascular smooth muscle cells (Yan and Li Citation2015). Furthermore, our results showed that the mitochondrial cristae were significantly broken, whereas the mitochondrial aspect ratio and area were significantly decreased in the d-Gal-treated SH-SY5Y cells, characteristics that can be attenuated after SPJ intervention. Aging particularly affects mitochondrial homeostasis and maintaining a well-functioning mitochondrial pool is imperative in order to avoid age-associated NDS. The brain is an organ with high energy requirements and impaired mitochondrial function has been associated with increased low-grade inflammation, excessive ROS production, and an accelerated aging phenotype. Zhou et al. (Citation2019) found that d-Gal causes abnormal morphologies and decreases the number of mitochondria in the brain, liver, and renal tubular epithelium of rats. Moreover, d-Gal-induced rats displayed higher levels of ROS and lower levels of MMP. Surprisingly, these morphologically and functionally insufficient mitochondria can remarkably be rescued by the application of chicken embryo extract and a nutritional mixture (Zhou et al. Citation2019). Meanwhile, curcumin and hesperidin also reversed d-Gal-induced mitochondrial dysfunction in a dose-dependent manner (Banji et al. Citation2014). Our previous studies showed that SPJ could ameliorate H2O2- or d-Gal-induced SH-SY5Y cell injury by modulating mitochondrial function (Deng et al. Citation2015; Wan et al. Citation2018). The present study also found that d-Gal stimulation led to an increase in ROS and a decrease in MMP and ATP, suggesting that d-Gal had an impairing effect on mitochondrial function in SH-SY5Y cells. In contrast, SPJ intervention reduced ROS production and MMP, but increased the ATP content in SH-SY5Y cells, indicating that SPJ had a protective effect on mitochondrial function in d-Gal-stimulated SH-SY5Y cells. The above studies suggest that SPJ can play a protective role against d-Gal-induced senescence in SH-SY5Y cells through the mitochondrial pathway.
Mitochondrial dynamics is a key process that regulates mitochondrial function and quality (Sebastian et al. Citation2017). Mutations of mitochondrial fission and fusion proteins lead to unbalanced mitochondrial dynamics, and have a pathogenic role in many age-related diseases. For example, Drp1 interacts with Aβ, leading to mitochondrial disruption, synaptic damage, and consequently AD (Manczak et al. Citation2011). Mfn2 mutations cause Charcot Marie Tooth disease type 2 A (CMT2A), one of the most common inherited axonal neuropathies (Stavropoulos et al. Citation2021). Opa1 mutation leads to autosomal dominant optic atrophy, the most common genetic form of optic nerve degeneration (Zaninello et al. Citation2022). Studies have found that d-Gal-induced up-regulates Drp1 protein expression in the hippocampal tissue of rapidly aging mice and senescent SH-SY5Y cells (Kou et al. Citation2017). d-Gal and TNF-α act on human embryonic hepatocyte line L02 cells, resulting in increased Fis1 and Drp1 protein expression and decreased protein levels of Mfn1, Mfn2 and Opa1 (Chen et al. Citation2020). Our results also revealed that Drp1 protein expressions increased whereas Mfn2 and Opa1 protein expression decreased in the hippocampus of aging rats, which can be reversed by SPJ intervention. Meanwhile, we also found that mitochondrial fission protein Drp1 was up-regulated, whereas fusion proteins such as Mfn2 and Opa1 were down-regulated in d-Gal-treated SH-SY5Y cells, and that SPJ could improve this imbalance in mitochondrial dynamics, which suggested that SPJ exerted neuroprotective effects by regulating the balance between mitochondrial fission and fusion and consequently restoring mitochondrial function.
Conclusions
Our study indicated that SPJ alleviated neuronal mitochondrial injuries in aging rats through regulating mitochondrial fission-fusion homeostasis, which may be regarded as a potential therapeutic target for the treatment of aging-related NDs.
Author contributions
Ting Wang and Zhi-Yong Zhou designed the study; Cheng Fan and Jin-Xin Wang conducted the experiments; Jin-Xin Wang and Zhang-E Xiong wrote the manuscript; Shan-Shan Hu, Ao-Jia Zhou, Ding Yuan, and Chang-Cheng Zhang carried out other experiments and analyzed the data. All the authors have read and approved the final manuscript prior to submission.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Baqami NM, Hamza RZ. 2021. Synergistic antioxidant capacities of vanillin and chitosan nanoparticles against reactive oxygen species, hepatotoxicity, and genotoxicity induced by aging in male Wistar rats. Hum Exp Toxicol. 40(1):183–202. doi: 10.1177/0960327120943267.
- Ali T, Kim MO. 2015. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J Pineal Res. 59(1):47–59. doi: 10.1111/jpi.12238.
- Balog J, Mehta SL, Vemuganti R. 2016. Mitochondrial fission and fusion in secondary brain damage after CNS insults. J Cereb Blood Flow Metab. 36(12):2022–2033. doi: 10.1177/0271678X16671528.
- Banji OJF, Banji D, Ch K. 2014. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by d-galactose in rat brain. Food Chem Toxicol. 74:51–59. doi: 10.1016/j.fct.2014.08.020.
- Chen Q, Wang Y, Jiao F, Shi C, Pei M, Wang L, Gong Z. 2020. Histone deacetylase 6 inhibitor ACY1215 ameliorates mitochondrial dynamic and function injury in hepatocytes by activating AMPK signaling pathway in acute liver failure mice. Histol Histopathol. 35:1047–1058.
- Delmotte P, Marin Mathieu N, Sieck GC. 2021. TNFα induces mitochondrial fragmentation and biogenesis in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 320(1):L137–L151. doi: 10.1152/ajplung.00305.2020.
- Deng LL, Wan JZ, Yuan D, Zhang CC, Li G, Wang T. 2015. Protective effects of total saponins from Panax japonicus on H2O2 induced injury via mitochondria pathway in SH-SY5Y cells. Zhong Yao Cai. 38(8):1690–1693.
- Deng LL, Yuan D, Zhou ZY, Wan JZ, Zhang CC, Liu CQ, Dun YY, Zhao HX, Zhao B, Yang YJ, et al. 2017. Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogen-activated protein kinase and nuclear factor kappa B signaling pathways. Neural Regen Res. 12(11):1877–1884. doi: 10.4103/1673-5374.219047.
- Du F, Yu Q, Yan SJ, Hu G, Lue LF, Walker DG, Wu L, Yan SF, Tieu K, Yan SS. 2017. PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain. 140(12):3233–3251. doi: 10.1093/brain/awx258.
- Durand MJ, Ait-Aissa K, Levchenko V, Staruschenko A, Gutterman DD, Beyer AM. 2019. Visualization and quantification of mitochondrial structure in the endothelium of intact arteries. Cardiovasc Res. 115(10):1546–1556. doi: 10.1093/cvr/cvy294.
- Esencan E, Jiang Z, Wang T, Zhang M, Soylemez-Imamoglu G, Seli E. 2020. Impaired mitochondrial stress response due to CLPP deletion is associated with altered mitochondrial dynamics and increased apoptosis in cumulus cells. Reprod Sci. 27(2):621–630. doi: 10.1007/s43032-019-00063-y.
- Friedland-Leuner K, Stockburger C, Denzer I, Eckert GP, Muller WE. 2014. Mitochondrial dysfunction: cause and consequence of Alzheimer’s disease. Prog Mol Biol Transl Sci. 127:183–210. doi: 10.1016/B978-0-12-394625-6.00007-6.
- Giacomello M, Pyakurel A, Glytsou C, Scorrano L. 2020. The cell biology of mitochondrial membrane dynamics. Nat Rev Mol Cell Biol. 21(4):204–224. doi: 10.1038/s41580-020-0210-7.
- He C, Yu Z, He Y, Xu C, Chang C, Yuan D. 2017. Purification of macroporous adsorption resin and decolorization of ion exchange resin of total saponins of Panax japonicus. Chia Tradit Herbal Drugs. 48:1146–1152.
- Hou YJ, Dan XL, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. 2019. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 15(10):565–581. doi: 10.1038/s41582-019-0244-7.
- Juan SMA, Adlard PA. 2019. Ageing and Cognition. Subcell Biochem. 91:107–122. doi: 10.1007/978-981-13-3681-2_5.
- Kou X, Li J, Liu X, Chang J, Zhao Q, Jia S, Fan J, Chen N. 2017. Swimming attenuated-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J Appl Physiol. 122(6):1462–1469. doi: 10.1152/japplphysiol.00018.2017.
- Li R, Robinson M, Ding X, Geetha T, Al-Nakkash L, Broderick TL, Babu JR. 2022. Genistein: a focus on several neurodegenerative diseases. J Food Biochem. 46(7):e14155. doi: 10.1111/jfbc.14155.
- Liu X, Cai Y, Cao X, Wei R, Li H, Zhou X, Zhang K, Wu S, Qian Q, Cheng B, et al. 2012. A new oncolytic adenoviral vector carrying dual tumour suppressor genes shows potent anti-tumour effect. J Cell Mol Med. 16(6):1298–1309. doi: 10.1111/j.1582-4934.2011.01396.x.
- Maharajan N, Cho GW. 2021. Camphorquinone promotes the antisenescence effect via activating AMPK/SIRT1 in stem cells and d-galactose-induced aging mice. Antioxidants. 10:1916. doi: 10.3390/antiox10121916.
- Manczak M, Calkins MJ, Hemachandra RP. 2011. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet. 20(13):2495–2509. doi: 10.1093/hmg/ddr139.
- Marcos-Rabal P, González-Fuentes J, Castro-Vázquez L, Lozano MV, Rodríguez-Robledo V, Santander-Ortega MJ, Selva-Clemente J, Villaseca-González N, Del Mar Arroyo-Jiménez M. 2021. Neurodegenerative diseases: a multidisciplinary approach. Curr Pharm Des. 27(30):3305–3336. doi: 10.2174/1381612827666210608152745.
- Mattson MP, Arumugam TV. 2018. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 27(6):1176–1199. doi: 10.1016/j.cmet.2018.05.011.
- Meng X, Wang M, Wang X, Sun G, Ye J, Xu H, Sun X. 2014. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res. 48(7):823–838. doi: 10.3109/10715762.2014.911853.
- OuYang LN, Xiang DW, Wu X, Xiang DX. 2010. Research progress on chemical constituents and pharmacological activities of Panax japonicus. Chinese Trad Herbal Drugs. 41:1023–1027.
- Panchal K, Tiwari AK. 2019. Mitochondrial dynamics, a key executioner in neurodegenerative diseases. Mitochondrion. 47:151–173. doi: 10.1016/j.mito.2018.11.002.
- Rana A, Oliveira MP, Khamoui AV, Aparicio R, Rera M, Rossiter HB, Walker DW. 2017. Promoting Drp1-mediated mitochondrial fission in midlife prolongs healthy lifespan of Drosophila melanogaster. Nat Commun. 8(1):448. doi: 10.1038/s41467-017-00525-4.
- Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ, Tieu K. 2014. Drp1 inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun. 5(1):5244. doi: 10.1038/ncomms6244.
- Reddy PH, Reddy TP. 2011. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res. 8(4):393–409. doi: 10.2174/156720511795745401.
- Sebastian D, Palacin M, Zorzano A. 2017. Mitochondrial dynamics: coupling mitochondrial fitness with healthy aging. Trends Mol Med. 23(3):201–215. doi: 10.1016/j.molmed.2017.01.003.
- Sharma A, Smith HJ, Yao P, Mair WB. 2019. Causal roles of mitochondrial dynamics in longevity and healthy aging. EMBO Rep. 20(12):e48395. doi: 10.15252/embr.201948395.
- Sisalli MJ, Ianniello G, Savoia C, Cuomo O, Annunziato L, Scorziello A. 2020. Knocking-out the Siah2 E3 ubiquitin ligase prevents mitochondrial NCX3 degradation, regulates mitochondrial fission and fusion, and restores mitochondrial function in hypoxic neurons. Cell Commun Signal. 18(1):42. doi: 10.1186/s12964-020-0529-x.
- Stavropoulos F, Sargiannidou I, Potamiti L, Kagiava A, Panayiotidis MI, Bae JH, Yeom SC, Lee JY, Kleopa KA. 2021. Aberrant mitochondrial dynamics and exacerbated response to neuroinflammation in a novel mouse model of CMT2A. Int J Mol Sci. 22:11569. doi: 10.3390/ijms222111569.
- Wan JZ, Wang R, Zhou ZY, Deng LL, Zhang CC, Liu CQ, Zhao HX, Yuan CF, He YM, Dun YY, et al. 2020. Saponins of Panax japonicus confer neuroprotection against brain aging through mitochondrial related oxidative stress and autophagy in rats. Curr Pharm Biotechnol. 21(8):667–680. doi: 10.2174/1389201021666191216114815.
- Wan JZ, Yuan D, Wang T. 2018. Study on the protective effect of Panax japonicus saponins on d-galactose induced SH-SY5Y neuron injury. Guizhou Med J. 42:387–389.
- Wan JZ, Deng LL, Zhang CC, Yuan Q, Liu J, Dun YY, Zhou ZY, Zhao HX, Liu C, Yuan D, et al. 2016. Chikusetsu saponin V attenuates H2O2-induced oxidative stress in human neuroblastoma SH-SY5Y cells through Sirt1/PGC-1alpha/Mn-SOD signaling pathways. Can J Physiol Pharmacol. 94(9):919–928. doi: 10.1139/cjpp-2015-0262.
- Wang K, Wu D, Ren X, Yu Q, Liu R, Huang S, Pan Y, Zhao S. 2021. Notoginsenoside R1 alleviates T EGDMA-induced mitochondrial apoptosis in preodontoblasts through activation of Akt/Nrf2 pathway-dependent mitophagy. Toxicol Appl Pharmacol;417:115482. doi: 10.1016/j.taap.2021.115482.
- Wang P, Deng JW, Dong J, Liu JH, Bigio EH, Mesulam M, Wang T, Sun L, Wang L, Lee AYL, et al. 2019. TDP-43 induces mitochondrial damage and activates the mitochondrial unfolded protein response. PLOS Genet. 15(5):e1007947. doi: 10.1371/journal.pgen.1007947.
- Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, Peng B, Liu C, Xiong G, Zhang C, et al. 2015. Saponins from Panax japonicus attenuate d-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 67(9):1284–1296. doi: 10.1111/jphp.12413.
- Wu YY, Liang CY, Liu TT, Liang YM, Li SJ, Lu YY, Liang J, Yuan X, Li CJ, Hou SZ, et al. 2018. Protective roles and mechanisms of polysaccharides from Dendrobium officinal on natural aging-induced premature ovarian failure. Biomed Pharmacother. 101:953–960. doi: 10.1016/j.biopha.2018.03.030.
- Yan J, Liu X, Han M, Wang Y, Sun X, Yu N, Li T, Su B, Chen Z. 2015. Blockage of GSK3β-mediated Drp1 phosphorylation provides neuroprotection in neuronal and mouse models of Alzheimer’s disease. Neurobiol Aging. 36(1):211–227. doi: 10.1016/j.neurobiolaging.2014.08.005.
- Yan MZ, Li SG. 2015. Effect of ginsenoside Rg3 on ageing of vascular smooth muscle cells and its mechanism. Chinese J Geriatric Heart Brain Vessel Dis. 17:1079–1082.
- Yuan D, Wan JZ, Deng LL, Zhang CC, Dun YY, Dai YW, Zhou ZY, Liu CQ, Wang T. 2014. Chikusetsu saponin V attenuates MPP+-induced neurotoxicity in SH-SY5Y cells via regulation of Sirt1/Mn-SOD and GRP78/caspase-12 pathways. Int J Mol Sci. 15(8):13209–13222. doi: 10.3390/ijms150813209.
- Zaninello M, Palikaras K, Sotiriou A, Tavernarakis N, Scorrano L. 2022. Sustained intracellular calcium rise mediates neuronal mitophagy in models of autosomal dominant optic atrophy. Cell Death Differ. 29(1):167–177. doi: 10.1038/s41418-021-00847-3.
- Zhou H, Ma J, Shan Y, Qi X, Wang H, Jia L. 2019. A combination of chicken embryo extract and a nutritional supplement protect a rat model of aging against d-galactose-induced dysfunction of mitochondria and autophagy. Food Funct. 10(5):2774–2784. doi: 10.1039/c8fo01734d.
