Abstract
Context
Duhuo Jisheng pill (DHJS) is a classic traditional Chinese medicine (TCM) formula for rheumatoid arthritis (RA). The effective components and therapeutic mechanisms of DHJS for treating RA are still unclear.
Objective
To explore the potential mechanism of DHJS against RA by means of network pharmacology and experimental verification.
Materials and methods
A network pharmacology and molecular docking analysis based on phytochemistry was used to elucidate the mechanism of DHJS against RA. The targets of DHJS anti-RA active ingredient were obtained by searching TCMSP, ETCM and TCMSID. The RA model induced by collagen was established in Wistar rats. The rats in the DHJS group were administered doses of 0.5, 1.0 and 2.0 g/kg for a period of 10 d. The expression of targets was measured with Western blot.
Results
Network pharmacology analysis showed that the anti-RA effect of DHJS was mediated by targets involved in immunity, inflammation and apoptosis, as well as PI3K-Akt and NF-κB signalling pathways. Of 2.0 g/kg DHJS significantly alleviated the ankle inflammation (IL-6: 62.73 ± 8.39 pg/mL, IL-1β: 50.49 ± 11.47 pg/mL, TNF-α: 16.88 ± 3.05 pg/mL, IL-17A: 12.55 ± 1.87 pg/mL, IL-10: 16.24 ± 3.00 pg/mL), comparing with the model group (IL-6: 92.02 ± 13.25 pg/mL, IL-1β: 71.85 ± 4.12 pg/mL, TNF-α: 25.64 ± 3.69 pg/mL, IL-17A: 22.14 ± 4.56 pg/mL, IL-10: 9.51 ± 3.03 pg/mL) (p < 0.05). Moreover, the protein expression of p-PI3K, p-AKT and p-p65 significantly decreased after DHJS administration.
Conclusions
DHJS could alleviate the collagen-induced arthritis (CIA) by the PI3K/AKT/NF-κB signalling pathway.
Introduction
Rheumatoid arthritis (RA), a complex autoimmune disease, is characterized pathologically by synovial hyperplasia and inflammatory cytokine-mediated joint destruction (Yang et al. Citation2018). According to the WHO, the morbidity of RA accounts for 0.5–1% of the global population (Firestein and McInnes Citation2017; Wang X et al. Citation2020). Despite real advances have been made in the treatment of RA over the past decade, a considerable number of patients have not yet achieved sustained remission (Liu, Huang, et al. Citation2018; Liu, Zhang, et al. Citation2018). Worse, the progression of RA can cause damage to the heart, lungs, kidneys and arteries (Liu and Wang Citation2014). The pathogenesis of RA is still not clarified completely, but it is thought to involve factors, such as genetic factors, environmental conditions and immunity or infection (Xin et al. Citation2020). The pathophysiological mechanisms of RA are mediated by inflammatory cytokines, including IL‑17, IL‑1β, IL‑6 and TNF‑α (Fernandez-Ruiz et al. Citation2018). At present, conventional treatments for patients with RA, including nonsteroidal anti-inflammatory drug, non-steroidal drugs, glucocorticoid and new biological agents, are unsatisfactory (Zheng et al. Citation2018). Therefore, it is urgent to deepen the understanding of the pathogenesis of RA and seek effective and safe treatment strategies.
According to the theory of traditional Chinese medicine (TCM), the occurrence and development of RA is stimulated by wind, cold and dampness, which belongs to arthralgia syndrome. Its symptoms are mainly hepatorenal insufficiency or deficiency of qi and blood. TCM has been used in clinic all over the world for more than 1000 years (Ding et al. Citation2016). Duhuo Jisheng pill (DHJS) is a Tang Dynasty prescription recorded in Bei Ji Qian Jin Yao Fang compiled by Sun Simiao. It consists of 15 commonly used herbs (Li et al. Citation2020; Liu et al. Citation2020). DHJS has the functions of expelling wind, dispersing cold and removing dampness, tonifying liver and kidney and nourishing qi and blood (Zhang et al. Citation2016). It can treat arthralgia syndrome, especially weakness, pain and stiffness in the joints, lower back and knees caused by rheumatism (Liu et al. Citation2021). But, the active compounds and potential molecular mechanisms of DHJS for the treatment of RA remain unclear.
At present, traditional herbal medicines have demonstrated promising anti-RA effects through multiple components, multiple targets and multiple approaches (Jo et al. Citation2022). However, the main components of herbal medicines used to treat RA are very complex, including alkaloids, flavonoids, terpenes, phenylalanine, etc. Moreover, their main pharmacological effects are to relieve pain, improve inflammatory reactions, regulate immune function, protect cartilage and inhibit synovial hyperplasia, etc. (Wang et al. Citation2021). Therefore, it is difficult to reveal the anti-RA mechanism of herbal medicines using traditional research methods.
In this study, the bioactive compounds of DHJS and its potential mechanisms against RA were investigated by the strategy of integrating network pharmacology and molecular docking. Finally, a collagen-induced arthritis (CIA) model was established in rats to verify the anti-RA effect and potential mechanism of DHJS, which will provide an experimental basis for DHJS to treat RA.
Materials and methods
Materials and reagents
DHJS (Batch No. 20200805) was supplied by Shanxi Huakang Pharmaceutical Co., Ltd., Shanxi, China. Type II collagen (CII) and incomplete Freund’s adjuvant (IFA) were supplied by Beijing Boyred Biotechnology Co., Ltd, Beijing, China. TNF-α, IL-1β, IL-6, IL-10 and IL-17A ELISA kits were obtained from Neobioscience Technology Company. Primary antibodies against p65, p-p65, PI3K, p-PI3K, AKT and p-AKT were supplied by Wuhan Boster Biological Technology, LTD., Wuhan, China. Goat anti-rabbit IgG antibody was obtained from Zhongshan Golden Bridge Biotechnology, Beijing, China.
UPLC-Q/TOF-MS analysis of DHJS
The principal component analysis of DHJS was carried out on a Waters ACQUITY UPLC using a Waters AcquityTM UPLC BEH HILIC column (1.7 μm particle size, 2.1 × 100 mm column). The mobile phase was designed as phase A (MeCN, 0.1% HCOOH) and phase B (Water, 10 mM HCOONH4, 0.1% HCOOH). The column temperature was 30 °C, and the flow rate in gradient mode was 0.4 mL/min, as shown in .
Table 1. Gradient elution conditions in RP-UPLC.
The mass spectrometer was run in V mode to obtain high sensitivity, with a capillary voltage of 2500 V in positive mode and a capillary voltage of 3000 V in negative mode, and the sample cone voltage in both modes was 100 V. Waters MassLynx version 4.1 software and Progenesis QI software (Massachusetts, USA) were used for data analysis.
Network pharmacology research
Screening of components and targets in DHJS
The main components of each herb in DHJS and their targets were collected from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, https://old.tcmsp-e.com/tcmsp.php), the Encyclopaedia of Traditional Chinese Medicine (ETCM, http://www.tcmip.cn/ETCM/) and Traditional Chinese Medicine Simplified Integrated Database (TCMSID, https://tcm.scbdd.com/). Oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18 were taken as the screening conditions, and the relevant targets were downloaded from TCMSP. The simplified molecular input line entry system (SMILES) of the compounds in ETCM was used for screening active ingredients by SwissADME according to Lipinski’s rule of five and high GI absorption while their targets were obtained from ETCM (Xu et al. Citation2019; Bakchi et al. Citation2022; Mukherjee et al. Citation2023). Screening principles in TCMSID are F-30 and druglikeness equal to 1 (represents OB ≥ 30% and druglike) while the targets of each active compounds were downloaded from SEA in TCMSID (Zhang, Dong, et al. Citation2022; Zhang, Wei, et al. Citation2022). Importantly, another 5 components (osthol, gentiopicrin, roburic acid, cimifugin and loganic acid) of DHJS with potential anti-RA research value were included as candidate active ingredients by searching related literature.
RA-related targets collection
RA-related targets were collected from the DrugBank (https://www.drugbank.ca/), GeneCards (https://www.genecards.org/), OMIM (https://omim.org/), TTD (http://db.idrblab.net/ttd/) and DisGeNET (https://www.disgenet.org/) databases. As a supplement, the microarray data of GSE1919, GSE55235 and GSE55457 in RA patients and healthy donors were downloaded from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/) database. The GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r/) based on R software was used to identify differentially expressed genes (DEGs) between normal and RA samples. The DEGs with p < 0.05 and |log2-fold change| > 1 were screened as the significant DEGs. Then the intersecting genes of the significant DEGs in the three data were added to RA targets.
GO and KEGG enrichment analysis
Potential therapeutic targets of DHJS for RA were obtained by Venn diagram online tool (http://jvenn.toulouse.inra.fr/app/example.html) between DHJS-related targets and RA-related targets, and then GO and KEGG enrichment analysis was conducted for these targets by the DAVID (https://david.ncifcrf.gov/) database. Next, the top 10 pathways in the above analysis were visualized through the bioinformatics platform (http://www.bioinformatics.com.cn/).
PPI network
Protein–protein interaction (PPI) network of the potential therapeutic targets was constructed by the STRING (https://cn.string-db.org/) database with a confidence score of 0.9 or higher and further visualized by Cytoscape version 3.9.1 (Massachusetts, USA). Then, CytoNCA, a plug-in of Cytoscape, was used to conduct a topological analysis of the PPI network. The targets were further screened based on topological analysis of degree centrality (DC), betweenness centrality (BC), closeness centrality (CC), eigenvector centrality (EC), network centrality (NC) and local average connectivity-based method centrality (LAC) (Tang et al. Citation2015; Fu et al. Citation2022). A target is deemed to be a ‘big hub’ when its DC surpasses twice the median DC of the network, thus, a sub-network was extracted based on two times the median DC. To further identify the hub targets and core network, the medians of the six parameters were set as the threshold for the second screening condition (Zhang, Dong, et al. Citation2022; Zhang, Wei, et al. Citation2022). Finally, the network of hub targets and their corresponding compounds and herbs were visualized by the Cytoscape.
Component-target molecular docking
The crystal structures of the target proteins were obtained from the RCSB PDB (PDB, https://www.rcsb.org/) database, and the MOL2 structures of candidate active compounds were downloaded from TCMSP. Before molecular docking, the proteins required a series of preparations, including molecular energy minimization, removal of water molecules, the addition of hydrogen atoms and provision of electric charges and magnetic fields by PyMOL version 2.4 (https://pymol.org/2/). Next, the docking grid box was constructed by AutoDockTools version 1.5.6 (California, USA) at the active site of each target. AutoDock Vina version 4.2 (California, USA) was further used to perform molecular docking and calculate binding energies between the proteins and ligands. Finally, the docking results were visualized by PyMOL and LigPlot+.
Experimental verification in vivo
Animals
Male Wistar rats weighting about 180–200 g (License No. SCXK-JI-2020-0002) were provided by Changchun Yisi Experimental Animal Technology Co., Ltd, Changchun, China. All animals were fed with standard chow diet ad libitum and maintained a 12 h light/dark cycle in a room with a relative humidity of 55% and a constant temperature of 23 ± 1 °C. The experimental procedure was conducted in accordance with NIH guidelines and approved by the experimental animal ethics committee of Harbin Medical University (HMUDQ20220517001).
Preparation of drug solution for animal experiment
DHJS and leflunomide were separately dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na) by ultrasonic vibration. DHJS is consisted of 15 commonly used herbs including Heracleum hemsleyanum (Pinyin name Duhuo), Taxillus sutchuenensis (Pinyin name Sangjisheng), Rehmannia glutinosa (Pinyin name Dihuang), Achyranthes bidentata (Pinyin name Niuxi), Asarum heterotropoides (Pinyin name Xixin), Gentiana macrophylla (Qinjiao), Wolfiporia cocos (Pinyin name Fuling), Cinnamomum cassia (Pinyin name Rougui), Saposhnikovia divaricata (Pinyin name Fangfeng), Ligusticum chuanxiong Hort (Pinyin name Chuanxiong), Codonopsis pilosula (Pinyin name Dangshen), Glycyrrhiza uralensis (Pinyin name Gancao), Angelica sinensis (Pinyin name Danggui), Paeonia lactiflora (Pinyin name Baishao) and Eucommia ulmoides (Pinyin name Duzhong).
Establishment and evaluation of the CIA rat model
Briefly, CII was mixed with the same amount of IFA to prepare the desired emulsion. In the first immunization, 200 μL of the emulsion was injected subcutaneously into the tail of rats. After 7 d, 100 μL of the emulsion was injected from the bottom of the tail of rats to enhance their immunity, and the paw volume was measured and the arthritis score was calculated every three days starting from day 8. The degree of arthritis was determined by the grade of 0–4: 0, no joint swelling; 1, swelling and/or redness of one to two interphalangeal joints; 2, swelling and/or redness of four interphalangeal joints or one larger joint; 3, redness/swelling of more than four joints; 4, claw deformities and joint stiffness. The arthritis index was the total score of the four paws of rats, with a maximum of 16 points and a minimum of 0 points (Yu et al. Citation2021).
Treatment of CIA
All rats were randomly divided into 6 groups (n = 10): the control group, CIA group, leflunomide group (LEF, 18.0 mg/kg), and DHJS group (low dose: 0.5 g/kg, medium dose: 1.0 g/kg, high dose: 2.0 g/kg). DHJS and LEF were administered orally for 10 d starting on day 21 after the primary immunization. The rats in the control and CIA groups were given equivalent 0.5% CMC-Na. The paw swelling and arthritis scores of rats were measured during the administration.
Histopathological assessment
After cervical dislocation, the rat’s ankle joints were taken and fixed in 4% paraformaldehyde for 24 h. The ankle was then decalcified with ethylenediaminetetraacetic acid (EDTA) for about 30 d, embedded in paraffin wax, sliced into 5 µm thick and stained with H&E. All sections of each rat were scored on a scale of 0–3 (0 – normal, 1 – inflammatory cell infiltration, 2 – synovial hyperplasia and vascular pannus formation and 3 – bone erosion and destruction).
Micro-CT
The left hind paw of the rat with ankle joint was preserved in 75% ethanol, and then its 3D structure was reconstructed by X-ray scanning in a micro-CT (PerkinElmer, Massachusetts, USA) system.
Cytokine levels assessment
The serum levels of TNF-α, IL-1β, IL-6, IL-17A and IL-10 in rats were determined by ELISA kits.
Western blotting
Spleen tissue proteins were isolated by 8% SDS-PAGE and then transferred to PVDF membranes. After blocking with 5% skimmed milk, the membrane was incubated with primary antibodies, including PI3K, p-PI3K, AKT, p-AKT, p65 and p-p65, at 4 °C overnight and followed by PBST wash. Next, the membranes were incubated with goat anti-rabbit IgG second antibody at room temperature for another 2 h. Ultimately, the target protein was visualized and analysed with Image J software.
Statistical analysis
The data were expressed as the mean ± SD. Differences between the groups were evaluated by one-way analysis of variance (ANOVA), followed by Bonferroni’s multiple comparison test. A value of p < 0.05 was defined as statistically significant.
Results
UPLC-Q-TOF-MS analysis
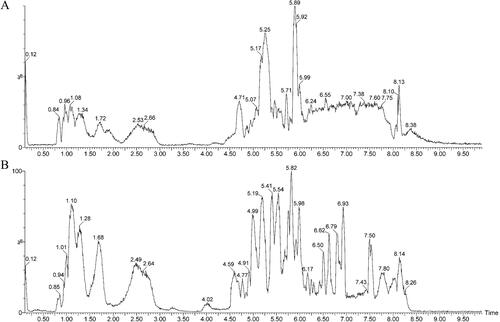
The principal components of DHJS were detected by UPLC-Q/TOF-MS in positive and negative ion mode. The basic peak intensity (BPI) chromatograms of DHJS are shown in . Through analysis, a total of 83 compounds were identified by different retention times, fragment ions, relevant literature as well as online databases, which included 43 terpenoids, 11 phenylpropanoids, 8 flavonoids, 8 saccharides, 5 steroids, 3 alkaloids, 3 chromones, 1 phenolic acid and 1 oxyacid.
Active components of DHJS and prediction of their targets
A total of 481 components were screened as active ingredients of DHJS, of which 41 were from Duhuo, 8 from Qinjiao, 38 from Fangfeng, 39 from Xixin, 50 from Rougui, 19 from Sangjisheng, 25 from Baishao, 9 from Dihuang, 26 from Niuxi, 14 from Fuling, 49 from Chuanxiong, 45 from Dangshen, 48 from Danggui, 42 from Duzhong and 115 from Gancao. A total of 1302 proteins are targeted by 481 active ingredients.
Acquisition of RA-related target genes
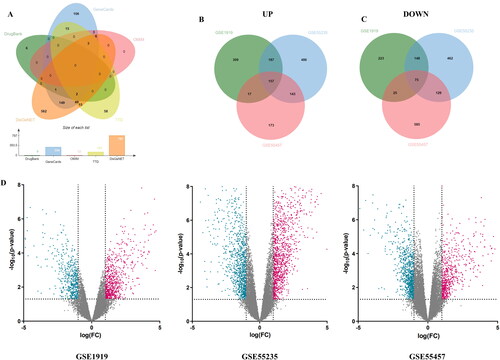
By searching five databases with the keyword ‘rheumatoid arthritis’, 9, 334, 12, 142 and 787 RA-related target genes were obtained from DrugBank, GeneCards, OMIM, TTD and DisGeNET databases, respectively. After combining the target genes from five databases and removing duplicates, a total of 975 RA-related target genes were obtained (). In addition, differential analysis of the gene series GSE1919, GSE55235 and GSE55457 identified 754, 1245 and 610 up-regulated DEGs and 518, 1020 and 901 down-regulated DEGs, respectively. The DEGs in each gene series were presented by the volcano plots (). Altogether there were 157 up-regulated DEGs and 75 down-regulated DEGs at the intersection of the 3 gene series (). Then, the 232 DEGs were added to RA-related target genes, and finally 1160 RA-related target genes were obtained.
Figure 2. Screening for RA-related targets. (A) Venn diagram of RA-related targets in DrugBank, GeneCards, OMIM, TTD and DisGeNET databases. (B) Venn diagrams of up-regulated DEGs and (C) Venn diagrams of down-regulated DEGs in GSE1919, GSE55235 and GSE55457, respectively. (D) Volcano plot of differential genes in RA samples. Red and green represent up-regulated and down-regulated genes, respectively, while black indicates no significant differences.
GO and KEGG enrichment analysis of potential therapeutic targets
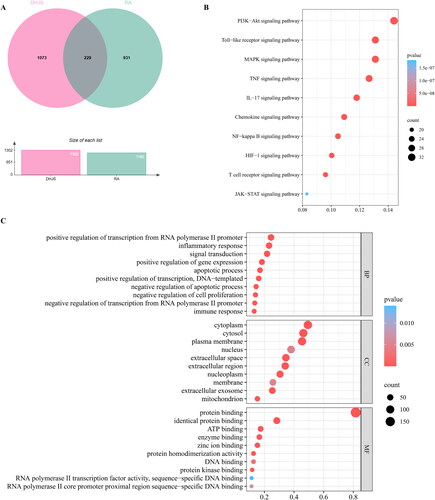
A total of 229 targets were screened as potential therapeutic targets of DHJS against RA by the intersection of active compound targets of DHJS and disease targets of RA (). To further explore the mechanisms of DHJS against RA, we performed GO and KEGG enrichment analysis of the potential therapeutic targets. GO analysis showed a total of 1127 items in three categories: 853 biological processes (BP) items, 83 cellular components (CC) items and 191 molecular functions (MF) items. The top 10 terms according to gene counts in every category are presented as column charts (). 178 pathways were enriched by KEGG pathway enrichment analysis. The top 10 RA-related pathways based on gene counts were PI3K-Akt signalling pathway, Toll-like receptor signalling pathway, MAPK signalling pathway, TNF signalling pathway, IL-17 signalling pathway, Chemokine signalling pathway, NF-κB signalling pathway, HIF-1 signalling pathway, T cell receptor signalling pathway and JAK-STAT signalling pathway ().
Figure 3. GO and KEGG enrichment analysis of potential therapeutic targets of DHJS against RA. (A) Venn diagram of DHJS targets and RA targets. (B) Top 10 terms of KEGG pathway enrichment analysis. (C) Top 10 terms of biological process, cellular component and molecular function enrichment analysis from GO enrichment analysis.
PPI network analysis
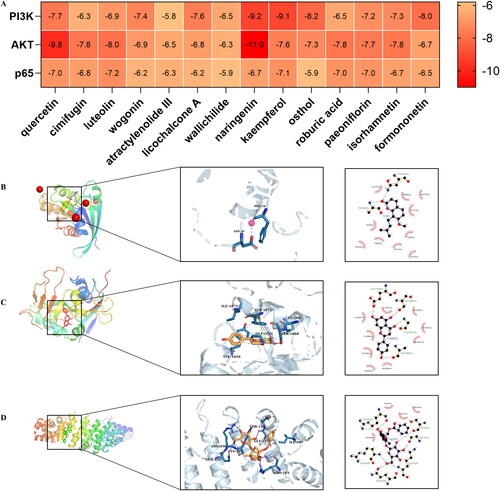
The PPI network consisting of 186 nodes and 1794 edges was constructed by importing the 229 potential therapeutic targets into the STRING database (). We further obtained the core PPI network, including 16 nodes and 156 edges by setting the DC, BC, CC, EC, NC and LAC thresholds to 40, 534.30347, 0.22182254, 0.11496056, 18.774586 and 15.428572, respectively (). The 16 hub targets and their corresponding compounds and herbs were imported into Cytoscape to construct an herb-compound-target network. A total of 14 compounds were screened as main key compounds according to the degree value (degree ≥ 3) (), and they were quercetin, cimifugin, luteolin, wogonin, atractylenolide III, licochalcone A, wallichilide, naringenin, kaempferol, osthol, roburic acid, paeoniflorin, isorhamnetin and formononetin.
Figure 4. Identification of core network and hub targets of DHJS against RA via topological analysis of PPI network. (A) PPI network of potential therapeutic targets. (B) PPI network of significant targets extracted from Panel A through DC. (C) PPI network of hub targets for RA treatment extracted from Panel B through DC, BC, CC, EC, NC and LAC.
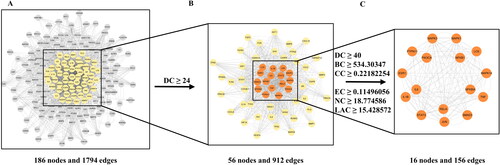
Table 2. Key compounds in DHJS against RA.
Molecular docking
Based on the PPI network and KEGG results, we selected three representative targets (PI3K, AKT and p65) among the 16 hub targets for molecular docking with 14 key compounds. The binding energies (kcal/mol) of the targets and compounds are shown in . Then, three representative compounds (osthol, naringenin and paeoniflorin) in DHJS with lower binding energy were further selected to visualize target-compound interactions and their modes of binding (). The results showed that osthol could bind to PI3K at PHE-28 and ASP-30. Naringenin was identified to bind to AKT at LYS-1067, SER-1068, TYR-1071, TYR-1050, GLY1032 and ILE-1075. Meanwhile, paeoniflorin was confirmed to bind to p65 at THR-146, ILE-107, GLY-145, ARG-436, LYS-437, GLN-435 and ARG-103.
The effect of DHJS on RA progression
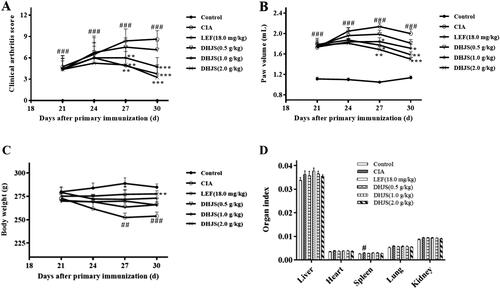
On the 30th day of initial immunization, LEF and DHJS (1.0 and 2.0 g/kg) could reduce the arthritis index of rats compared with CIA model rats (p < 0.001) (). As can be seen from , compared with the CIA model group, LEF and DHJS (2.0 g/kg) could significantly reduce paw volume of rats (p < 0.05 and p < 0.01) on the 30th day. The body weight of rats in each group was measured during the administration period. As shown in , on the 30th day of the primary immunization, the body weight of the rats in the CIA group was significantly lower than that in the control group (p < 0.001). The body weight of rats in DHJS (2.0 g/kg) group increased prominently (p < 0.01), compared with the CIA group. After sample collection, the organ index of the rats was calculated. Compared with the control group, the spleen index of CIA rats was remarkably increased (p < 0.05), but there was no significant difference in other organ indexes, as shown in .
Figure 6. Effect of DHJS on the development of disease in CIA rats. Effect of DHJS on (A) Arthritis index, (B) Paw swelling, (C) Body weight and (D) Organ index in CIA rats. Data were presented as means ± SE (n = 8), #p < 0.05, ##p < 0.01 and ###p < 0.001, vs. control group *p < 0.05, **p < 0.01 and ***p < 0.001, vs. CIA group.
The effect of DHJS on ankle and tarsometatarsal joints of CIA rats
At the end of the experiment, the hind paws of the rats in each group were photographed to observe the swelling and joint deformation. As shown in , compared with the control group, the paws of the rats in the model group were obviously red and swollen, and the intraplantar joints were severely deformed. Compared with CIA model group, administration of LEF and DHJS (2.0 g/kg) markedly inhibited paw swelling and improved joint deformation. DHJS (0.5 and 1.0 g/kg) also alleviated the redness and oedema of rat paws to a certain extent, but the tarsometatarsal joints of rats were still swollen and deformed.
Figure 7. Therapeutic effects of DHJS in CIA rats. (A) Representative photographs of hind paws. (B) Typical pathological sections of the ankle joint stained with H&E (original magnification ×200). (C) Representative micro-CT images of the left hind paw. (D) Histological scores were determined after H&E section observation. Data were presented as means ± SE (n = 8), ##p < 0.01, vs. control group; *p < 0.05 and **p < 0.01, vs. CIA group.
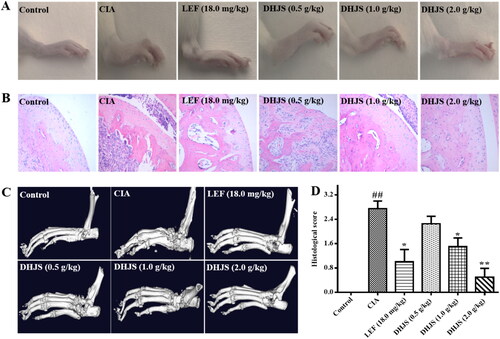
As shown in , histological evaluation of the ankle joints in the CIA group indicated inflammatory cell infiltration, synovial lining hyperplasia, articular cartilage damage and bone erosion, while treatment with DHJS and LEF was effective in reducing the extent of inflammatory cell infiltration, cartilage and bone damage. In addition, the HE score of DHJS was significantly lower than that of CIA rats ().
By analysing the micro-CT images of the left hind paw of the rats in each group, it was found that the tarsometatarsal joints of the rats in the control group had no bone destruction, while the bones of rats in the CIA group were noticeably rough and uneven, and the destruction of the ankle bones was aggravated. Compared with the CIA model group, LEF and different doses of DHJS could significantly inhibit bone erosion and joint destruction in CIA rats, and DHJS (2.0 g/kg) improved bone destruction caused by arthritis most obviously. These results revealed that DHJS could improve joint and bone destruction caused by arthritis, as shown in .
Effects of DHJS on serum cytokine levels in CIA rats
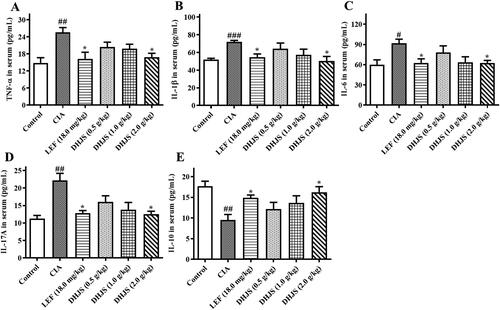
Cytokines, such as TNF-α, IL-1β, IL-6, IL-10 and IL-17A, are key factors in regulating inflammation and joint injury in the process of RA (Yang et al. Citation2018; Zhai et al. Citation2018). As the key targets of network pharmacological analysis, the expression of TNF-α, IL-1β, IL-6, IL-10 and IL-17A in serum of rats was determined by ELISA. The results () showed that compared with the control group, the expressions of TNF-α, IL-1β, IL-6 and IL-17A in serum were apparently increased (p < 0.01, p < 0.001, p < 0.01 and p < 0.01), while IL-10 was decreased (p < 0.01) in the CIA group. LEF and DHJS (1.0 and 2.0 g/kg) could dramatically decrease the levels of TNF-α, IL-1β, IL-6 and IL-17A (p < 0.05), and remarkably increase the level of IL-10 (p < 0.05) compared with the CIA group. These results showed that DHJS could prevent CIA inflammation by inhibiting the secretion of TNF-α, IL-1β, IL-6 and IL-17A and promoting the production of IL-10.
Figure 8. Effects of DHJS on serum inflammatory cytokines in CIA rats. (A) The serum level of IL-6. (B) The serum level of IL-1β. (C) The serum level of TNF-α. (D) The serum level of IL-17A. (E) The serum level of IL-10. Data were presented as means ± SE (n = 6), ##p < 0.01 and ###p < 0.001, vs. control group, *p < 0.05 and **p < 0.01 vs. CIA group.
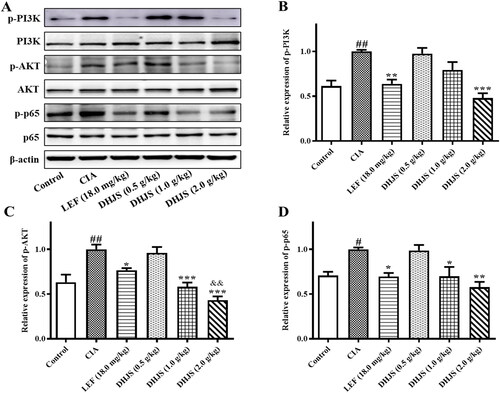
Effects of DHJS on the expression of the PI3K/AKT/NF‐κB pathway in CIA rats
To verify the anti-RA mechanism of DHJS previously predicted based on network pharmacology, we further evaluated the effect of DHJS on the expression of PI3K/AKT/NF-κB pathway-related proteins using Western blotting. As shown in , the protein expression of p-PI3K, p-AKT and p-p65 in the spleen tissue of CIA rats increased significantly (p < 0.01, p < 0.05 and p < 0.05) compared with the control group. The increase was decreased remarkably by DHJS (2.0 g/kg).
Figure 9. Effects of DHJS on the expression of PI3K/AKT/NF‐κB signalling pathway in spleen of CIA rats. (A) Western blot analysis of p-PI3K, PI3K, p-AKT, AKT, p-p65, p65 and β-actin protein expression in spleen. (B) Quantitative analysis of p-PI3K/β-actin. (C) Quantitative analysis of p-AKT/β-actin. (D) Quantitative analysis of p-p65/β-actin. Data were presented as means ± SE, #p < 0.05 and ##p < 0.01, vs. Control group; *p < 0.05 and **p < 0.01 vs. CIA group.
Discussion
DHJS is one of the commonly prescribed TCM formulas for the treatment of RA in China. Considering the complexity of active components in DHJS and the variety of potential regulatory targets of diseases, DHJS compounds and corresponding targets were collected from several databases through network pharmacology, and RA therapeutic targets were collected simultaneously. Through target screening, 16 hub targets were obtained and a PPI network was established. Among these targets, some were key factors in inflammatory responses, such as TNF, IL-6, IL-1β and IL-10 (An et al. Citation2018; Ni et al. Citation2018; Wang, Cheng, et al. Citation2018; Wang, Wang, et al. Citation2018; Ge et al. Citation2019). Pro-inflammatory cytokines including IL-6, IL-1β and TNF-α mediate the occurrence and progression of RA disease (Huang et al. Citation2019). T cells and macrophages can secrete the pro-inflammatory cytokine IL-6, which contributes to the pannus formation in rheumatoid synovium (Kaneshiro et al. Citation2019). IL-1β is a critical inflammatory mediator of the innate immune response, and its expression is elevated in all phases of RA (Dong et al. Citation2020). IL-1β can induce the proliferation of synovial fibroblastic cells and accelerate the release of pro-inflammatory mediators in the pathogenic process of RA (Chang et al. Citation2014). TNF-α is one of the main mediators in the pathogenesis of RA. RA synovial fibroblasts in synovial intima play an important regulatory role via TNF-α, leading to cartilage and bone damage (Huang et al. Citation2019). IL-17A is a direct cytokine released by Th17 cells, which can promote the production of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-1 and human GM-CSF, and plays a destructive role in joints (Leipe et al. Citation2014; He et al. Citation2020). IL-10 plays a crucial role in many autoimmune inflammatory diseases, such as RA. And Treg cells in RA patients have impaired ability to produce IL-10 (Wang et al. Citation2019). Consequently, the present findings indicated that DHJS could efficiently decrease the content of TNF-α, IL-1β, IL-6 and IL-17A and increase the expression of IL-10 in the serum of CIA rats. This result suggested that DHJS might prevent the inflammation of CIA by inhibiting the secretion of IL-6, IL-1β, IL-17A and TNF-α, and promoting the production of IL-10.
In addition, GO analysis of key targets showed that DHJS participated in both immune and inflammatory responses. KEGG analysis results revealed that more candidate pathway was involved, including the TNF, NF-κB and PI3K/AKT signalling pathway. The NF-κB pathway, a key pathway in the process of inflammatory response (Jia et al. Citation2015), accelerates RA development by increasing tissue damage, matrix metalloprotein expression and immune cell proliferation (Shu et al. Citation2015). The NF-κB signalling pathway can be activated by IL-1β, TNF-α and CD40L, etc. Subsequently, the activated NF-κB pathway promotes the release of downstream inflammatory cytokines and regulates B-cell lymphoma 2 protein, and thus participates in the process of apoptosis and inflammatory response. The level of AKT in the synovial tissue of RA was prominently higher than that in osteoarthritis, which indicated that the increased AKT level might be caused by RA-mediated inflammation (Tas et al. Citation2016). The PI3K/AKT pathway also plays an essential role in the pathological process of RA (Meng et al. Citation2017). The PI3K/AKT pathway can be activated by growth factors and cytokines, thereby activating downstream Bcl-2 and participating in the apoptotic response. Furthermore, the PI3K/AKT signalling pathway can also mediate the NF-κB pathway. Hence, PI3K/AKT/NF-κB signalling pathway can affect the production of inflammatory cytokines, synovial cell proliferation and the progression of RA.
Fourteen potential active compounds, including naringenin, osthol and paeoniflorin, were further selected from DHJS with degree ≥ 3 as the screening condition. Research (Zhang et al. Citation2021) has revealed that naringenin was effective in relieving collagen-induced RA in rats by reducing inflammatory cell infiltration and synovial damage. In addition, rats that were given naringenin displayed reduced inflammation in the paw and C-reactive protein levels, as well as lower serum myeloperoxidase, nitric oxide and total antioxidant capacity (Hajizadeh et al. Citation2021). Osthol is the main effective component of DHJS. In vitro, osthol apparently inhibits the inflammatory response of macrophages, bronchial epithelial cells, rat peritoneal cells and human peripheral monocytes (Hershberg Citation2002; Iwasaki and Medzhitov Citation2004), and significantly reduce the inflammation caused by physical and chemical factors in animals (Rakoff-Nahoum et al. Citation2004). Mechanistically, Osthol can inhibit the high expression of COX-2 protein and iNOS protein to some extent, and further block the phosphorylation of MAPK/p38, thus achieving the anti-inflammatory effect (Sun Citation2017). Paeoniflorin is a bioactive monomeric component isolated from Cynanchum otophyllum Schneid, which is an important drug in DHJS. It was previously reported that the levels of IL-1, TNF-α and PGE 2 decreased after administration of different concentrations of paeoniflorin in the type II collagen-induced arthritis rat model. The mechanism of paeoniflorin treating RA may be through G protein-coupled signal transduction pathway (Zhang et al. Citation2008). Another study showed that paeoniflorin inhibited the proliferation of rat synovial fibroblasts by regulating PGE2 receptor, thereby reducing the expression of various inflammatory cytokines (Wang et al. Citation2012). In this study, the results of molecular docking indicated that the characteristic components of DHJS, including naringenin, osthol and paeoniflorin, were bond well with the core targets PI3K, AKT and p65.
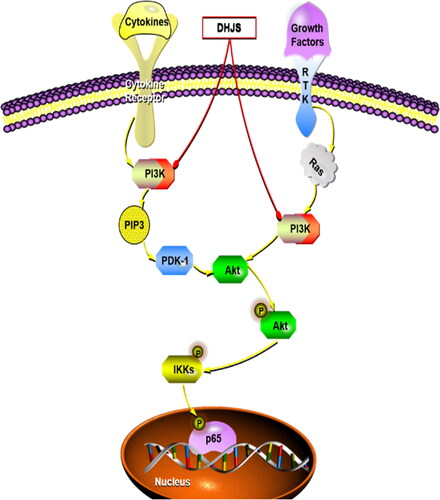
To date, studies have indicated that the PI3K/AKT pathway may be the main upstream target of the NF-κB signalling cascade, and PI3K antagonists can induce anti-inflammatory effects by inhibiting the activation of NF-κB (Dan et al. Citation2008; Venkatesan et al. Citation2010; Gambhir et al. Citation2015; Wang, Cheng, et al. Citation2018; Wang, Wang, et al. Citation2018). Recently, many studies have shown that the PI3K/AKT/NF-κB pathway has a crucial effect on the pathogenesis of inflammation (Lee et al. Citation2018; Liu, Huang, et al. Citation2018; Liu, Zhang, et al. Citation2018; Wang, Cheng, et al. Citation2018; Wang, Wang, et al. Citation2018; Kalantary-Charvadeh et al. Citation2019). Combined with literature and network pharmacological results, the PI3K/AKT/NF-κB pathway was verified in CIA rats. The results demonstrated that in contrast to the CIA rats, the expression of p-PI3K, p-AKT and p-p65 protein in the spleen tissue of the DHJS-treated group was significantly inhibited. In conclusion, these results showed that the inhibition of PI3K/AKT/NF-κB signalling pathway might be one of the underlying molecular mechanisms of the anti-RA effect of DHJS, as shown in .
Figure 10. Diagram of the underlying mechanism of DHJS in RA treatment. Red arrows indicate blocking and yellow arrows indicate activation.
This investigation has uncovered the material basis and mechanism of action of DHJS against RA. However, there are still certain issues that need to be addressed in subsequent studies. It is noteworthy that the volatile oil components present in some drugs in DHJS, such as Heracleum hemsleyanum Diels and Taxillus sutchuenensis (Lecomte) Danser, cannot be identified using UPLC-Q/TOF-MS. Therefore, in order to thoroughly explore the material basis of DHJS, upcoming research will utilize QC-MS to examine the volatile components in DHJS. In addition, a validation of these biomarkers and potential targets in humans is necessary, and should be the subject of future studies.
Conclusions
This study analysed the potential active components, target proteins, signalling pathways and mechanisms of DHJS in treating RA by virtue of network pharmacology and molecular docking technology. Furthermore, in vivo experiments confirmed that DHJS could reduce the expression of PI3K/AKT/NF-κB signalling pathway, and regulate the secretion of related cytokines to attenuate RA. The present research offers a theoretical foundation for the comprehensive investigation of the therapeutic mechanism of DHJS in the treatment of RA. Additionally, it has provided significant progress in elucidating the pharmacological basis and mechanism of TCM in ameliorating RA.
Author’s contributions
Ping Xin designed the study and wrote the manuscript, Xiaoyun Xu performed the experimental validation, Huaxi Zhang organized and analysed the public data, Yuezhou Hu was responsible for data visualization, Chengjie Deng, Shiqin Sun and Shuang Liu assisted with the pharmacology experiments, Xiaoliang Li assisted in the design of the experiment and revised the manuscript, and Hongxing Ma and Xuegang Zhou jointly directed and supervised the entire experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- An Q, Yan W, Zhao Y, Yu K. 2018. Enhanced neutrophil autophagy and increased concentrations of IL-6, IL-8, IL-10 and MCP-1 in rheumatoid arthritis. Int Immunopharmacol. 65:119–128. doi: 10.1016/j.intimp.2018.09.011.
- Bakchi B, Krishna AD, Sreecharan E, Ganesh VBJ, Niharika M, Maharshi S, Puttagunta SB, Sigalapalli DK, Bhandare RR, Shaik AB. 2022. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: a medicinal chemist’s perspective. J Mol Struct. 1259:132712. doi: 10.1016/j.molstruc.2022.132712.
- Chang Y, Zhao Y, Cao Y, Gu W, Pang J, Zhan H. 2014. Bufalin exerts inhibitory effects on IL-1β-mediated proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 37(5):1552–1559. doi: 10.1007/s10753-014-9882-5.
- Dan H, Cooper M, Cogswell P, Duncan J, Ting J, Baldwin A. 2008. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 22(11):1490–1500. doi: 10.1101/gad.1662308.
- Ding H, Gao G, Zhang L, Shen G, Sun W, Gu Z, Fan W. 2016. The protective effects of curculigoside A on adjuvant-induced arthritis by inhibiting NF-small ka, CyrillicB/NLRP3 activation in rats. Int Immunopharmacol. 30:43–49. doi: 10.1016/j.intimp.2015.11.026.
- Dong X, Zheng Z, Lin P, Fu X, Li F, Jiang J, Zhu P. 2020. ACPAs promote IL-1beta production in rheumatoid arthritis by activating the NLRP3 inflammasome. Cell Mol Immunol. 17(3):261–271. doi: 10.1038/s41423-019-0201-9.
- Fernandez-Ruiz J, Ramos-Remus C, Sanchez-Corona J, Castillo-Ortiz JD, Castaneda-Sanchez JJ, Bastian Y, Romo-Garcia MF, Ochoa-Gonzalez F, Monsivais-Urenda AE, Gonzalez-Amaro R, et al. 2018. Analysis of miRNA expression in patients with rheumatoid arthritis during remission and relapse after a 5-year trial of tofacitinib treatment. Int Immunopharmacol. 63:35–42. doi: 10.1016/j.intimp.2018.07.028.
- Firestein GS, McInnes IB. 2017. Immunopathogenesis of rheumatoid arthritis. Immunity. 46(2):183–196. doi: 10.1016/j.immuni.2017.02.006.
- Fu B, Li Y, Shi X, Liu P, Zhang Y, Tian H. 2022. NCAPG promotes pulmonary artery smooth muscle cell proliferation as a promising therapeutic target of idiopathic pulmonary hypertension: bioinformatics analysis and experiment verification. Int J Mol Sci. 23(19):11762. doi: 10.3390/ijms231911762.
- Gambhir S, Vyas D, Hollis M, Aekka A, Vyas A. 2015. Nuclear factor kappa B role in inflammation associated gastrointestinal malignancies. World J Gastroenterol. 21(11):3174–3183. doi: 10.3748/wjg.v21.i11.3174.
- Ge Z, Zhu X, Wang B, Hu J, Sun J, Wang S, Chen X, Meng S, Liu L, Cheng Z. 2019. MicroRNA-26b relieves inflammatory response and myocardial remodeling of mice with myocardial infarction by suppression of MAPK pathway through binding to PTGS2. Int J Cardiol. 280:152–159. doi: 10.1016/j.ijcard.2018.12.077.
- Hajizadeh A, Abtahi Froushani S, Tehrani A, Azizi S, Bani Hashemi S. 2021. Effects of naringenin on experimentally induced rheumatoid arthritis in Wistar rats. Arch Razi Inst. 76:903–912.
- He Y, Tang J, Wu B, Yang B, Ou Q, Lin J. 2020. Correlation between albumin to fibrinogen ratio, C-reactive protein to albumin ratio and Th17 cells in patients with rheumatoid arthritis. Clin Chim Acta. 500:149–154. doi: 10.1016/j.cca.2019.10.009.
- Hershberg RM. 2002. The epithelial cell cytoskeleton and intracellular trafficking. V. Polarized compartmentalization of antigen processing and Toll-like receptor signaling in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 283(4):G833–839.
- Huang C, Chiou C, Liu S, Hu S, Su C, Tsai C, Tang C. 2019. Melatonin attenuates TNF-alpha and IL-1beta expression in synovial fibroblasts and diminishes cartilage degradation: implications for the treatment of rheumatoid arthritis. J Pineal Res. 66(3):e12560. doi: 10.1111/jpi.12560.
- Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 5(10):987–995. doi: 10.1038/ni1112.
- Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan X, Xu Z, Zhang P. 2015. Cucurbitacin E inhibits TNF-alpha-induced inflammatory cytokine production in human synoviocyte MH7A cells via suppression of PI3K/Akt/NF-kappaB pathways. Int Immunopharmacol. 29(2):884–890. doi: 10.1016/j.intimp.2015.08.026.
- Jo HG, Seo J, Lee D. 2022. Clinical evidence construction of East Asian herbal medicine for inflammatory pain in rheumatoid arthritis based on integrative data mining approach. Pharmacol Res. 185:106460.
- Kalantary-Charvadeh A, Sanajou D, Hemmati-Dinarvand M, Marandi Y, Khojastehfard M, Hajipour H, Mesgari-Abbasi M, Roshangar L, Nazari Soltan Ahmad S. 2019. Micheliolide protects against doxorubicin-induced cardiotoxicity in mice by regulating PI3K/Akt/NF-kB signaling pathway. Cardiovasc Toxicol. 19(4):297–305. doi: 10.1007/s12012-019-09511-2.
- Kaneshiro K, Sakai Y, Suzuki K, Uchida K, Tateishi K, Terashima Y, Kawasaki Y, Shibanuma N, Yoshida K, Hashiramoto A. 2019. Interleukin-6 and tumour necrosis factor-alpha cooperatively promote cell cycle regulators and proliferate rheumatoid arthritis fibroblast-like synovial cells. Scand J Rheumatol. 48(5):353–361. doi: 10.1080/03009742.2019.1602164.
- Lee J, Kim G, Choi J, Choi Y, Jeong N, Park P, Choi H, Kim S. 2018. 4-(Hydroxymethyl)catechol extracted from fungi in marine sponges attenuates rheumatoid arthritis by inhibiting PI3K/Akt/NF-kappaB signaling. Front Pharmacol. 9:726. doi: 10.3389/fphar.2018.00726.
- Leipe J, Schramm MA, Prots I, Schulze-Koops H, Skapenko A. 2014. Increased Th17 cell frequency and poor clinical outcome in rheumatoid arthritis are associated with a genetic variant in the IL4RGene, rs1805010. Arthritis Rheumatol. 66(5):1165–1175. doi: 10.1002/art.38343.
- Li J, Wang W, Feng G, Du J, Kang S, Li Z, Zhu W, Shang H. 2020. Efficacy and safety of Duhuo Jisheng decoction for postmenopausal osteoporosis: a systematic review and Meta-Analysis. Evid Based Complement Alternat Med. 2020:6957825.
- Liu H, Wang K. 2014. Therapeutic effect of captopril on rheumatoid arthritis in rats. Asian Pac J Trop Med. 7(12):996–999. doi: 10.1016/S1995-7645(14)60175-9.
- Liu L, Xu L, Wang S, Wang L, Wang X, Xu H, Li X, Ye H. 2021. Confirmation of inhibitingTLR4/MyD88/NF-kappaB signalling pathway by Duhuo Jisheng decoction on osteoarthritis: a network pharmacology approach-integrated experimental study. Front Pharmacol. 12:784822. doi: 10.3389/fphar.2021.784822.
- Liu W, Huang S, Li Y, Li Y, Li D, Wu P, Wang Q, Zheng X, Zhang K. 2018. Glycyrrhizic acid from licorice down-regulates inflammatory responses via blocking MAPK and PI3K/Akt-dependent NF-kappaB signalling pathways in TPA-induced skin inflammation. Medchemcomm. 9(9):1502–1510. doi: 10.1039/c8md00288f.
- Liu W, Jin S, Huang M, Li Y, Wang Z, Wang P, Zhao X, Xia P, Feng J. 2020. Duhuo jisheng decoction suppresses matrix degradation and apoptosis in human nucleus pulposus cells and ameliorates disc degeneration in a rat model. J Ethnopharmacol. 250:112494. doi: 10.1016/j.jep.2019.112494.
- Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long H, Wang Y. 2018. Sinomenine inhibits the progression of rheumatoid arthritis by regulating the secretion of inflammatory cytokines and monocyte/macrophage subsets. Front Immunol. 9:2228. doi: 10.3389/fimmu.2018.02228.
- Meng Q, Du X, Wang H, Gu H, Zhan J, Zhou Z. 2017. Astragalus polysaccharides inhibits cell growth and pro-inflammatory response in IL-1beta-stimulated fibroblast-like synoviocytes by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Apoptosis. 22(9):1138–1146. doi: 10.1007/s10495-017-1387-x.
- Mukherjee D, Ghosal I, Dhar D, Das S, Chakraborty S. 2023. Bioactive compounds from four Indian medicinal plants have different potency to induce sex reversal in Nile tilapia: a chromatographic, molecular docking and in silico analysis. J Ethnopharmacol. 307:116263. doi: 10.1016/j.jep.2023.116263.
- Ni S, Li C, Xu N, Liu X, Wang W, Chen W, Wang Y, van Wijnen A. 2018. Follistatin-like protein 1 induction of matrix metalloproteinase 1, 3 and 13 gene expression in rheumatoid arthritis synoviocytes requires MAPK, JAK/STAT3 and NF-kappaB pathways. J Cell Physiol. 234(1):454–463. doi: 10.1002/jcp.26580.
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. 2004. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 118(2):229–241. doi: 10.1016/j.cell.2004.07.002.
- Shu Z, Shi X, Nie D, Guan B. 2015. Low-molecular-weight fucoidan inhibits the viability and invasiveness and triggers apoptosis in IL-1beta-treated human rheumatoid arthritis fibroblast synoviocytes. Inflammation. 38(5):1777–1786. doi: 10.1007/s10753-015-0155-8.
- Sun W. 2017. Study on the anti-inflammatory activity and mechanism of osthole. Nanjing, China: Nanjing Medical University.
- Tang Y, Li M, Wang J, Pan Y, Wu F. 2015. CytoNCA: a cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems. 127:67–72. doi: 10.1016/j.biosystems.2014.11.005.
- Tas S, Maracle C, Balogh E, Szekanecz Z. 2016. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol. 12(2):111–122. doi: 10.1038/nrrheum.2015.164.
- Venkatesan B, Valente A, Prabhu S, Shanmugam P, Delafontaine P, Chandrasekar B. 2010. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB and MKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 49(4):655–663. doi: 10.1016/j.yjmcc.2010.05.007.
- Wang H, Li S, Zhang G, Wu H, Chang X. 2019. Potential therapeutic effects of cyanidin-3-O-glucoside on rheumatoid arthritis by relieving inhibition of CD38+ NK cells on Treg cell differentiation. Arthritis Res Ther. 21(1):220. doi: 10.1186/s13075-019-2001-0.
- Wang Q, Ma Y, Huang B, Wei W. 2012. Inhibitory effect of paeoniflorin on rat fibroblast-like synoviocytes hyperplasia induced by PGE2 via β-arrestin 2 regulating EP2 receptor. Chin Pharmacol Bull. 28:43–47.
- Wang S, Cheng Z, Hu Y, Liu T. 2018. Sotetsuflavone suppresses invasion and metastasis in non-small-cell lung cancer A549 cells by reversing EMT via the TNF-alpha/NF-kappaB and PI3K/AKT signaling pathway. Cell Death Discov. 4:26.
- Wang S, Wang L, Wu C, Sun S, Pan J. 2018. E2F2 directly regulates the STAT1 and PI3K/AKT/NF-kappaB pathways to exacerbate the inflammatory phenotype in rheumatoid arthritis synovial fibroblasts and mouse embryonic fibroblasts. Arthritis Res Ther. 20(1):225. doi: 10.1186/s13075-018-1713-x.
- Wang X, Gong S, Pu D, Hu N, Wang Y, Fan P, Zhang J, Lu X. 2020. Up-regulation of miR-365 promotes the apoptosis and restrains proliferation of synoviocytes through downregulation of IGF1 and the inactivation of the PI3K/AKT/mTOR pathway in mice with rheumatoid arthritis. Int Immunopharmacol. 79:106067. doi: 10.1016/j.intimp.2019.106067.
- Wang Y, Chen S, Du K, Liang C, Wang S, Boadi EO, Li J, Pang X, He J, Chang Y. 2021. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. J Ethnopharmacol. 279:114368. doi: 10.1016/j.jep.2021.114368.
- Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou X, Ma H, Wei D, Sun S. 2020. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int Immunopharmacol. 80:106210. doi: 10.1016/j.intimp.2020.106210.
- Xu HY, Zhang YQ, Liu ZM, Chen T, Lv CY, Tang SH, Zhang XB, Zhang W, Li ZY, Zhou RR, et al. 2019. ETCM: an encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 47(D1):D976–d982. doi: 10.1093/nar/gky987.
- Yang Y, Zhang X, Xu M, Wu X, Zhao F, Zhao C. 2018. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int Immunopharmacol. 54:153–162. doi: 10.1016/j.intimp.2017.11.013.
- Yu X, Zhou J, Zhao F, Liu X, Mao Y, Diao L, Wen C, Liu M. 2021. Tomatidine suppresses the destructive behaviors of fibroblast-like synoviocytes and ameliorates type II collagen-induced arthritis in rats. Front Pharmacol. 12:670707. doi: 10.3389/fphar.2021.670707.
- Zhai K, Duan H, Khan G, Xu H, Han F, Cao W, Gao G, Shan L, Wei Z. 2018. Salicin from alangium Chinense ameliorates rheumatoid arthritis by modulating the Nrf2-HO-1-ROS pathways. J Agric Food Chem. 66(24):6073–6082. doi: 10.1021/acs.jafc.8b02241.
- Zhang G, Sun G, Guan H, Li M, Liu Y, Tian B, He Z, Fu Q. 2021. Naringenin nanocrystals for improving anti-rheumatoid arthritis activity. Asian J Pharm Sci. 16(6):816–825. doi: 10.1016/j.ajps.2021.09.001.
- Zhang L, Dong J, Wei H, Shi S, Lu A, Deng G, Cao D. 2022. TCMSID: a simplified integrated database for drug discovery from traditional Chinese medicine. J Cheminform. 14(1):89. doi: 10.1186/s13321-022-00670-z.
- Zhang L, Wei W, Wang N, Wang Q, Chen J, Chen Y, Wu H, Hu X. 2008. Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflamm Res. 57(8):388–395. doi: 10.1007/s00011-007-7240-x.
- Zhang T, Wei W, Chang S, Liu N, Li H. 2022. Integrated network pharmacology and comprehensive bioinformatics identifying the mechanisms and molecular targets of yizhiqingxin formula for treatment of comorbidity with Alzheimer’s disease and depression. Front Pharmacol. 13:853375. doi: 10.3389/fphar.2022.853375.
- Zhang W, Wang S, Zhang R, Zhang Y, Li X, Lin Y, Wei X. 2016. Evidence of Chinese herbal medicine Duhuo Jisheng decoction for knee osteoarthritis: a systematic review of randomised clinical trials. BMJ Open. 6(1):e008973. doi: 10.1136/bmjopen-2015-008973.
- Zheng T, Su S, Dai X, Zhang L, Duan J, Zhen Q. 2018. Metabolomic analysis of biochemical changes in the serum and urine of Freund’s adjuvant-induced arthritis in rats after treatment with silkworm excrement. Molecules. 23(6):1490. doi: 10.3390/molecules23061490.