Abstract
Context
Si-Miao-Yong-An (SMYA) has been widely used for the clinical treatment of atherosclerosis (AS). Yet, its complete mechanism of action is not fully understood.
Objective
To investigate the mechanism by which SMYA stabilizes AS plaques from the perspective of inhibiting vasa vasorum (VV) angiogenesis.
Materials and methods
We used male ApoE-/- mice to establish an AS model. The mice were divided into model, SMYA (11.7 mg/kg/d), and simvastatin (SVTT) (2.6 mg/kg/d) groups. Mice were given SMYA or SVTT by daily gavage for 8 weeks. HE staining, immunofluorescence double-labelling staining, and immunohistochemical staining were used to observe the pathological changes in the plaques. Finally, the protein and mRNA expression levels of the Wnt1/β-catenin signalling pathway were detected by Western blot and qRT-PCR, respectively.
Results
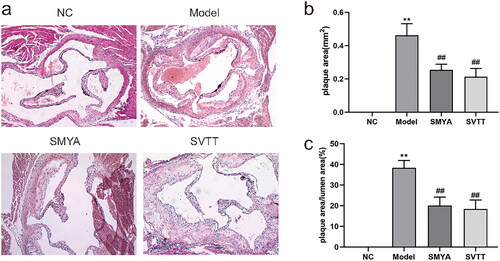
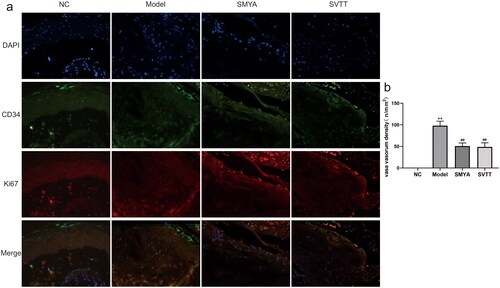
SMYA significantly attenuated cholesterol crystallization, and lipid accumulation in AS plaques, resulting in smaller plaque size (0.25 mm2 vs. 0.46 mm2), and lowering ratio of plaque to lumen area (20.04% vs. 38.33%) and VV density (50.64/mm2 vs. 98.02/mm2). Meanwhile, SMYA suppressed both the positive area percentage of Wnt1 (2.53 vs. 3.56), β-catenin (3.33 vs. 5.65) and Cyclin D1 (2.10 vs. 3.27) proteins in the aortic root plaques, and mRNA expression of Wnt1 (1.38 vs. 2.09), β-catenin (2.05 vs. 3.25) and Cyclin D1 (1.39 vs. 2.57).
Discussion and conclusions
SMYA has a protective effect against AS, which may be related to its anti-VV angiogenesis in plaques, suggesting that SMYA has the potential as a novel botanical formulation in the treatment of AS.
Introduction
Atherosclerosis (AS) is a chronic pathological process characterized by subintima lipid deposition and proliferation of vascular smooth muscle cells in large and medium arteries, ultimately leading to plaque formation and vascular stenosis, which is the common pathological basis of various cardiovascular diseases and has become one of the major threats to human health (Baumer et al. Citation2017). The current research on AS mainly involves autophagy and apoptosis, vascular smooth muscle cell proliferation, immune and inflammatory response, and other aspects (Cai et al. Citation2019; Fang et al. Citation2021). With the deepening of research, more and more studies have found that preventing plaque rupture and maintaining plaque stability is significant for inhibiting the development of AS and reducing the incidence of cardiovascular adverse events (Jinnouchi et al. Citation2020; Zhang et al. Citation2022).
Vasa vasorum (VV) angiogenesis within the plaque is significantly related to the stability of the plaque (Su et al. Citation2017; Huisman et al. Citation2021). Under physiological conditions, the vasa vasorum is only distributed in the adventitia and outer third of the media, and the nutrition and oxygen of the rest are provided by the arterial lumen. Under pathological conditions, compensatory thickening of the arterial intima can lead to local tissue hypoxia in the intima and media, and induce VV to extend into the AS plaque. However, the development of VV angiogenesis is not perfect, and it is easy to leak and bleed, aggravating the plaque’s instability (Sedding et al. Citation2018; Zorc-Pleskovic et al. Citation2018). The Wnt1/β-catenin signaling pathway is conserved during biological evolution and plays an important regulatory role in cell differentiation, growth and proliferation. When the Wnt1/β-catenin signaling pathway is inhibited in vascular endothelial cells, it can suppress cell proliferation and serve to enhance the stability of AS plaques (Yan et al. Citation2013; Birdsey et al. Citation2015).
Si-Miao-Yong-An (SMYA) is a traditional Chinese medicine (TCM) prescription. It consists of Lonicera japonica Thunb (Caprifoliaceae), Scrophularia microdonta Franch (Scrophulariaceae), Angelica sinensis Diels (Apiaceae), and Glycyrrhiza uralensis Fisch (Leguminosae), at a proportion of 3:3:2:1. Chlorogenic acid (flower buds from Lonicera japonica), harpagoside (dried root of Scrophularia microdonta), ferulic acid (angelica body from Angelica sinensis), and glycyrrhizic acid (root and rhizome from Glycyrrhiza uralensis) are the main active ingredients of SMYA, among which chlorogenic acid can inhibit the production of reactive oxygen species (ROS) and protect vascular endothelium. Glycyrrhizic acid can inhibit the proliferation of vascular smooth muscle cells and reduce the content of triglycerides and low-density lipoprotein in the blood (Fogelman et al. Citation2016; Jung et al. Citation2017; Qi J et al. Citation2020). Based on the structural characteristics of VV, clinical intervention of new VV can target two pathways: inhibiting new VV and promoting the maturation of new VV. Mature trophoblastic vessels have low permeability, low inflammatory status and rich recruitment of parietal cells (pericytes, smooth muscle cells) to new blood vessels. Our previous studies have clarified that SMYA decoction can promote the maturation of new VV and stabilize vulnerable plaques by recruiting pericytes and smooth muscle cells to migrate into vessels in plaques (Peng et al. Citation2012; Qi et al. Citation2019). Conversely, whether SMYA can stabilize vulnerable plaques by inhibiting VV angiogenesis has not been further investigated. In this study, ApoE-/- mice were used to establish an AS model and to explore the mechanism of SMYA stabilizing AS vulnerable plaques through Wnt1/β-catenin signaling pathway-mediated VV angiogenesis.
Materials and methods
Animals and experimental groups
Healthy 7-8 week-old male C57BL/6J mice (23.12 ± 1.18 g) and male ApoE-/- mice (22.97 ± 1.35 g) at the same age, were provided by Beijing Huafukang Bioscience Co., Ltd. (Beijing, China). The mice were raised in a specific-pathogen-free (SPF) environment, room temperature 22 ± 2 °C, relative humidity 50 ± 3%, defined circadian rhythm of 12 h light/dark cycle, free to eat and drink, and adaptive feeding for a week. The Animal Ethics Committee approved all animal experiments at Tianjin University of TCM (approval number: TCM-LAEC2018032).
ApoE-/- mice were fed with a high-fat diet (21% fat, 0.15% cholesterol) with 1.1% L-methionine for 16 weeks to establish an AS model (Zhou et al. Citation2001), and 10 C57BL/6J mice were fed with a regular diet as a normal control (NC) group. After 16 weeks, ApoE-/- mice were randomly divided into model, SMYA, and SVTT groups (n = 10). The SMYA group was given 11.7 mg/kg/d by gavage, and the SVTT group was given 2.6 mg/kg/d by gavage. The model and NC groups were given an equal volume of 20 mL/kg/d by gavage for 8 consecutive weeks. The administered dose follows the equivalent dose converted from the body surface area between humans and animals (Xu M et al. Citation2002). Each mouse was anesthetized with diethyl ether inhalation, blood was collected from the abdominal aorta, and the aortic root and part of the myocardial tissue were taken and stored at −80 °C refrigerator or fixed in 4% paraformaldehyde for later use.
Main drugs and reagents
The raw materials (Lot No. 151002) of SMYA were purchased in November 2019 from Anhui Xiehecheng Co., Ltd. (Bozhou, China), and verified by Professor Fang Liu of the Department of Pharmacy of the First Teaching Hospital of Tianjin University of TCM. Freeze-dried powder production was completed by the Department of Pharmacy of the First Teaching Hospital of Tianjin University of TCM, according to the original dose ratio (Lonicera japonica: Scrophularia microdonta: Angelica sinensis: Glycyrrhiza glabra = 3:3:2:1). The specific preparation method was carried out according to the method of Zhao et al. (Citation2020). We analyzed the freeze-dried powder by high-performance liquid chromatography (HPLC), the results were similar to those of Ren et al. (Citation2019), then tested the content of the active ingredients (chlorogenic acid, harpagoside, ferulic acid, and glycyrrhizic acid). The test results were in line with the standards of the Pharmacopoeia of the People's Republic of China published by China Science and Technology Publishing House (formulated by the Chinese Pharmacopoeia Commission in Beijing in 2015) (2015 edition).
SVTT was purchased from Mosadong Pharmaceutical Co., Ltd. (Hangzhou, China). Hematoxylin-eosin (HE) staining kit was purchased from Baihao Biotechnology Co., Ltd. (Tianjin, China). CD34 antibody was purchased from GeneTex (USA). DAB chromogenic kit was purchased from Zhongshan Jinqiao Biotechnology Co., Ltd. (Beijing, China). Ki67 antibody, β-catenin antibody and Fluoroshield mounting medium were purchased from Abcam (UK). Wnt1, Gsk-3β and Cyclin D1 antibodies were purchased from Boosen Biotechnology Co., Ltd. (Beijing, China). GAPDH antibody was purchased from Sanying Biotechnology Co., Ltd. (Wuhan, China). The Goat anti-rabbit IgG kit was purchased from Boster Biological Engineering Co., Ltd. (Wuhan, China). cDNA PrimeScript RT and SYBR Premix Ex Taq II were purchased from Baori Doctor Technology Co., Ltd. (Beijing, China).
Histomorphological observation
The prepared paraffin section of the aortic root tissue was first deparaffinized in xylene, dehydrated with concentration gradient ethanol (100%, 95%, 75%) and rinsed with distilled water. Then staining with hematoxylin, differentiation with 1% hydrochloric acid ethanol for 3 s, and 0.5% eosin staining after rinsing. Finally, the sections were dehydrated with ethanol, made to be transparent with xylene and sealed. The histomorphological changes were observed under an optical microscope (Olympus, BH-2, Japan).
Measurement of plaque/lumen area ratio
The prepared HE-stained sections of aortic root tissue were observed under a 100× optical microscope and the images were analyzed by Image-Pro Plus software (version 6.0). Then the ratio of plaque area (PA) to lumen area (LA) (PA/LA) was measured and statistical analysis.
Measurement of vasa vasorum density
Paraffin sections of dehydrated aortic roots were repaired with EDTA antigen retrieval solution (dilution 1:50) for 3 min, after being washed with PBS, incubated with 3% H2O2, then washed with PBS and drop 5% BSA blocking solution. The primary antibody solution was added (CD34 was diluted at 1:100, Ki67 diluted at 1:250. The NC group was replaced with 0.01 M, Ph = 7.4 PBS), and allowed to incubate overnight at 4 °C. On the second day, wash the slide with PBS, then add the secondary antibody (diluted 1:600). After incubating for 1 h, wash the slide with PBS. Finally, slide with Fluoroshield Mounting Medium with DAPI.
The images were observed and collected under a fluorescence upright microscope (Olympus, BX-51, Japan) and analyzed with Image-Pro Plus software (version 6.0). Randomly select 5 visual fields (400×) in the plaque area to measure the plaque area (PA, mm2), use CD34 as an endothelial cell marker and Ki67 as a proliferative cell marker to detect the number of VV (Q, n) in the plaque area. According to the formula microvascular density (MVD) = Q/PA, calculate the density of VV (n/mm2) (Passaro et al. Citation2017), and take the average value as the density of the VV of the specimen.
Immunohistochemistry
Drop primary antibody (Wnt1 was diluted at 1:600, β-catenin diluted at 1:500, and Cyclin D1 diluted at 1:600. The NC group was replaced with 0.01 M, pH = 7.4 PBS) into aortic root tissue treated with 5% BSA blocking solution, and allowed to incubate overnight at 4 °C. The next day, wash the slide with PBS and add biotin-labeled goat anti-rabbit IgG secondary antibody dropwise, incubate for 1 h, and then wash the slide with PBS. After adding the SABC reagent, incubate for 30 min, and wash the slide with PBS. Finally, DAB was used for chromogenic staining, followed by counterstain with hematoxylin, 3 s differentiation with 1% hydrochloric acid ethanol, dehydrated with ethanol and xylene after running water washing, and neutral gum sealing.
The images were observed and collected under an optical microscope. Randomly select 5 high-power visual fields (400×) plaque areas, and use Image-Pro Plus software (version 6.0) for semi-quantitative analysis. Calculate the percentage of Wnt1, β-catenin, Cyclin D1 protein positive expression area and plaque area, and calculate the average value for statistical analysis.
Western blot analysis
Protein was extracted from the aortic root tissue of each group using tissue protein lysate (RIPA: PMSF = 97:3) and denatured at 100 °C for 5 min. Equal amounts of protein were then separated by SDS-PAGE gel electrophoresis and transferred to PVDF membranes. Primary antibodies Wnt1 (1:1000), Gsk-3β (1:1000), β-catenin (1:1000), and Cyclin D1 (1:1000) were added. GAPDH (1:8000) was used for Wnt1, Gsk-3β, and β-catenin internal reference, and β-actin (1:8000) was used for Cyclin D1 internal reference, which was incubated overnight at 4 °C. After washing with 1 × TBST, they were incubated with horseradish peroxidase-labeled secondary antibody (goat anti-rabbit IgG, 1:5000) for 1 h at room temperature, placed in ECL developer (1:1), mixed well in the dark, and exposed with a Bio-Rad gel imager. The protein bands were analyzed by Gel-Pro software, and the results were expressed as the relative expression levels of the target protein and the internal reference. Each experiment was repeated at least three times.
Quantitative real-time PCR (qRT-PCR)
Portions of the aortic root tissues from mice were removed from the −80 °C freezer, and total RNA was extracted with a Trizol kit. The absorbance ratios (OD260/OD280) at 260 nm and 280 nm were measured by UV spectrophotometer and purified, and the RNA purity was evaluated by the A260/A280 ratio. When the ratio is 1.8–2.0, the RNA purity is high and suitable for qRT-PCR. Reverse transcription was performed using cDNA PrimeScript RT reagent and amplification was performed using SYBR Premix Ex Taq II according to the manufacturer’s instructions. According to the original qRT-PCR test results, the relative mRNA levels were calculated using the 2-ΔΔCt method. The qRT-PCR primer sequences are shown in .
Table 1. Primer sequences used for qRT-PCR analysis.
Statistical analysis
Statistical analysis of all results was performed using Prism8.0 software (GraphPad Software Inc., La Jolla, CA, USA), all data were expressed as mean ± standard deviation (SD), multiple comparisons were performed using One-Way ANOVA, Use Ordinary Test or Brown-Forsythe and Welch ANOVA Tests, and p < 0.05 was considered statistically significant.
Results
SMYA reduces the ratio of aortic PA/LA and stabilizes plaques in AS mice
The results of HE staining showed that the aortic wall of mice in the NC group had a clear structure hierarchy, smooth and intact intima, no lipid deposition and vascular stenosis. Compared with the NC group, the aortic intima of mice in the model group was significantly thickened, with AS plaque formation, a large number of foam cells, cholesterol crystallization and lipid accumulation in the plaque, and plaque rupture and bleeding. Compared with the model group, the aortic plaque area was significantly reduced, foam cell infiltration in the plaque was alleviated, cholesterol crystals and lipid accumulation were reduced, and the degree of intimal thickening was mild in the SMYA and SVTT groups (). The ratio of aortic PA and PA/LA was significantly decreased (p < 0.01) ().
Figure 1. Effects of Si-Miao-Yong-an (SMYA) on aortic root plaque stability and the ratio of PA/LA in mice. (a) HE staining was used to observe as in ApoE−/− mice treated with SMYA. In the model group, the aortic intima was significantly thickened, as plaques were formed, a large number of foam cells, cholesterol crystals and lipids accumulated in the plaques, and plaques ruptured and bled. After the intervention of SMYA, the plaque area was reduced, the foam cell infiltration in the plaque was alleviated, the cholesterol crystals and lipid accumulation were reduced, and the degree of intimal thickening was mild (optical microscope, ×100). (b) after the intervention of SMYA, the ratio of aortic plaque area (PA) was significantly decreased. (c) after the intervention of SMYA, the ratio of aortic plaque area (PA) to lumen area (LA) (PA/LA) was significantly decreased. Data are shown as mean ± SD. **p < 0.01, compared with the normal control (NC) group. ##p < 0.01, compared with the model group.
SMYA reduces the density of vasa vasorum in aortic plaques of as mice
The results of immunofluorescence double labelling staining showed that no plaques were observed in the aortic root of the NC group. The positive staining density of CD34 and Ki67 in plaques was significantly higher in the model group, but significantly lower in the SMYA and SVTT groups (). The density of VV was calculated by an upright fluorescence microscope. Compared with the NC group, the density of VV was significantly increased in the model group. Compared with the model group, the density of VV in the plaques decreased significantly after SMYA intervention (p < 0.01) ().
Figure 2. Effects of SMYA on vasa vasorum angiogenesis in aortic root plaques of as mice. (a) Immunofluorescence double staining was used to observe the density of vasa vasorum in aortic root plaques, CD34 was used as an endothelial cell marker, and Ki67 was used as a proliferating cell marker. The results showed that the positive staining density of CD34 and Ki67 in as plaques were significantly reduced after SMYA intervention (fluorescent microscopy, ×400). (b) after SMYA intervention, the density of vasa vasorum in the aortic root plaque was significantly reduced. Data are shown as mean ± SD. **p < 0.01, compared with the normal control (NC) group. ##p < 0.01, compared with the model group.
SMYA inhibits the expression of Wnt1, β-catenin and Cyclin D1 protein in aortic plaques of as mice
Immunohistochemical staining of paraffin sections of aortic roots was observed under an optical microscope, and the positive expression of Wnt1 protein was mainly localized in the cell membrane and cytoplasm, the positive expression of the β-catenin protein was mainly localized in the cytoplasm and nucleus, and the positive expression of Cyclin D1 protein was mainly localized in the nucleus, all of which showed brownish-yellow granules ().
Figure 3. Effects of Si-Miao-Yong-an (SMYA) on the percentage of the positive area of Wnt1, β-catenin and Cyclin D1 proteins in the aortic root plaques of mice. (a) the Localization of Wnt1, β-catenin and Cyclin D1 proteins in mice as plaques were observed by immunohistochemical staining. Wnt1 protein was mainly located in the cell membrane and cytoplasm, the β-catenin protein was mainly located in the cytoplasm and nucleus, and Cyclin D1 protein was mainly located in the nucleus, all of which showed brown granules (optical microscope, ×400). (b-d) after SMYA intervention, the percentages of Wnt1, β-catenin and Cyclin D1 protein-positive areas were significantly reduced. Data are shown as mean ± SD. **p < 0.01, compared with the normal control (NC) group. ##p < 0.01, compared with the model group.
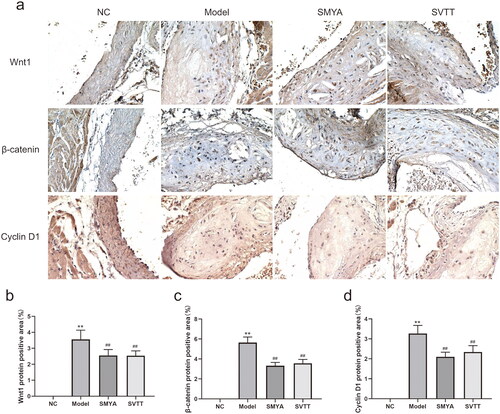
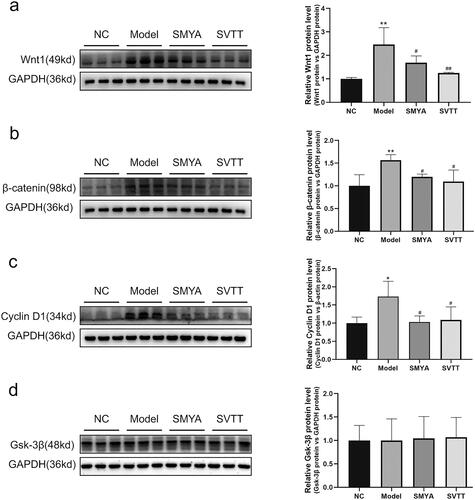
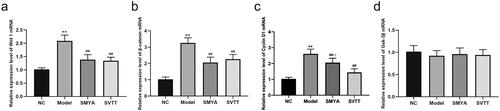
Compared with the NC group, the percentage of positive areas of Wnt1, β-catenin, and Cyclin D1 proteins in the aortic root plaques of mice in the model group was significantly increased (p < 0.01). Compared with the model group, the percentage of positive areas of Wnt1, β-catenin, and Cyclin D1 proteins in the aortic root plaques of mice in the SMYA and SVTT groups was significantly decreased (p < 0.01) (). At the same time, the expression of Wnt1, β-catenin, and Cyclin D1 proteins was quantitatively analyzed by Western blot, and the results were consistent with immunohistochemical staining (). In addition, there was no significant change in the expression of the Gsk-3β protein ().
Figure 4. Effects of Si-Miao-Yong-an (SMYA) on protein expression of the wnt1/β-catenin signaling pathway in aortic of as mice. Western blot analysis of Wnt1, β-catenin, Cyclin D1 and Gsk-3β. After SMYA intervention, the protein expression of Wnt1, β-catenin and Cyclin D1 was significantly decreased, while the expression of Gsk-3β protein had no significant change among the groups. Data are shown as mean ± SD. **p < 0.01, *p < 0.05, compared with the normal control (NC) group. ##p < 0.01, #p < 0.05, compared with the model group.
SMYA inhibits the expression of wnt1/β-catenin signaling pathway mRNA in the aortic of AS mice
Compared with the NC group, the aortic Wnt1, β-catenin, and CyclinD1 mRNA expression levels were significantly increased in the model group (p < 0.01), while the Gsk-3β mRNA expression level was not significantly different (p > 0.05). Compared with the model group, the aortic Wnt1, β-catenin, and CyclinD1 mRNA expression levels were significantly decreased in the SMYA and SVTT groups (p < 0.01), but there was no significant difference in the Gsk-3β mRNA expression (p > 0.05). Compared with the SVTT group, the aortic Cyclin D1 mRNA expression level was more significantly decreased in the SMYA group (p < 0.05) ().
Figure 5. Effects of Si-Miao-Yong-an (SMYA) on mRNA expression of the wnt1/β-catenin signalling pathway in aortic of AS mice. After SMYA intervention, the aortic Wnt1, β-catenin, and Cyclin D1 mRNA expression levels were significantly decreased in mice. while the expression of Gsk-3β mRNA had no significant change among the groups. Data are shown as mean ± SD. **p < 0.01, compared with the normal control (NC) group. ##p < 0.01, compared with the model group. △p < 0.05, compared with the SVTT group.
Discussion
AS is the common pathological basis of various cardiovascular and cerebrovascular diseases such as coronary heart disease and stroke, and the pathological mechanism is more complex, which is currently recognized as the injury response theory, the infiltration theory, the chronic inflammation theory and the thrombosis theory (Chen W et al. Citation2018). AS mainly occurs in the large and middle arteries, and its pathological manifestations are lipid deposition in the intima and subintima of the arterial wall and its branches, accompanied by the proliferation and migration of medial smooth muscle cells to the subintima, which thickens the intima and forms AS plaque. AS is a chronic dynamic process, and different development stages from AS to plaque formation are accompanied by different pathological changes such as endothelial dysfunction, lipid deposition, inflammation, immune response, and thrombosis, and plaque rupture or bleeding is the main cause of adverse cardiovascular events caused by AS (Chiu et al. Citation2012; Lin L et al. Citation2021).
TCM espouses that SMYA has the functions of clearing heat and resolving toxins, promoting blood circulation and alleviating pain. It was originally a special prescription for the treatment of gangrene. In recent years, integrated traditional Chinese and Western medicine has provided a deeper understanding of AS disease, SMYA has been used to treat a variety of vascular diseases, such as coronary heart disease, stroke, hypertension, and peripheral vascular disease, with significant clinical efficacy (Li L et al. Citation2020). After previous studies, our team found that SMYA could stabilize AS vulnerable plaques by reducing intraplaque lipid content and increasing fibrous cap thickness (Qi Z et al. Citation2019; Li M et al. Citation2021). In addition, SMYA can also inhibit the serum levels of interleukin 1β (IL-1β) and interleukin 8 (IL-8), reduce the expression of soluble intercellular adhesion molecule-1 (ICAM-1) in the rabbit AS model (Peng et al. Citation2012). These results suggest that SMYA can antagonize AS inflammation and stabilize AS plaques. This study showed that SMYA could significantly improve the morphological changes of the aortic root in AS mice, reduce the degree of foam cell infiltration and accumulation, diminish the area of AS plaques and the ratio of PA/LA, and alleviate the degree of intimal thickening, suggesting that SMYA may have an effect on the stability of coronary vulnerable plaques.
VV refers to a layer of the tiny vascular network surrounding the adventitia of arterial vessels, which can provide oxygen and nutrients for the vascular wall and maintain the metabolism and energy balance of the vascular wall. Upon stimulation by factors such as a hypoxic environment and inflammation, the VV will gradually extend into the media or even intima and grow into the interior of AS plaques (Xu et al. Citation2015; Sedding et al. Citation2018). The density of intracoronary VV increases during the early stages of AS development when endothelial function impairment is aggravated (Mollmark et al. Citation2012). The VV in the plaque belong to pathological neovascularization, which is not well-developed, the structure of the vascular wall is immature, there is only endothelial cell composition, lack of smooth muscle cell and pericyte support, it is easy to rupture and bleed, and then cause plaque rupture (Sedding et al. Citation2018). We used immunofluorescence double-labelled staining to observe intraplaque VV angiogenesis. CD34 is a commonly used vascular endothelial cell marker (Siemerink et al. Citation2012). Ki67 is closely associated with cell growth and proliferation, which is widely used to label nascent cells (Li LT et al. Citation2015). We performed double labelling of tissue sections for CD34 and Ki67 enabling the observation of VV angiogenesis within the plaque. The results of immunofluorescence double labelling staining in this study showed that SMYA could reduce the density of VV in aortic plaques of AS mice, suggesting that SMYA can inhibit intraplaque VV angiogenesis to improve the stability of vulnerable plaques. In previously relevant animal models, SVTT was found to reduce VV density in the left anterior descending coronary artery of porcine, alleviate hypoxic conditions in the coronary vessel wall, and reduce HIF-1α and VEGF expression in the coronary adventitia, thereby stabilizing AS plaques (Wilson et al. Citation2002). Another study also found that SVTT increased microvascular density in myocardial ischemia area and improved cardiac function, it also reduced the density of new VV in aortic plaques and inhibited HIF-1α and VEGF protein expression in aorta, suggesting that SVTT has a dual regulatory effect on angiogenesis in ischemic myocardium and AS plaques after myocardial infarction (Shen et al. Citation2011). Combined with our results, we identified that SMYA, a classic prescription of TCM, also has a similar bidirectional regulation of VV by SVTT.
Angiogenesis is a complex process that is regulated by both pro-angiogenic factors and anti-angiogenic factors (Li L et al. Citation2020). Wnt protein is a cysteine-rich secreted protein consisting of 350-400 amino acids that exhibit their distinct functions at various stages of body growth and development (MacDonald et al. Citation2009; Chen et al. Citation2019). Wnt proteins have been discovered, which are divided into Wnt1 classes with high transforming ability and Wnt5a classes with moderate or no transforming ability (Tepekoy et al. Citation2015). Wnt1 class proteins tend to propagate signals through the canonical Wnt pathway, while Wnt5a class mostly tends to activate the Wnt/Ca2+ pathway, which is a non-canonical Wnt pathway (Kawano et al. Citation2009; van Loon et al. Citation2021). To investigate whether SMYA intervenes in intraplaque VV angiogenesis by regulating the Wnt1/β-catenin signalling pathway, we selected several key points, Wnt1, Gsk-3β, β-catenin and Cyclin D1 for the study. Among them, Wnt1 is the initiation factor, Gsk-3β is a negative regulator of this pathway, β-catenin is the most critical node factor, and Cyclin D1 is the target gene (Chen et al. Citation2019; Guo et al. Citation2019).
Gsk-3β is a serine kinase that plays a regulatory role in cell proliferation, growth, migration, apoptosis as well as gene expression. Activation of Gsk-3β phosphorylates and degrades β-catenin, the most critical protein factor in the Wnt pathway, which inhibits downstream of the Wnt1/β-catenin signalling pathway (Lin et al. Citation2020). Cyclin D1 is a key protein that regulates the G1 phase, which can push the cell cycle from the G1 phase into the S phase and accelerate the rate of cell proliferation (Tchakarska and Sola Citation2020). In endothelial cells, when stimulated by Wnt1 signalling, Wnt1 protein will bind to frizzled (FZD) or low-density lipoprotein-receptor-related protein (LRP), inhibit the degradation of β-catenin in the cytoplasm, increase the number of β-catenin in the cytoplasm to promote its transfer into the nucleus, and interact with T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) in the nucleus to activate the target gene Cyclin D1, shorten the growth cycle of endothelial cells, accelerate the proliferation rate of endothelial cells, and promote angiogenesis (Liu et al. Citation2011; Li et al. Citation2016; van Loon et al. Citation2021). The results of this study showed that SMYA could significantly reduce the expression levels of Wnt1, β-catenin, and Cyclin D1 protein in aortic plaques of AS mice, as well as Wnt1, β-catenin, and Cyclin D1 mRNA levels in aortic tissues. Moreover, SMYA was more significant with Simvastatin in reducing Cyclin D1 mRNA in the aorta. In this study, there was no significant difference in Gsk-3β mRNA levels in the aortic tissues of AS mice among the groups, suggesting that Gsk-3β was not significantly abnormally expressed at the genetic level in the ApoE−/− mice AS model. These results indicate that in the ApoE −/− AS model, SMYA can inhibit the expression of Wnt1/β-catenin signalling pathway to intervene in intraplaque VV angiogenesis and thus stabilize AS vulnerable plaques (). What is the role of SMYA in angiogenesis and stabilizing atherosclerotic plaque when Wnt1/β-catenin signaling pathway is inhibited or activated? This is a deficiency of the present study, in the future, the specific mechanism of interaction of SMYA will be investigated in depth by adding Wnt1 activators or inhibitors.
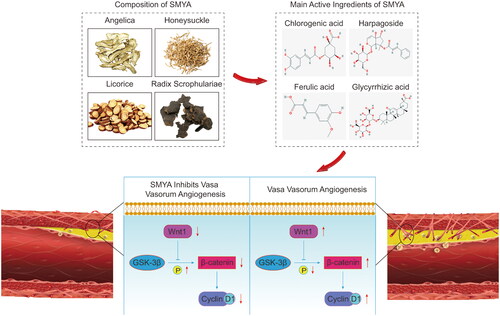
Figure 6. Si-Miao-Yong-an (SMYA) inhibits vasa vasorum angiogenesis through the Wnt1/β-catenin signalling pathway. SMYA is Composed of honeysuckle, Radix Scrophulariae, Angelica, and licorice. Chlorogenic acid, harpagoside, ferulic acid, and glycyrrhizic acid are the main active components of SMYA in the treatment of atherosclerosis. This study confirmed that SMYA can reduce foam cell infiltration, lipid deposition, and smooth muscle cell proliferation, thereby reducing the ratio of plaque area (PA) to lumen area (LA) (PA/LA), which may be related to SMYA inhibiting vasa vasorum angiogenesis through the Wnt1/β-catenin signalling pathway. The molecular structure of the main active components of SMYA is derived from the Traditional Chinese Medicine systems pharmacology database and analysis platform (https://old.tcmsp-e.com/tcmsp.php).
Conclusions
In this study, ApoE−/− mice were used to establish an AS vulnerable plaque model, and by observing the effect of SMYA on plaque morphology in the aortic root of mice, our findings showed that SMYA had the effect of stabilizing AS plaques, and intraplaque VV angiogenesis was used as the research direction, and the Wnt1/β-catenin signalling pathway was used as the starting point to carry out a mechanistic study, providing a basis for revealing the protective effect of SMYA in AS diseases.
Authors contributions
Zhongwen Qi, Zhipeng Yan, Ke Zhu, and Junping Zhang participated in the design and implementation of the study. Zhongwen Qi and Zhipeng Yan participated in the processing of research data, Figures production and manuscript writing. Yueyao Wang, Yajie Fang and Tingting Li participated in the collection of references, Junping Zhang participated in the revision of the manuscript.
Acknowledgments
The authors thank the Experimental Center of the First Teaching Hospital of Tianjin University of Traditional Chinese Medicine and the Animal Center of Tianjin Hospital of Integrative Medicine for their expertise and technical help.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Baumer Y, McCurdy S, Weatherby TM, Mehta NN, Halbherr S, Halbherr P, Yamazaki N, Boisvert WA. 2017. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun. 8(1):1129–1142. doi: 10.1038/s41467-017-01186-z.
- Birdsey GM, Shah AV, Dufton N, Reynolds LE, Osuna Almagro L, Yang Y, Aspalter IM, Khan ST, Mason JC, Dejana E, et al. 2015. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/beta-catenin signaling. Dev Cell. 32(1):82–96. doi: 10.1016/j.devcel.2014.11.016.
- Cai T, Cui X, Zhang K, Zhang A, Liu B, Mu JJ. 2019. LncRNA TNK2-AS1 regulated ox-LDL-stimulated HASMC proliferation and migration via modulating VEGFA and FGF1 expression by sponging miR-150-5p. J Cell Mol Med. 23(11):7289–7298. doi: 10.1111/jcmm.14575.
- Chen W, Newman AB, Fried LF, Rifkin DE, Shlipak MG, Sarnak MJ, Katz R, Madero M, Raphael KL, Bushinsky DA, et al. 2018. Relationship of acid-base status with arterial stiffness in community-living elders: the health ABC study. Nephrol Dial Transplant. 33:1572–1579.
- Chen WR, Zhou YJ, Yang JQ, Liu F, Zhao YX, Sha Y. 2019. Melatonin attenuates beta-glycerophosphate-induced calcification of vascular smooth muscle cells via a Wnt1/beta-catenin signaling pathway. Biomed Res Int. 2019:3139496. doi: 10.1155/2019/3139496.
- Chiu CA, Yu TH, Hung WC, Lu LF, Chung FM, Tsai IT, Yang CY, Hsu CC, Lu YC, Houng JY, et al. 2012. Increased expression of visfatin in monocytes and macrophages in male acute myocardial infarction patients. Mediators Inflamm. 2012:469852. doi: 10.1155/2012/469852.
- Fang S, Wan X, Zou X, Sun S, Hao X, Liang C, Zhang Z, Zhang F, Sun B, Li H, et al. 2021. Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis. 12(1):88. doi: 10.1038/s41419-020-03357-1.
- Fogelman Y, Gaitini D, Carmeli E. 2016. Antiatherosclerotic effects of licorice extract supplementation on hypercholesterolemic patients: decreased CIMT, reduced plasma lipid levels, and decreased blood pressure. Food Nutr Res. 60:30830. doi: 10.3402/fnr.v60.30830.
- Guo Q, Zhong W, Duan A, Sun G, Cui W, Zhuang X, Liu L. 2019. Protective or deleterious role of Wnt/beta-catenin signaling in diabetic nephropathy: an unresolved issue. Pharmacol Res. 144:151–157. doi: 10.1016/j.phrs.2019.03.022.
- Huisman LA, Steinkamp PJ, Hillebrands JL, Zeebregts CJ, Linssen MD, Jorritsma-Smit A, Slart R, van Dam GM, Boersma HH. 2021. Feasibility of ex vivo fluorescence imaging of angiogenesis in (non-) culprit human carotid atherosclerotic plaques using bevacizumab-800CW. Sci Rep. 11(1):2899–2909. doi: 10.1038/s41598-021-82568-8.
- Jinnouchi H, Sato Y, Sakamoto A, Cornelissen A, Mori M, Kawakami R, Gadhoke NV, Kolodgie FD, Virmani R, Finn AV. 2020. Calcium deposition within coronary atherosclerotic lesion: implications for plaque stability. Atherosclerosis. 306:85–95. doi: 10.1016/j.atherosclerosis.2020.05.017.
- Jung HJ, Im SS, Song DK, Bae JH. 2017. Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Rep. 50(6):323–328. doi: 10.5483/bmbrep.2017.50.6.182.
- Kawano Y, Diez S, Uysal-Onganer P, Darrington RS, Waxman J, Kypta RM. 2009. Secreted Frizzled-related protein-1 is a negative regulator of androgen receptor activity in prostate cancer. Br J Cancer. 100(7):1165–1174. doi: 10.1038/sj.bjc.6604976.
- Li L, Chen X, Su C, Wang Q, Li R, Jiao W, Luo H, Tian Y, Tang J, Li X, et al. 2020. Si-Miao-Yong-An decoction preserves cardiac function and regulates GLC/AMPK/NF-kappaB and GLC/PPARalpha/PGC-1alpha pathways in diabetic mice. Biomed Pharmacother. 132:110817. doi: 10.1016/j.biopha.2020.110817.
- Li LT, Jiang G, Chen Q, Zheng JN. 2015. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 11(3):1566–1572. doi: 10.3892/mmr.2014.2914.
- Li M, Qi Z, Zhang J, Zhu K, Wang Y. 2021. Effect and mechanism of Si-Miao-Yong-An on vasa vasorum remodeling in ApoE(-/-) mice with atherosclerosis vulnerable plague. Front Pharmacol. 12:634611. doi: 10.3389/fphar.2021.634611.
- Li Y, Jin K, van Pelt GW, van Dam H, Yu X, Mesker WE, Ten Dijke P, Zhou F, Zhang L. 2016. c-Myb Enhances breast cancer invasion and metastasis through the Wnt/β-catenin/axin2 pathway. Cancer Res. 76(11):3364–3375. doi: 10.1158/0008-5472.CAN-15-2302.
- Lin J, Song T, Li C, Mao W. 2020. GSK-3beta in DNA repair, apoptosis, and resistance of chemotherapy, radiotherapy of cancer. Biochim Biophys Acta Mol Cell Res. 1867(5):118659. doi: 10.1016/j.bbamcr.2020.118659.
- Lin L, Xie Z, Xu M, Wang Y, Li S, Yang N, Gong X, Liang P, Zhang X, Song L, et al. 2021. IVUS\IVPA hybrid intravascular molecular imaging of angiogenesis in atherosclerotic plaques via RGDfk peptide-targeted nanoprobes. Photoacoustics. 22:100262. doi: 10.1016/j.pacs.2021.100262.
- Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X, Li Z, Bian A, Wang X, Liu D, et al. 2011. Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med. 11(2):105–112. doi: 10.1007/s10238-010-0110-9.
- MacDonald BT, Tamai K, He X. 2009. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 17(1):9–26. doi: 10.1016/j.devcel.2009.06.016.
- Mollmark JI, Park AJ, Kim J, Wang TZ, Katzenell S, Shipman SL, Zagorchev LG, Simons M, Mulligan-Kehoe MJ. 2012. Fibroblast growth factor-2 is required for vasa vasorum plexus stability in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 32(11):2644–2651. doi: 10.1161/ATVBAHA.112.252544.
- Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, Montaner B, Chakravarty P, Bhaw L, Diana G, et al. 2017. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell. 32(3):324–341.e6. doi: 10.1016/j.ccell.2017.08.001.
- Peng L, Li M, Xu YZ, Zhang GY, Yang C, Zhou YN, Li LJ, Zhang JP. 2012. Effect of Si-Miao-Yong-An on the stability of atherosclerotic plaque in a diet-induced rabbit model. J Ethnopharmacol. 143(1):241–248. doi: 10.1016/j.jep.2012.06.030.
- Qi J, Cui J, Mi B, Yan X, Xu W, Ma H, Zhang Q, Xu F. 2020. Isoliquiritigenin inhibits atherosclerosis by blocking TRPC5 channel expression. Cardiovasc Ther. 2020:1926249. doi: 10.1155/2020/1926249.
- Qi Z, Li M, Zhu K, Zhang J. 2019. Si-Miao-Yong-An on promoting the maturation of vasa vasorum and stabilizing atherosclerotic plaque in ApoE(-/-) mice: an experimental study. Biomed Pharmacother. 114:108785. doi: 10.1016/j.biopha.2019.108785.
- Ren Y, Chen X, Li P, Zhang H, Su C, Zeng Z, Wu Y, Xie X, Wang Q, Han J, et al. 2019. Si-Miao-Yong-An decoction ameliorates cardiac function through restoring the equilibrium of SOD and NOX2 in heart failure mice. Pharmacol Res. 146:104318. doi: 10.1016/j.phrs.2019.104318.
- Sedding DG, Boyle EC, Demandt JAF, Sluimer JC, Dutzmann J, Haverich A, Bauersachs J. 2018. Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol. 9:706. doi: 10.3389/fimmu.2018.00706.
- Shen W, Shi HM, Fan WH, Luo XP, Jin B, Li Y. 2011. The effects of simvastatin on angiogenesis: studied by an original model of atherosclerosis and acute myocardial infarction in rabbit. Mol Biol Rep. 38(6):3821–3828. doi: 10.1007/s11033-010-0497-0.
- Siemerink MJ, Klaassen I, Vogels IM, Griffioen AW, Van Noorden CJ, Schlingemann RO. 2012. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis. 15(1):151–163. doi: 10.1007/s10456-011-9251-z.
- Su T, Wang YB, Han D, Wang J, Qi S, Gao L, Shao YH, Qiao HY, Chen JW, Liang SH, et al. 2017. Multimodality imaging of angiogenesis in a rabbit atherosclerotic model by GEBP11 peptide targeted nanoparticles. Theranostics. 7(19):4791–4804. doi: 10.7150/thno.20767.
- Tchakarska G, Sola B. 2020. The double dealing of cyclin D1. Cell Cycle. 19(2):163–178. doi: 10.1080/15384101.2019.1706903.
- Tepekoy F, Akkoyunlu G, Demir R. 2015. The role of Wnt signaling members in the uterus and embryo during pre-implantation and implantation. J Assist Reprod Genet. 32(3):337–346. doi: 10.1007/s10815-014-0409-7.
- Van Loon K, Huijbers EJM, Griffioen AW. 2021. Secreted frizzled-related protein 2: a key player in noncanonical Wnt signaling and tumor angiogenesis. Cancer Metastasis Rev. 40(1):191–203. doi: 10.1007/s10555-020-09941-3.
- Wilson SH, Herrmann J, Lerman LO, Holmes DR, Jr., Napoli C, Ritman EL, Lerman A. 2002. Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation. 105(4):415–418. doi: 10.1161/hc0402.104119.
- Xu J, Lu X, Shi GP. 2015. Vasa vasorum in atherosclerosis and clinical significance. Int J Mol Sci. 16(5):11574–11608. doi: 10.3390/ijms160511574.
- Xu M, Jin YL, Fu J, Huang H, Chen SZ, Qu P, Tian HM, Liu ZY, Zhang W. 2002. The abnormal expression of retinoic acid receptor-beta, p53 and Ki67 protein in normal, premalignant and malignant esophageal tissues. World J Gastroenterol. 8(2):200–202. doi: 10.3748/wjg.v8.i2.200.
- Yan Z, Zhu Z, Wang J, Sun J, Chen Y, Yang G, Chen W, Deng Y. 2013. Synthesis, characterization, and evaluation of a novel inhibitor of WNT/beta-catenin signaling pathway. Mol Cancer. 12(1):116. doi: 10.1186/1476-4598-12-116.
- Zhang S, Liu Y, Cao Y, Zhang S, Sun J, Wang Y, Song S, Zhang H. 2022. Targeting the microenvironment of vulnerable atherosclerotic plaques: an emerging diagnosis and therapy strategy for atherosclerosis. Adv Mater. 34(29):e2110660. doi: 10.1002/adma.202110660.
- Zhao Y, Sun D, Chen Y, Zhan K, Meng Q, Zhang X, Zhu L, Yao X. 2020. Si-Miao-Yong-An Decoction attenuates isoprenaline-induced myocardial fibrosis in AMPK-driven Akt/mTOR and TGF-β/SMAD3 pathways. Biomed Pharmacother. 130:110522. doi: 10.1016/j.biopha.2020.110522.
- Zhou J, Moller J, Danielsen CC, Bentzon J, Ravn HB, Austin RC, Falk E. 2001. Dietary supplementation with methionine and homocysteine promotes early atherosclerosis but not plaque rupture in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 21(9):1470–1476. doi: 10.1161/hq0901.096582.
- Zorc-Pleskovic R, Pleskovic A, Vraspir-Porenta O, Zorc M, Milutinovic A. 2018. Immune cells and vasa vasorum in the tunica media of atherosclerotic coronary arteries. Bosn J Basic Med Sci. 18(3):240–245. doi: 10.17305/bjbms.2018.2951.
