Abstract
Context
Diabetic peripheral neuropathy (DPN) results in an enormous burden and reduces the quality of life for patients. Considering there is no specific drug for the management of DPN, traditional Chinese medicine (TCM) has increasingly drawn attention of clinicians and researchers around the world due to its characteristics of multiple targets, active components, and exemplary safety.
Objective
To summarize the current status of TCM in the treatment of DPN and provide directions for novel drug development, the clinical effects and potential mechanisms of TCM used in treating DPN were comprehensively reviewed.
Methods
Existing evidence on TCM interventions for DPN was screened from databases such as PubMed, the Cochrane Neuromuscular Disease Group Specialized Register (CENTRAL), and the Chinese National Knowledge Infrastructure Database (CNKI). The focus was on summarizing and analyzing representative preclinical and clinical TCM studies published before 2023.
Results
This review identified the ameliorative effects of about 22 single herbal extracts, more than 30 herbal compound prescriptions, and four Chinese patent medicines on DPN in preclinical and clinical research. The latest advances in the mechanism highlight that TCM exerts its beneficial effects on DPN by inhibiting inflammation, oxidative stress and apoptosis, endoplasmic reticulum stress and improving mitochondrial function.
Conclusions
TCM has shown the power latent capacity in treating DPN. It is proposed that more large-scale and multi-center randomized controlled clinical trials and fundamental experiments should be conducted to further verify these findings.
Introduction
Diabetes mellitus (DM) is a collection of metabolic disorders characterized by incomplete glucose utilization and excessive production due to abnormal gluconeogenesis and glycogenolysis, leading to hyperglycemia (Sacks et al. Citation2023). In 2021, approximately 537 million adults (20 ∼ 79 years) lived with diabetes. It’s estimated to increase to 643 million by 2030 and 783 million by 2045 (Magliano et al. 2021). Individuals with diabetes are at increased risk of developing complications that commonly affect the heart, blood vessels, eyes, kidneys, nerves, teeth, and gums (Magliano et al. 2021). These complications, especially microvascular (predominantly neuropathy) and macrovascular issues, contribute to diabetes being the fourth leading cause of death in developed countries (Roglic et al. Citation2005). About 6.7 million deaths worldwide in 2021 were attributed to diabetes-related causes (Magliano et al. 2021). Consequently, effective strategies to combat diabetes complications are urgently needed. Diabetic peripheral neuropathy (DPN), a prevalent and debilitating microvascular complication of type 2 DM (T2DM), affects nearly 50% of patients after 10 years of the disease (Ang et al. Citation2014). It manifests as a heterogeneous group of symptoms affecting the peripheral nervous system (Iqbal et al. Citation2018) with various clinical features (Faselis et al. Citation2020), including distal symmetric polyneuropathy, characterized by numbness, tingling, pain, or weakness that begins in the feet and progresses proximally (Yovera-Aldana et al. Citation2021). DPN significantly reduces quality of life and predisposes individuals to falls, injuries, infections, and amputations (Feldman et al. Citation2019). Its prevalence is between 21.3% with 34.5% in T2DM, and 7% to 34.2% in type 1 DM (T1DM) (Yovera-Aldana et al. Citation2021). DPN is commonly categorized into symmetric polyneuropathies (with motor, sensory, and autonomic manifestations), focal, and multifocal neuropathies, and mixed forms (Thomas Citation1997). Its renewed classifications include typical DPN (mainly distal symmetric sensorimotor polyneuropathy) and atypical conditions (such as mononeuritis multiplex, and thoracic radiculopathy) (Tesfaye et al. Citation2010). In the theories of traditional Chinese medicine (TCM), DPN was classified into the following four distinct categories: cold coagulation blood stasis syndrome, blood deficiency cold coagulation syndrome, Qi deficiency blood stasis syndrome, and cold dampness spleen syndrome. (Tian et al. Citation2020).
Management of DPN typically involves glycemic control, lipid management, and pharmaceutical interventions for neuropathic pain (American Diabetes Association Professional Practice Committee Citation2024). Early glycemic control in T1DM can delay or prevent DPN (DCCT Research Group Citation1995; Albers et al. Citation2010), yet its effectiveness is less pronounced in T2DM (Callaghan et al. Citation2012). Dyslipidemia is a key factor in neuropathy development (Andersen et al. Citation2018), and lifestyle interventions such as weight loss and physical activity have shown positive effects in DPN management, rather than conventional lipid-lowering medications (Afshinnia et al. Citation2022). Pharmaceutical treatments such as gabapentinoids, sodium channel blockers, serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants (TCAs) are recommended for neuropathic pain (Price et al. Citation2022), and combination therapy is often more effective than monotherapy (Tesfaye et al. Citation2022). However, optimal management of DPN remains a challenge, necessitating the exploration of innovative therapeutic approaches.
Emerging TCM-based treatments offer innovative and effective strategies for DPN management. TCM, with its long history in disease prevention and treatment, has garnered global attention. Recent studies have developed novel herbal products to address the growing prevalence of DPN (Hu M et al. Citation2023; Jiang et al. Citation2023; Liu Q et al. Citation2023; Wang Q et al. Citation2023; Zhang et al. Citation2024). Knowledge of the properties and scientific evidence supporting TCM’s role in DPN management is crucial. This review focused on the latest TCM approaches for DPN management, delving into contemporary research on TCM applications for DPN treatment.
Methods
Study selection
A literature review was conducted using PubMed, the Cochrane Neuromuscular Disease Group Specialized Register (CENTRAL) and the Chinese National Knowledge Infrastructure Database (CNKI), with TCM, DPN, and TCM treatment of DPN as keywords. Eventually, about 130 studies published before 2023 were included, focusing on preclinical studies and clinical trials assessing the effectiveness of TCM in treating DPN.
Inclusion criteria: (1) Clinical studies on treatment of diabetic peripheral neuropathy with traditional Chinese medicine; (2) Preclinical studies on treatment of diabetic peripheral neuropathy with traditional Chinese medicine; (3) The mechanism of treating diabetic peripheral neuropathy with traditional Chinese medicine.
Exclusion criteria: (1) External use of traditional Chinese medicine in treating diabetic peripheral neuropathy; (2) Treatment of diabetic peripheral neuropathy with the combination of traditional Chinese medicine.
Classification of traditional Chinese medicine for DPN treatment
TCM is classified into three categories: single herbal extracts, herbal compound prescriptions, and Chinese patent medicines. The Mudan granule is the only drug currently marketed by the National Medical Products Administration for DPN, while most herbal extracts, compounds, and patent medicines remain in the preclinical or clinical study phases.
Single herbal extracts
Based on their chemical structures, single herbal extracts in TCM are broadly categorized into glycosides, phenols, alkaloids, flavonoids, and other types. Despite the differences in their chemical composition, these extracts commonly target pathological indicators of DPN, such as the structure of the sciatic nerve, nerve conduction velocity (NCV), and nerve-related proteins. Only a few representative herbs are presented here; details of others are provided in .
Table 1. Single herbal extracts for the treatment of DPN.
Preclinical research - glycosides
Glycosides generally refer to compounds composed of aglycones and monosaccharides. This paper mainly introduced saponins. Saponins are a group of compounds widely distributed in the plant kingdom, characterized by their structure containing triterpenoid or steroid glycoside ligands and one or more sugar chains (Güçlü-Ustündağ and Mazza Citation2007). At present, glycosides are widely studied in metabolic syndrome, cardiovascular disease, neuropathy and cancer (Guo et al. Citation2023).
Panax notoginseng, derived from the root of Panax notoginseng (Burk.) F. H. Chen (Araliaceae), is a perennial herb. It is deemed to promote blood circulation and treat cardiovascular disease (Hao et al. Citation2017). Research studies have highlighted the benefits of active saponin components extracted from Panax notoginseng in treating various diseases, including diabetic retinopathy, cardiac lipotoxicity, and rheumatoid arthritis (Zhou et al. Citation2019; Jiao et al. Citation2021; Tian et al. Citation2022). In a study conducted by Wang W et al. (Citation2019), RSC96 cells were cultured with notoginsenoside R1 (NGR1) at concentrations ranging from 10 ∼ 100 µM, followed by treatment with 100 mM glucose to simulate hyperglycemic conditions. The results showed that NGR1 treatment in RSC96 cells under high glucose conditions led to a reduction in reactive oxygen species (ROS) generation and an increase in the expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), compared to the high glucose group without treatment.
Diosgenin, a natural steroidal saponin extracted from Dioscorea nipponica Makino, Dioscorea opposite Thunb., and Dioscorea zingiberensis Wright (Dioscoreaceae), has a variety of beneficial effects, including hypoglycemic (Xu et al. Citation2020), cardiovascular protective (Li et al. Citation2021), and hypolipidemic properties (Gong et al. Citation2010). Leng et al. (Citation2020) showed that DPN mice received low (50 mg/kg) and high (100 mg/kg) doses of diosgenin through continuous gavage for 8 weeks. They concluded that diosgenin treatment led to an improvement in blood glucose levels and body weight in diabetic mice. Additionally, diosgenin significantly increased tail withdrawal latency, relieved mechanical hyperalgesia in diabetic animals and improved the pathological changes of the sciatic nerve. Similarly, it also reduced malondialdehyde (MDA) levels while enhancing the activities of superoxide dismutase (SOD) and glutathione peroxidase (Gpx).
Several other glycosides derived from TCM extracts may play therapeutic roles in treating DPN through various pharmacological mechanisms. These include astragaloside IV (AS-IV) (Ben et al. Citation2021; Yin et al. Citation2021), saikosaponin D (SSD) (Law et al. Citation2014; Zhou et al. Citation2021; Xiang et al. Citation2023; Xu X et al. Citation2023), salidroside (Li R et al. Citation2019; Xue et al. Citation2019; Zheng et al. Citation2021; Jin et al. Citation2022), paeoniflorin (Yang X et al. Citation2016; Yang X et al. Citation2022), gentiopicroside (Gent) (Lu et al. Citation2018; Xiao et al. Citation2020, Citation2022), and polydatin (Ji et al. Citation2012; Jiang et al. Citation2013; Gong et al. Citation2017).
Preclinical research - phenols
Phenols have different chemical structures and activities with more than 8,000 kinds which can be found in vegetables, seeds, fruits, nuts, red wine, tea, and many other food sources. In structure, phenols are metabolites of secondary plants, characterized by at least one aromatic ring connected with one or more hydroxyl groups (Eseberri et al. Citation2022). Phenols are widely used in disease research due to their outstanding anti-inflammatory and antioxidant effects (Azuma et al. Citation1986).
Curcuma longa L. (Zingiberaceae), with its long history of use as a traditional medicine in Asian countries, has attracted significant scientific interest (Memarzia et al. Citation2021). The main pharmacological component of Curcuma longa is curcumin. During the past decade, numerous studies have identified that curcumin has a wide range of biological effects, including antioxidant, neuroprotective, anti-inflammatory, and anticancer properties (Hussain et al. Citation2017; Kunnumakkara et al. Citation2017; Tanrıkulu-Küçük et al. Citation2019; Bhat et al. Citation2019). In vitro and in vivo studies have been conducted to investigate the efficacy of curcumin in treating peripheral neuropathies. It was noted that curcumin facilitates nerve regeneration and enhances NGF expression in rats with DPN (Ma et al. Citation2016; Zhang WX et al. Citation2022). Electron microscopy analyses have shown that in curcumin-treated groups, the sciatic nerve cross-sections are structurally intact, with improved segregation of myelinated nerve fibers. Meanwhile, both the mechanical withdrawal threshold (MWT) and NCV have been observed to increase proportionally with the duration and dosage of curcumin administration (Zhang WX et al. Citation2022).
The extracts of Magnolia officinalis Rehd. et Wils. (Magnoliaceae), specifically magnolol (MG) and honokiol (HNK), are lignin compounds recognized for their broad application value. Recent studies have demonstrated their anti-inflammatory, antioxidant, anticancer, and other pharmacological effects (Zhang and Tang Citation2012; Rauf et al. Citation2021). Both magnolol and honokiol have been proven to possess neuroprotective effects, with mechanisms related to their antioxidant properties and the antagonism of excitatory amino acids, making them potential therapeutic agents for neurodegenerative diseases (Lin et al. Citation2006).
In a study by Yang J et al. (Citation2022), both T1DM and T2DM mice were used as models for DPN. They revealed that MG enhanced neurological functions in DPN mice by improving sensitivity to thermal and mechanical stimuli, and significantly increasing motor nerve conduction velocity (MNCV). In addition, MG was observed to enhance the density of intraepidermal nerve fibers (IENF) on the foot skin of DPN mice and to protect the myelin sheath structure of the sciatic nerve, as determined by immunofluorescence analysis. Similarly, honokiol, whose structure is similar to MG, exhibited comparable effects. Hu M et al. (Citation2023) indicated honokiol improvements in sciatic NCV, increased thermal and mechanical sensitivity in diabetic rats, and relief from neuropathy symptoms. Moreover, honokiol mitigated high glucose-induced damage in Schwann cells.
Myelin basic protein (MBP) is essential for myelination and nerve health. Similarly, resveratrol, a compound widely distributed in plants, has also been identified to play a role in neuroprotection (Koushki et al. Citation2018; Nadile et al. Citation2022). Treatment of resveratrol in mice induced by streptozotocin revealed that resveratrol could enhance the expression of MBP to exact a protective effect on myelin (Zhang et al. Citation2021). Moreover, salvianolic acid A (Xu Citation2020) and salvianolic acid B (Wang QQ et al. Citation2019; Wang SX et al. Citation2010), derived from Salvia miltiorrhiza Bunge (Lamiaceae), were proved to improve DPN in vivo and in vitro.
Preclinical research - alkaloids
Alkaloids have a great influence on the lives of animals and human beings. However, their biosynthesis in plants involves many catalytic steps, and the diversification of structure is only associated with the appearance of nitrogen atoms in heterocyclic rings (Ziegler and Facchini Citation2008). At present, it is demonstrated that plant alkaloids not only have anticancer and antibacterial effects but also have the potential to ameliorate hypertension and neurological function (Bhambhani et al. Citation2021).
Vincamine, a monoterpene indole alkaloid extracted from Madagascar periwinkle (Apocynaceae), is clinically used to treat cerebrovascular diseases by increasing cerebral blood flow and oxygen consumption to improve cognitive dysfunction (Zhao et al. Citation2023). Researchers have hypothesized that vincamine could potentially aid in the treatment of DPN in mice. The proposed effects included improving blood flow velocity and perfusion in foot pads and sciatic nerve tissues, preventing myelin sheath damage in sciatic nerves, and ameliorating the density damage of intraepidermal nerve fibers (IENF) in foot skin (Xu JW et al. Citation2023).
Lycorine, an isoquinoline alkaloid extracted from the Lycoris radiata herb (Amaryllidaceae), exhibits multiple pharmacological effects, such as anticancer, anti-inflammation, and antioxidative stress (Roy et al. Citation2018; Wu et al. Citation2021). Studies involving in vivo and in vitro administration of lycorine have shown that it can reduce the thermal threshold by 36.12% and reverse MWT, demonstrating its potential effectiveness in treating neuropathy (p < 0.05, both) (Yuan et al. Citation2022).
Rhizoma coptidis refers to the dried rhizome of plants such as Coptis chinensis Franch., Coptis deltoidea C. Y. Cheng et Hsiao, or Coptis teeta Wall. (Ranunculaceae) (Wang J et al. Citation2019). The main active components extracted from these plants are berberine and jatrorrhizine (JAT), both isoquinoline alkaloids known to alleviate DPN. Berberine is used primarily in clinical settings for treating intestinal infections, due to its anti-inflammatory properties. Behavioral assays demonstrated increases in thresholds for mechanical allodynia and thermal hyperalgesia during berberine treatment, as measured by the von Frey test and the tail-flick test (Zan et al. Citation2017; Yerra et al. Citation2018). In addition, Gong et al. (Citation2023) found that JAT offers more protection against DPN than other flavonoid alkaloids. After the treatment of JAT (50, 100 and 200 mg/kg) in db/db mice for eight consecutive weeks, it significantly improved the general physiological functions and nerve morphology. Treatment significantly promoted myelin formation by improving sciatic nerve conduction velocity, increasing heat and pain sensitivity, and elevating myelin protein levels.
Preclinical research - flavonoids
It’s well known that flavonoids are the most abundant and widely distributed secondary plant metabolites in fruits and vegetables (Čižmárová et al. Citation2023). They have various pharmacological properties like antibacterial, antiviral, antitumor, anti-inflammatory, antiallergic, vasodilation, and cardioprotective effects (Chiorcea-Paquim Citation2023).
Pueraria lobata, officially recognized in the Chinese pharmacopeia and known for its benefits to muscles, brain, liver, and kidneys, is the dried root of Pueraria lobata (Willd.) Ohwi (Fabaceae) or Pueraria thomsonii Benth (Fabaceae) (Wang et al. Citation2020; Li et al. Citation2023). Puerarin, the primary isoflavonoid bioactive ingredient isolated from Pueraria lobata, has been widely used in treating cardiovascular diseases, diabetes, diabetic complications, and neurodegenerative disorders (Zhou et al. Citation2014). In vivo, studies verified that puerarin can enhance the activity of SOD in sciatic nerve tissue and helps reduce oxidative stress-related damage, inhibit excessive free radical generation, improve metabolic status, and consequently repair damaged nerve fibers. Additionally, puerarin has been shown to increase nerve conduction velocity and improve neurosensory symptoms (Wu et al. Citation2012).
Quercetin, another significant flavonoid abundantly found in fruits and vegetables, is known for its anti-inflammatory, anticancer, neuroprotective, and anti-hyperglycemic properties (Hosseini et al. Citation2021). It’s proved that quercetin can mitigate high glucose-induced damage to Schwann cells by modulating autophagy (Qu et al. Citation2014). Xie et al. (Citation2020b) identified that quercetin improves peripheral nerve function by alleviating the imbalance of the gut microbiota associated with DPN phenotypes. In the research conducted by Zhao et al. (Citation2021), DPN rats were treated daily with low (30 mg/kg) and high (60 mg/kg) doses of quercetin for six weeks. Subsequent observations of pathomorphological changes in sciatic nerves revealed that nerve fibers in DPN rats were loosely arranged, disordered, less intensely stained, and varied in size. Further investigation revealed that quercetin significantly reduced the levels of tumor necrosis factor-α (TNF-α) (p < 0.05) and interleukin-1β (IL-1β) (p < 0.001) which were negatively correlated with MWT and NCV. Overall, this study suggested that quercetin could contribute to improving nerve function by reducing inflammatory factors.
Preclinical research - others
A substantial number of studies have demonstrated that polysaccharides found in certain Chinese medicinal herbs, such as Lycium barbarum polysaccharide and Hedysarum polysaccharide, can effectively improve DPN. Both Lycium barbarum polysaccharide and Hedysarum polysaccharide not only reduced blood glucose levels but also alleviated symptoms of allodynia and hyperalgesia. Furthermore, they helped preserve NCV and mitigate structural damage to nerve fibers (Liu et al. Citation2018; He et al. Citation2022). Additionally, researchers (Dong et al. Citation2019) discovered that decreased cell viability and increased apoptosis in RSC96 cells, induced by high glucose levels, could be reversed by applying muscone. However, the extent of muscone effectiveness on DPN still requires confirmation through further in vivo experiments.
Clinical research
Although most studies on herbal extract monomers, such as those derived from Panax notoginseng, curcumin, and puerarin, have been conducted in vitro and in vivo, there are fewer clinical trials. Moreover, products like the Panax notoginsen saponins capsule and puerarin injection have shown promising clinical efficacy in DPN patients. A clinical investigation involving 50 cases demonstrated that the Panax notoginsen saponin capsule effectively improved total bilirubin levels (p < 0.05) and vibrating perception threshold (VPT) (p < 0.05). While there were no significant differences in serum iron levels (p > 0.05) between the treatment and control groups, the levels of hepcidin and ferritin in the treatment group [17.87 ± 5.27 ng/mL, 224.24 ± 207.44 ng/mL] were significantly lower than those of the mecobalamin group [23.06 ± 5.97 ng/mL, 422.19 ± 296.31 ng/mL] (Sun and Liu Citation2020).
Numerous studies have indicated that the hydrophobic nature of curcumin leads to poor absorption and rapid metabolism. To address this, nano-curcumin, known for its greater bioavailability, was used in a double-blind, randomized, parallel, placebo-controlled clinical trial (Asadi et al. Citation2019). The trial included 80 patients with diabetic sensorimotor polyneuropathy, aged 30 ∼ 60 years. Participants in the intervention group were instructed to take an 80 mg capsule of nano-curcumin after a meal for 8 weeks. Comparing the placebo and curcumin groups revealed that curcumin effectively reduced fasting blood sugar (FBG, p = 0.004), glycated hemoglobin (HbA1c, p < 0.001), the total neuropathy score (p < 0.001), and total reflex score (p = 0.04). Moreover, the reduction in FBG and HbA1c was correlated with a decrease in the severity of diabetic sensorimotor polyneuropathy (DSPN) (FBG: p = 0.02, r = 0.26; HbA1c: p = 0.04, r = 0.22). While the study showed that nano-curcumin supplementation could mitigate the severity of DSPN, the lack of male participants and the use of a single dose suggest the need for more comprehensive clinical studies in the future.
Puerarin injection, initially launched in China in 1993 as an adjunctive treatment for coronary heart disease, angina pectoris, and myocardial infarction, has also reflected its efficacy in treating DPN (Dong Citation2015; Yang Citation2016; Zhang Citation2017; Wang et al. Citation2018; Zeng Citation2020). In clinical studies comparing puerarin treatment with conventional therapies which receiving hypoglycemic therapy, α-lipoic acid, or vitamin B supplementation only, the observation group was administered an intravenous infusion of puerarin daily, with their general condition, symptom scores, and NCV closely monitored. The results indicated several significant findings: 1) the total effective rate of treatment in the observation group (91.67%) was markedly higher than in the control group (81.25%, p < 0.05), 2) patients receiving puerarin injection showed better improvement in symptom scores compared to before treatment (p < 0.05), 3) treatment positively influenced blood rheology (p < 0.05), enhancing nerve blood supply and reduced nerve damage, and 4) electromyogram (EMG) analysis revealed significant improvements in the motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV) of the median and common peroneal nerves after treatment (p < 0.05) (Wang et al. Citation2018).
Self-composed Chinese herbal compound prescription
Chinese herbal compounding, a traditional therapeutic method with a rich history in China, has received increasing attention in recent years, particularly for treating complex and multifaceted diseases such as DPN. The preclinical and clinical studies were reviewed.
Preclinical research
Preclinical studies have demonstrated improvements in DPN with the combination of herbal medicines, including Tang-luo-ning (TLN), Jinmaitong, Jiaweibugan decoction, Huangqi Guizhi Wuwu decoction, Modified Huangqi Guizhi Wuwu decoction, Danggui Sini decoction, Mongolian prescriptions, Long-Qi decoction, and the Tangtong formula. The details are shown in .
Table 2. TCM compound formula for the treatment of DPN(Preclinical).
Tang-luo-ning (TLN) is a traditional Chinese herbal compound prescription consisting of eight herbs. Both in vivo and in vitro studies demonstrated that TLN downregulates the expression of apoptosis-related proteins, such as the caspase-3 and Bcl-2-associated X protein (Bax). It inhibits sciatic nerve apoptosis, improves motor and sensory nerve conductance, and enhances thermal sensory threshold in DPN rats. Additionally, TLN attenuates demyelination and axonal atrophy (Yang et al. Citation2017). In DPN rats, TLN showed improvement in the thermal perception threshold (TPT) of the rat tail, NCV, and demyelination, and reversed the expression levels of apoptosis-related proteins (Li X et al. Citation2019).
Jinmaitong (JMT), consisting of 12 drugs, is a prescription from Peking Union Medical College Hospital of the China Academy of Medical Sciences. It has been reported to inhibit inflammatory reactions, modulate the gut microbiota, and affect neuregulin 1 in DPN rats (Sun et al. Citation2019; Xie et al. Citation2020a). JMT improves NCV and pain and temperature sensation in rats. Pharmacological studies suggest that its potential mechanism involves repairing nerve damage and enhancing nerve regeneration. This is achieved by increasing the content of insulin-like growth factor 1 (IGF-1), insulin-like growth factor 1 receptor (IGF-1R), myelin protein zero (MPZ), and peripheral myelin protein 22 (PMP22) expression, as well as related mRNAs in tissues under high glucose conditions (Song et al. Citation2019, Citation2021).
Jiaweibugan decoction, which primarily contains ingredients from nine well-known traditional Chinese herbs, exhibits significant effects on DPN through antioxidative stress. Research has verified that Jiaweibugan decoction markedly decreases serum MDA level (p < 0.05) and increases the glutathione (GSH) level (p < 0.05) compared to the model group (Wang et al. Citation2013).
Huangqi Guizhi Wuwu decoction (HGW), comprising five traditional medicines of the ‘Synopsis of Golden Chamber’ also demonstrates efficacy in treating DPN by inhibiting oxidative stress. Under hyperglycemic conditions, HGW treatment in RSC96 cells has been shown to reduce the level of ROS while upregulating the activity of total superoxide dismutase (T-SOD) (Gao Citation2022). It’s revealed that HGW ameliorates the typical phenotypes of DPN in mice by changing the gut microbiota and plasma metabolism, which include an increase in tactile perception levels and thermal latency, as well as a decrease in SNCV and MNCV (Zhang et al. Citation2024).
Similarly, the modified Huangqi Guizhi Wuwu decoction (MHGW) has been the subject of study. Lucas Fast Blue staining (LFB) of sciatic nerve samples from rats in the administration group indicated more regular myelin sheath morphology, less swelling of the myelin sheath, more orderly nerve fibers, and a more uniform density (Zhang et al. Citation2023a, Citation2023b).
In addition to decoctions, various other dosage forms of proprietary Chinese medicines, such as Tang Bi Kang granule (TBK), Compound Qiying granule, QiGui TangTongNing granule, Liuweiluobi granule, Chaihu Shugan powder, and Compound XiongShao capsule (CXSC), have been developed in hospitals or research institutes for DPN treatment.
The Tang Bi Kang granule, based on the Huangqi Guizhi Wuwu decoction, is an empirical formula developed by Professor Liu Tonghua of Beijing University of Chinese Medicine. It has shown significant efficacy in improving DPN. Studies on TBK (Yang XW et al. Citation2015; Liu G et al. Citation2023) indicate that it can significantly reverse MNCV and SNCV deficits and reduce axonal atrophy and demyelination. TBK achieves this by markedly decreasing FBG, inflammatory cytokines, and oxidative stress-related indices, as well as by lowering thermal perception thresholds.
Similarly, the Compound XiongShao capsule (CXSC) (Yu et al. Citation2021), derived from the traditional classical herb formula Buyang Huanwu Decoction, comprises 12 traditional Chinese herbs. A study on CXSC confirmed its neuroprotective effects in DPN rats by maintaining the integrity of myelin and axonal structures, and alleviating NCV impairments (MNCV: F5,36 = 7.644, p < 0.0001; SNCV: F5,36 = 12.83, p < 0.0001). Furthermore, CXSC was also effective in reducing mechanical hyperalgesia (F5,36 = 18.24, p < 0.0001) and thermal hyperalgesia (F5,36 = 8.45, p < 0.0001).
Clinical research
There is growing clinical interest in the use of empirical compound formulas for treating DPN. These include Xiaoketongbi formula (XF), Danggui Sini decoction and its modifications, Fuyang Tongluo decoction, modified Huangqi Guizhi Wuwu decoction, Tangmoning decoction, Buyang Huanwu decoction, and Fuyuan Huoxue decoction ().
Table 3. TCM compound formula for the treatment of DPN(clinical).
The Xiaoketongbi formula (XF), derived from the Tao-He-Cheng-Qi decoction in the ‘Treatise on Typhoid Fever’, primarily comprises Persicae semen, Rheum officinale Baill. (Polygonaceae), and Astragali radix. To assess its effectiveness in treating painful diabetic neuropathy, a single-center, randomized, single-blind, double-dummy, and parallel-controlled clinical trial involving 68 patients was conducted. After 10 weeks of treatment, the Brief Pain Inventory for Diabetic Peripheral Neuropathy (BPI-DPN) scores showed a significant decrease in both the XF and pregabalin groups, dropping from 42.44 ± 17.56 to 26.47 ± 22.22 and from 52.03 ± 14.30 to 37.85 ± 17.23 points, respectively (p < 0.001). However, there were no significant differences in the BPI-DPN score reduction between the two groups. Compared to the pregabalin group, the XF group demonstrated a significantly higher number of patients showing improvement [35.3% (12/34) vs. 11.8% (4/34), p = 0.045] and a more substantial absolute change in MNCV of the right median nerve (0.7 ± 2.3 in the XF group vs. −2.2 ± 4.1 in the pregabalin group, p = 0.004). No serious adverse effects were reported during the trial indicating the safety of XF (Lu et al. Citation2022).
Danggui Sini decoction (DGS), also originating from the ‘Treatise on Typhoid Fever,’ consists primarily of seven Chinese herbs. Clinical studies have demonstrated the efficacy of DGS and its modifications in treating DPN patients with symptoms of cold coagulation blood stasis syndrome, blood deficiency cold coagulation syndrome, qi deficiency blood stasis syndrome, and cold dampness spleen syndrome. Cao (Citation2018) showed that DPN patients characterized by cold coagulation and blood stasis were selected as the observation group and treated with 350 mL/day of DGS juice for four weeks. The results indicated a higher total effective rate in the observation group compared to the control group. Additionally, there was a more significant improvement in MNCV and SNCV of the common peroneal and tibial nerves in the limbs of the observation group.
Furthermore, treating elderly diabetic peripheral neuropathy of the blood deficiency and cold coagulation type with the modified Danggui Sini decoction (MDGS) not only improved MNCV and SNCV, but also enhanced hemorheological indexes, reduced platelet aggregation, and promoted blood circulation after four weeks of treatment (Yang Citation2017a). Combining MDGS with acupressure was found to improve clinical symptoms and MNCV in treating DPN with qi deficiency and blood stasis (Yu Citation2016). Chen (Citation2018) reported that improving renal function and hemorheology could alleviate the clinical symptoms of DPN patients with cold dampness and spleen trapping syndrome.
Fuyang Tongluo decoction (FYTL), a formula comprising six Chinese herbs based on DGS and modified Baizhu Fuzi Tang, was administered to 60 patients diagnosed with DPN and cold congealed blood stasis syndrome (Zhang Dong, Feng, Cui et al. Citation2019; Zhang, Dong, Feng, Guo Citation2019). It was revealed that FYTL significantly reduced blood glucose and lipid levels than the control groups and showed good therapeutic efficacy in the total clinical effectiveness rate (71.67% vs. 48.33%, p < 0.05) and the Toronto Clinical Scoring System (TCSS) scores (5.5 ± 2.4 vs. 6.5 ± 1.7, p < 0.05) than the control group. Additionally, FYTL can also increase serum NGF and insulin-like growth factor (IGF) mRNA expression to alleviate neuronal damage.
Another prescription, Fuyuan Huoxue decoction (FYHX), demonstrated significant efficacy in relieving symptoms related to tingling, burning, and numbness in patients compared to the control group, as well as in improving TCSS scores (Zhang and Zhang Citation2018). A clinical trial reported that FYHX was shown to accelerate muscle nerve conduction speed and regulate blood glucose levels (Zhang Citation2018).
Clinical evidence supports the therapeutic effects of some proprietary Chinese medicines, developed based on hospital experiences, on DPN. These include Jiangtang Huoluo pill, Jiangtang Tongmailing capsule, Xiongshao capsule, and Danshen and Gegen granule. Compound Xiongshao capsule (XS) (Cui et al. Citation2018) has shown a significant therapeutic effect in treating DPN by improving NCV, the incubation period, and amplitude of the median and common peroneal nerves, as well as reducing the whole blood high-shear viscosity in patients. Its efficacy is comparable to that of the combination of epoprostenol with beziridine sodium and is superior to that of the combination of furadithiamine with methylcobalamin and epoprostenol monotherapy.
In a clinical study involving the Jiangtang Huoluo pill combined with TCM fumigation (Wu et al. Citation2019), the total effective rate of the treatment group was 90.0%, significantly higher than that of the control group (p < 0.05). Furthermore, both the Jiangtang Tongmailing capsule (Mu Citation2016) and the Danshen and Gegen granule (Wen Citation2017) have been shown to aid recovery in DPN patients and are recommended for clinical use.
Chinese traditional patent medicine
Chinese patent medicines are a category of drugs that have a fixed prescription composition and ratio and are available on the market. Among them, the Mudan granule is currently used to treat DPN. Others currently undergoing clinical studies for DPN include the Mongolian medicine Zhenbao Pill, the compound Dangshen dropping pill, and the Tongxinluo capsule. While these drugs have been available on the market for an extended period, their therapeutic effects on DPN have not been fully validated. Therefore, more preclinical and clinical research is necessary to substantiate its efficacy in treating DPN.
The Mudan granule, composed of nine herbs, was approved in 2008 by the National Medical Products Administration as a specific treatment for DPN. Pharmacological experiments confirmed that Mudan granule can alleviate symptoms of DPN in rats by improving myelinated large fibers, addressing early small nerve fiber lesions, and accelerating NCV (Yang W et al. Citation2015). Additionally, it has been shown to increase potassium channel kv7.3 mRNA expression and decrease N-type calcium channel protein mRNA expression, aiding in the treatment of painful diabetic peripheral neuropathy (PDPN) (Qi and Yu Citation2015a).
In the clinical realm, several studies have been conducted to evaluate the efficacy of Mudan granule in DPN patients. In a clinical trial (Qi and Yu Citation2015b), the treatment group (n = 32) received Mudan granule for four weeks, in addition to the standard treatment received by the control group. The results indicated a higher clinical efficacy rate in the Mudan granule group (84.4% vs. 62.5%, p < 0.05). It was also found to improve lipid metabolism and antioxidant stress. Another clinical trial observed the effects of Mudan granule administration over eight weeks. Statistical analyses of the left and right common peroneal nerves, as well as the conduction rates of the left and right tibial nerves, showed more significant improvements in the observation group compared to the control group (p < 0.05) (Yao Citation2018; Zhang Citation2019).
The Mongolian medicine Zhenbao pill (MMZ), which contains 17 herbs, has traditionally been used to treat cerebral hemorrhage, nerve injury, and radiculitis. Recently, it has been discovered that MMZ can also promote the division and proliferation of Schwann cells. Consequently, two research groups (Yi et al. Citation2019; Zhao Citation2021) conducted randomized controlled clinical studies to observe the therapeutic effect of MMZ on DPN patients. Although no significant differences were found in blood glucose, lipid levels, TCM syndrome, or Michigan Diabetic Neuropathy Score (MDNS) scores between the MMZ-administered group and the control group, there was a significant increase in MNCV and SNCV of the median and common peroneal nerves in the MMZ group (p < 0.05).
In addition, the compound Danshen dropping pill (Yu Citation2022) and the Tongxinluo capsule (Du Citation2018), previously used for coronary artery disease and angina pectoris, have been clinically combined with methylcobalamin in the treatment of DPN. This combination was found to alleviate clinical symptoms such as pain and numbness in DPN patients.
Mechanisms of chinese medicine in treating DPN
There are many mechanisms for treating DPN with traditional Chinese medicine, and the main ones are anti-inflammatory, antioxidation, improvement of mitochondrial function and endoplasmic reticulum stress. These aspects were introduced respectively and summarized in .
Figure 1. Mechanisms and representative drugs of TCM in treating DPN. Inflammation (blue): alleviated through AMPK, GPR40, TLR4 and PPARγ signal pathways. Oxidative stress and apoptosis (red): inhibit oxidative stress, and apoptosis through Keap1/Nrf2 and PI3K/AKT pathways. Mitochondrial dysfunction (purple): the regulation of mitochondria can be divided into mitochondrial biogenesis, dynamics, oxidative stress and autophagy. Endoplasmic reticulum stress (green): TCM inhibits endoplasmic reticulum stress through the IRE1α/XBP1/CHOP signaling pathway. AMPK: the adenosine 5′-monophosphate (AMP)-activated protein kinase; GPR40: G protein-coupled receptor 40; TLR4: Toll-like receptor 4; PPAR: the peroxisome proliferator-activated receptor; Nrf2: the nuclear factor erythroid 2-related factor 2; PI3K: Class I phosphoinositide 3-kinase; AKT: serine/threonine kinase; Keap1: Kelch-like ECH-associating protein 1; IRE1α: inositol requiring enzyme 1; XBP1: X-box binding protein1; CHOP: CCAAT/enhancer binding protein homologous protein.
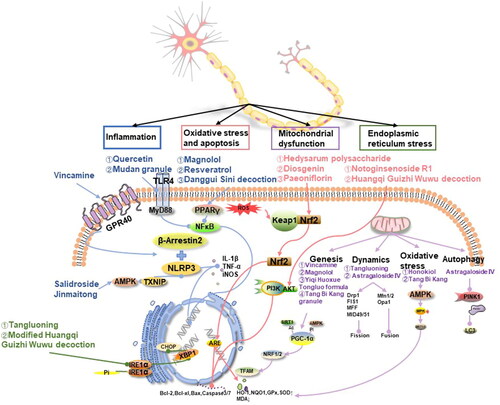
Inflammatory reaction
A growing body of research suggests that neuroinflammation plays a critical role in the pathogenesis of DPN. The systemic inflammatory response, often accompanying insulin signaling dysfunction in hyperglycemic states, activates transcription factors such as nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) and NF-κB. This activation triggers a cascade of cytokines and chemokines, leading to an increase in pro-inflammatory cytokines. These cytokines not only contribute to decreased NCV, impaired blood supply to nerves, and degeneration of the axon terminals but also play a significant role in the onset and progression of DPN (Feldman et al. Citation2019). The mechanism of TCM in treating DPN may involve inhibiting the inflammatory response in nerves by regulating the NLRP3 and NF-κB pathways.
NLRP3 is a central regulator of inflammation and is highly expressed in glial cells and neurons. TCM may exert anti-inflammatory effects by modulating NLRP3. Vincamine, an activator of the G protein-coupled receptor 40 (GPR40), and β-arrestin2, a key regulator of GPR40 signaling, have been implicated in NLRP3 activation. Experimental evidence has shown that in mice treated with vincamine, GPR40 and β-arrestin2 were increased in sciatic nerves and RSC96 cells compared to those of a DPN mouse model. Immunoprecipitation experiments have indicated that β-arrestin2 can bind to NLRP3. Thus, vincamine promotes the binding of NLRP3 to β-arrestin2 and inhibits the expression of pro-inflammatory factors such as IL-1β and TNF-α, as well as the pro-inflammatory enzyme iNOS (Xu JW et al. Citation2023).
The thioredoxin-interacting protein (TXNIP) is recognized as a central molecule in the inflammatory process leading to the death of insulin-producing cells in the human pancreas. It is upstream of NLRP3, and the TXNIP-NLRP3 complex is essential for the activation of inflammatory vesicles (Sun et al. Citation2019). Salidroside has been shown to enhance the activity of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK), reduce TXNIP levels and inhibit NLRP3 inflammatory vesicle activation. This results in a decrease in serum expression of caspase-1 and IL-1β, thereby alleviating diabetic neuropathic pain (Zheng et al. Citation2021). Furthermore, Jinmaitong compound has also been found to ameliorate DPN by inhibiting the TXNIP/NLRP3 pathway (Sun et al. Citation2019).
NF-κB, a transcription factor that mediates immune and inflammatory responses, is composed of subunits including p65, p50, and IκBα. It acts by activating the IκB kinases complex, subsequently releasing heterodimers p65 and p50, which then rapidly enter the nucleus (Yu et al. Citation2020). High levels of glucose and free fatty acids (FFA) can activate NF-κB and downstream inflammatory pathways, leading to upregulation of pro-inflammatory genes such as IL-1β and TNF-α. Toll-like receptor 4 (TLR4), a transmembrane recognition receptor, recruits myeloid differential protein-88 (MyD88) to activate NF-κB. In studies exploring the mechanism of action of quercetin and Mudan granule in treating DPN, Western Blot analysis (WB) and immunohistochemical experiments showed significant reductions in the expression of TLR4, MyD88, p65, and NF-κB in the sciatic nerve, as well as decreases in inflammatory factors like TNF-α, IL-1β, and IL-6 in the serum and sciatic nerve. These findings suggest that quercetin and Mudan granule may ameliorate typical myelin, axonal, and neuronal damage in rats by inhibiting the inflammatory response through modulatory effects on critical proteins in the TLR-related pathway, including TLR4, MyD88, and NF-κB (Zhao et al. Citation2021; Zhou and Yao Citation2021).
The peroxisome proliferator-activated receptor (PPAR) has three isoforms, among which PPARγ is specifically involved in neural tissues, playing a role in glucose and lipid metabolism, insulin sensitivity, and cell growth. Magnolol has been identified as an agonist of PPARγ. Using a PPARγ-specific knockdown mouse model of DPN, it has been shown that PPARγ acts as an upstream pathway of NF-κB, inhibiting NF-κB expression and nuclear translocation, and consequently downregulating TNF-α or iNOS. This leads to attenuation of neuronal damage from the inflammatory response in DPN (Yang J et al. Citation2022). Similar mechanistic studies on resveratrol and Danggui Sini decoction (Kumar and Sharma Citation2010; Cheng Citation2020) for DPN treatment have found that NF-κB protein and mRNA expression, along with inflammatory cascade responses, are downregulated, thus ameliorating elevated levels of TNF-α, IL-6, and COX-2.
Oxidative stress and apoptosis
Hyperglycemia-induced oxidative stress is a key contributor to neuronal apoptosis and the subsequent development of neuropathies such as DPN. Studies have established a positive correlation between the occurrence of DPN and the level of oxidative stress. TCM has been shown to potentially inhibit the production of downstream factors involved in oxidative stress. This inhibition is achieved by activating key pathways, namely the nuclear factor erythroid 2-related factor 2 (Nrf2) and the class I phosphoinositide 3-kinase (PI3K)/serine/threonine kinase (AKT) pathways. By modulating these pathways, TCM could alleviate neurotrophic reduction, axonal degeneration, and neuronal apoptosis caused by oxidative stress, thus offering a potential therapeutic approach for DPN.
Nrf2, a crucial transcription factor that regulates cellular oxidative stress, plays a beneficial role in ameliorating oxidative stress and promoting cell survival by maintaining cellular redox homeostasis (Leng et al. Citation2020). Under normal conditions, Nrf2 is bound to Kelch-like ECH-Associating protein 1 (Keap1) in the cytoplasm. During cellular stress, ROS oxidizes Keap1, leading to the release and nuclear translocation of Nrf2 (He et al. Citation2022). Nrf2 regulates downstream antioxidant response elements (AREs), including nucleotide adenosine diphosphate hydrolase (NADH), haem oxygenase-1 (HO-1), quinone oxidoreductase-1 (NQO1), and glutamylcysteine ligase (GCLC) (Leng et al. Citation2020). Additionally, it upregulates anti-apoptotic proteins such as B cell lymphoma-2 (Bcl-2) and Bcl-xL, and downregulates pro-apoptotic protein Bax, thus reducing caspase 3/7 activity and minimizing apoptotic cell death.
Immunofluorescence and WB assays have shown that Hedysarum polysaccharide significantly increases Nrf2 protein expression by inhibiting Keap1 expression. This modulation attenuates MDA production, a marker of oxidative stress, in the serum of ob/ob mice, and increases the expression of the glutamate-cysteine ligase catalytic subunit (GCLC), a rate-limiting enzyme in glutathione (GSH) synthesis (He et al. Citation2022). Moreover, glutathione reductase, an enzyme critical to converting oxidized glutathione disulfide to its reduced form, saw increased mRNA and protein expression under Hedysarum polysaccharide treatment, thus mitigating oxidative stress damage to nerves.
Further research into the mechanism of diosgenin in DPN treatment revealed its ability to increase the activity of SOD and glutathione peroxidase (GPx). This is achieved by reversing the decrease in Nrf2 levels under high glucose conditions, thus increasing the expression of downstream HO-1 and NQO1 and reducing MDA levels (Leng et al. Citation2020). Additionally, paeoniflorin administration to RSC96 cells promoted the upregulation of Nrf2 protein levels, enhancing nuclear translocation and activation of the Nrf2/ARE pathway to alleviate oxidative stress. The Keap1/Nrf2 pathway’s influence on the anti-apoptotic factor Bcl2 was also observed, with increased levels of Bcl2 and decreased levels of Bax and caspase-3 (Yang et al. Citation2016).
The PI3K/AKT pathway is a classic insulin signaling pathway that plays a crucial role in regulating oxidative stress and apoptosis in neurotoxicity. PI3K is a key regulatory signal in glucolipid metabolism, oxidative stress modulation, and neuroprotection. It activates downstream target proteins, including protein kinase B (AKT), through the phosphorylation of threonine 308 or serine 473 (Tan et al. Citation2022). In a high glucose state, cellular phosphorylation of PI3K and AKT is inhibited, leading to an increase in pro-apoptosis-related protein expression. Experimental studies have shown that notoginsenoside R1 (NGR1) can activate the PI3K/AKT pathway in a high glucose state. This is achieved by downregulating the expression of miR-503, which is sensitive to high glucose. As a result, NGR1 reduces ROS and caspase-3 expression, improving cell viability, and reducing apoptosis (Wang W et al. Citation2019).
Similarly, protein expression levels of PI3K, AKT, and phosphorylated AKT (p-AKT) were elevated in the treatment group receiving the Huangqi Guizhi Wuwu decoction. However, when this treatment was combined with LY294002, an inhibitor of the PI3K/AKT pathway, there was a significant decrease in the protein expression of PI3K, AKT, and p-AKT compared to the group treated with TCM alone. The oxidative stress-related indices in this combined treatment group were similar to those observed in the model group. This suggests that the Huangqi Guizhi Wuwu decoction might decrease the ROS content and increase total superoxide dismutase (T-SOD) activity by activating the PI3K/AKT pathway. Therefore, it could effectively enhance the antioxidant capacity of cells and attenuate oxidative stress-induced cellular damage (Gao Citation2022).
Mitochondrial dysfunction
As the survival of neurons in the peripheral nervous system and their associated distal axons is highly dependent on energy, the core pathogenesis of DPN can be viewed as a state of impaired metabolism and bioenergetic exhaustion (Eid et al. Citation2023). In high glucose states, mitochondrial dysfunction leads to maladaptive glucose and lipid energy metabolism in human sensory neurons, axons, and Schwann cells (Chandrasekaran et al. Citation2019). Mitochondria are pivotal in cellular energy metabolism and possess multiple regulatory mechanisms, including mitochondrial biogenesis, dynamics, oxidative stress, and autophagy (Huang et al. Citation2024). Therefore, Chinese medicine, by targeting these mitochondrial aspects, may play a role in the treatment of DPN and be shown in .
Figure 2. Mechanisms of traditional chinese medicine in treating DPN by improving mitochondrial dysfunction, including mitochondrial biogenesis (①), mitochondrial dynamics (② and ③), mitochondrial oxidative stress (④) and mitochondrial autophagy (⑤) (by figdraw). PGC-1α: PPARγ coactivator-1α; AMPK: the adenosine 5′-monophosphate (AMP)-activated protein kinase; NRF1 and NRF2: nuclear respiratory factors 1 and 2; TFAM: the mitochondrial transcription factor A; SIRT1: Sirtuin 1; Drp1: dynamin-related protein 1; Mfn1/2: mitochondrial protein 1/2; Opa1: Optic atrophy 1; AGEs: advanced glycosylation end products; PINK1: PTEN-inducible kinase 1; parkin: Parkinson’s disease protein.
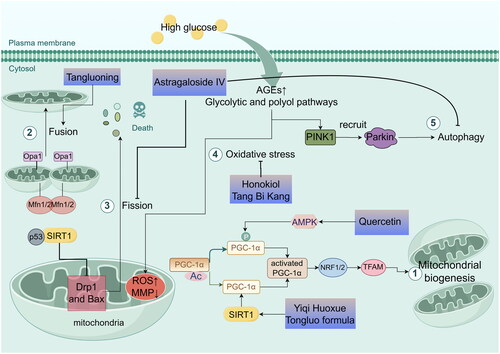
Mitochondrial biogenesis, the process of generating new mitochondria from pre-existing ones through division, is regulated by PPARγ coactivator-1α (PGC-1α). This regulation occurs upon activation of PGC-1α through phosphorylation or deacetylation. Activated PGC-1α subsequently stimulates nuclear respiratory factors 1 and 2 (NRF1 and NRF2), which then activate mitochondrial transcription factor A (TFAM), promoting mitochondrial DNA and protein synthesis (Li et al. Citation2017). Interestingly, PGC-1α activation can be triggered by phosphorylation through AMPK, as well as deacetylation through Sirtuin 1 (SIRT1) (Cardanho-Ramos and Morais Citation2021). Quercetin, identified as a potential agent for treating DPN, is believed to correct pathological changes such as oxidative stress, reduced ATP production, and mitochondrial morphology damage in DPN rats. It does this by activating the AMPK/PGC-1α pathway and upregulating the expression of AMPK, PGC-1α, NRF1, and TFAM, thus playing a crucial role in protecting neuro mitochondrial biological functions. Furthermore, the compound Yiqi Huoxue Tongluo formula (Zhang L et al. Citation2022) has been shown to ameliorate DPN by regulating energy metabolism in nerves, as evidenced by the upregulation of Na+/K+-ATPase activity and increased SIRT1, PGC1α, TFAM mRNA, and protein expression. Additionally, herbal medicines such as magnolol (Yang J et al. Citation2022), vincamine (Xu JW et al. Citation2023), and Tang Bi Kang granule (Yu et al. Citation2023) have demonstrated similar effects.
Mitochondrial dynamics, which include mitochondrial fusion, fission, and degradation, play a crucial role in energy production. Regulation of mitochondrial fission involves primarily dynamin-related protein 1 (Drp1), fission protein 1 (FIS1), mitochondrial fission factor (MFF), and mitochondrial dynamics proteins 49/51 (MID49/51). In contrast, fusion is regulated by mitochondrial proteins 1 and 2 (Mfn1 and Mfn2) for the outer mitochondrial membrane (OMM), and by optic atrophy 1 (Opa1) for the inner mitochondrial membrane (IMM) (Chan Citation2020). AS-IV (Ben et al. Citation2021) exhibits a protective effect against mitochondrial damage by regulating the SIRT1/p53 pathway, downregulating the expression of Drp1 and Bax, and upregulating the anti-apoptotic protein Bcl-2. This modulation inhibits mitochondrial fission and concurrently reduces apoptosis. Tangluoning (Zhu et al. Citation2021) significantly increases ATP and matrix metalloproteinases (MMP) levels in DPN rats by reducing calcium ions. This effect is achieved by upregulation of Mfn1, Mfn2, and Opa1 expression and reduction of the mitochondrial fission-associated Drp1, which improves mitochondrial dyskinesia, enhances the expression of sciatic nerve myelin basic protein (MBP) and myelin protein zero (MPZ), and mitigates neural sheath lesions and demyelination. Moreover, the Mudan granule downregulates the expression of mitochondrial Drp-1, leading to decreased Bax and MDA content, and increased activity of Bcl-2, GSH, and catalase (CAT) indicators (Chen et al. Citation2017).
Mitochondria are the primary source of ROS in most mammalian cells. In the diabetic state, autoxidative glycosylation, the formation of advanced glycosylation end products (AGEs), and increased activity of the glycolytic and polyol pathways lead to excess ROS production. This excess in mitochondrial ROS contributes to reduced MMP, the release of apoptotic proteins, and, ultimately, mitochondrial dysfunction (Bhatti et al. Citation2017). When mitochondrial damage and ROS overproduction occur, there is a loss of Schwann cells, myelinated axons, and sensory neurons. This is accompanied by insufficient ATP production, further exacerbating axonal injury and leading to the development of chronic degenerative diseases (Chowdhury et al. Citation2013). The AMPK/SIRT1 pathway is crucial in countering HG-induced oxidative stress in the nervous system. In RSC96 cells treated with honokiol, there was a reversal of the inhibitory effect of high glucose on AMPK, increased AMPK phosphorylation, and a significant increase in SIRT1 and SIRT3 mRNA and protein levels. This was accompanied by similar enhancements in the protein expression of downstream target genes (SOD1 and SOD2) (Hu M et al. Citation2023). Similarly, the Chinese medicine Tang Bi Kang has been shown to improve mitochondrial oxidative stress by activating the AMPK/SIRT3 pathway, promoting SOD expression, reducing MDA levels, and increasing the NAD+/NADH ratio, as well as ATP synthesis (Yu et al. Citation2023).
Neural tissues, which are rich in mitochondria, undergo significant changes under oxidative stress under high glucose conditions. Selective autophagy in mitochondria reduces ROS production in these organelles, thus protecting cells from damage. However, neurons are particularly sensitive to autophagy. A sudden increase in autophagic activity can lead to dramatic intracellular changes, enhancing neuronal autophagy and potentially resulting in cell necrosis and neuronal disintegration (Li and Zhang Citation2023). The PTEN-inducible kinase 1 (PINK1)/Parkinson’s disease protein (Parkin) pathways are among the most extensively studied in mitochondrial autophagy. PINK1 kinase activity is essential for the rapid and efficient recruitment of Parkin to damaged mitochondria (Geisler et al. Citation2010). Experimental evidence has shown that AS-IV (Wei et al. Citation2022) significantly downregulates LC3, PINK, and Parkin expression. This downregulation ameliorates oxidative stress and inhibits overactivation of autophagy in Schwann cells.
Endoplasmic reticulum stress
The endoplasmic reticulum plays a crucial role in various metabolic processes in vivo, including protein synthesis, folding, modification, and transport. When protein misfolding exceeds a certain threshold, it disrupts endoplasmic reticulum homeostasis, inducing endoplasmic reticulum stress (ERS) and leading to neurological injury (O’Brien et al. Citation2014). During ERS, the accumulation of unfolded proteins causes GRP78 to dissociate from membrane proteins and bind to these unfolded proteins. Inositol requiring enzyme 1 (IRE1α), a transmembrane protein in the endoplasmic reticulum, forms dimers and undergoes autophosphorylation in its free state. The phosphorylated IRE1α (p-IRE1α) splices the mRNA precursor of transcription factor X-box binding protein 1 (XBP1), removing an intron. This sustained expression of XBP1 activates the ERS apoptotic transcription factor CCAAT/enhancer-binding protein homologous protein (CHOP) in the nucleus (Lin and Popko Citation2009). CHOP can then directly or indirectly induce apoptosis.
Tangluoning and the modified Huangqi Guizhi Wuwu decoction have been found to reduce ERS and improve the structure and function of the sciatic nerve through this pathway. Researchers (Li X et al. Citation2019) observed that after 12 weeks of administering Tangluoning to DPN rats by gavage, the expression of GRP78 in the treated group was significantly higher compared to the model group, as shown by the WB analysis (p < 0.05). Additionally, the expressions of IRE1α/P-IRE1α and XBP-1 were significantly lower in the treated group compared to the model group (p < 0.05, p < 0.01). These changes in protein content may be related to the observed improvements in sciatic nerve function and myelin structure in rats. Similarly, treatment of DPN with modified Huangqi Guizhi Wuwu decoction (Zhang et al. Citation2023b) significantly reduced the expression of ERS-related proteins, such as p-IRE1α and CHOP (p < 0.05, p < 0.01), compared to the model group, according to WB analysis findings. Real-time fluorescence quantitative polymerase chain reaction techniques confirmed these results at the mRNA level for these two proteins. This suggests that the compound could ameliorate neuromyelin structure damage in diabetic conditions by regulating the expression of ERS-related proteins and mRNAs. The potential upstream targets of the compound warrant further investigation.
Discussion
DPN is a chronic metabolic disorder characterized by a complex etiology and pathogenesis. The risk of microvascular complications begins to manifest even during the prediabetes stage. Patients with prolonged hyperglycemia often develop lipid metabolism disorders, leading to thickening of the capillary basement membranes, endothelial hyperplasia, and swelling of vascular and endothelial cells. These changes contribute to the deposition of glycoproteins in the vessel walls, narrowing of the lumen, microcirculation disorders, and ultimately ischemia and hypoxia-induced neural tissue damage, characteristic of DPN. Nerve damage associated with DPN is evident in both structural and functional changes, including deficiency of constituent proteins and trophic factors.
Preclinical experiments using TCM to examine the structure of nerves in DPN rats have shown that most TCM treatments can improve peripheral fiber degeneration, myelin sheath swelling, and nerve fiber demyelination, resulting in better-aligned nerve fibers. Although these structural improvements are not visually observable in clinical settings, functional evaluations of sciatic nerve performance, including tests for NCV, TPT, and MWT, have shown that TCM can enhance NCV and improve TPT and MWT sensitivity in DPN. Furthermore, certain herbal medicines have been found to increase resistance to injury and regeneration of peripheral nerves by increasing the expression of neurotrophic factors, such as NGF and BDNF.
This review comprehensively summarizes the potential mechanistic pathways through which TCM treats DPN. TCM has been found to alleviate oxidative stress and inhibit apoptosis through the Keap1/Nrf1/HO-1 and PI3K/AKT pathways. Additionally, it can suppress the production of inflammatory factors in nerves through the NLRP3 inflammasome and NF-κB pathways. TCM also enhances mitochondrial function via the AMPK/SIRT1/PGC-1α pathway, mitigates endoplasmic reticulum stress by inhibiting the IRE1α/XBP-1/CHOP pathway, and attenuates autophagy-related nerve damage. By modulating one or more of these pathways and their interactions, TCM demonstrates a multifaceted effect, often showing superior preclinical and clinical efficacy compared to Western medications such as methylcobalamin and α-lipoic acid. Future research is essential to unravel the underlying pathogenesis of DPN and to identify the key targets and mechanisms of TCM treatment.
Conclusions
DPN is a complex metabolic disorder with multifaceted pathogenesis, beginning at the prediabetes stage. TCM has demonstrated efficacy in the treatment of DPN, with promising outcomes observed in the improvement of nerve structure and function. These effects appear to be mediated by mechanisms such as the reduction of oxidative stress and inflammation, and the enhancement of mitochondrial function. TCM, often used in conjunction with Western medications such as methylcobalamin and α-lipoic acid, has demonstrated superior efficacy and a higher safety profile in clinical trials. The future treatment of DPN lies in understanding its pathogenesis and revealing the concrete mechanisms of TCM, which could lead to more effective and integrated treatment.
Authors’ contribution
The review was designed and performed by Xuansheng Ding, Yuna Zhang, Xianglong Wu, Wenhui Yao, and Yadong Ni. The manuscript was written by Yuna Zhang and Xianglong Wu. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- American Diabetes Association Professional Practice Committee. 2024. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2024. 2024. Diabetes Care. 47(Suppl 1): s 231–s243. doi:10.2337/dc24-S012.
- Afshinnia F, Reynolds EL, Rajendiran TM, Soni T, Byun J, Savelieff MG, Looker HC, Nelson RG, Michailidis G, Callaghan BC, et al. 2022. Serum lipidomic determinants of human diabetic neuropathy in type 2 diabetes. Ann Clin Transl Neurol. 9(9):1392–1404. doi:10.1002/acn3.51639.
- Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM, Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group. 2010. Effect of prior intensive insulin treatment during the diabetes control and complications trial (DCCT) on peripheral neuropathy in type 1 diabetes during the epidemiology of diabetes interventions and complications (EDIC) study. Diabetes Care. 33(5):1090–1096. doi:10.2337/dc09-1941.
- Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, Jensen TM, Finnerup NB, Jensen TS, Lauritzen T, et al. 2018. Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: addition-Denmark. Diabetes Care. 41(5):1068–1075. doi:10.2337/dc17-2062.
- Ang L, Jaiswal M, Martin C, Pop-Busui R. 2014. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 14(9):528. doi:10.1007/s11892-014-0528-7.
- Asadi S, Gholami MS, Siassi F, Qorbani M, Khamoshian K, Sotoudeh G. 2019. Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: a randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 43:253–260. doi:10.1016/j.ctim.2019.02.014.
- Azuma Y, Ozasa N, Ueda Y, Takagi N. 1986. Pharmacological studies on the anti-inflammatory action of phenolic compounds. J Dent Res. 65(1):53–56. doi:10.1177/00220345860650010901.
- Ben Y, Hao J, Zhang Z, Xiong Y, Zhang C, Chang Y, Yang F, Li H, Zhang T, Wang X, et al. 2021. Astragaloside IV inhibits mitochondrial-dependent apoptosis of the dorsal root ganglion in diabetic peripheral neuropathy rats through modulation of the Sirt1/p53 signaling pathway. Diabetes Metab Syndr Obes. 14:1647–1661. doi:10.2147/DMSO.S301068.
- Bhambhani S, Kondhare KR, Giri AP. 2021. Diversity in chemical structures and biological properties of plant alkaloids. Molecules. 26(11):3374. doi:10.3390/molecules26113374.
- Bhat A, Mahalakshmi AM, Ray B, Tuladhar S, Hediyal TA, Manthiannem E, Padamati J, Chandra R, Chidambaram SB, Sakharkar MK. 2019. Benefits of curcumin in brain disorders. Biofactors. 45(5):666–689. doi:10.1002/biof.1533.
- Bhatti JS, Bhatti GK, Reddy PH. 2017. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 1863(5):1066–1077. doi:10.1016/j.bbadis.2016.11.010.
- Callaghan BC, Hur J, Feldman EL. 2012. Diabetic neuropathy: one disease or two? Curr Opin Neurol. 25(5):536–541. doi:10.1097/WCO.0b013e328357a797.
- Cao B. 2018. Clinical observation on Danggui Sini decoction in treating diabetic peripheral neuropathy of cold coagulation and blood stasis type. Guide China Med. 16:188–189. Chinese.
- Cardanho-Ramos C, Morais VA. 2021. Mitochondrial biogenesis in neurons: how and where. Int J Mol Sci. 22(23):13059. doi:10.3390/ijms222313059.
- Chan DC. 2020. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 15(1):235–259. doi:10.1146/annurev-pathmechdis-012419-032711.
- Chandrasekaran K, Anjaneyulu M, Choi J, Kumar P, Salimian M, Ho CY, Russell JW. 2019. Role of mitochondria in diabetic peripheral neuropathy: influencing the NAD(+)-dependent Sirt1-PGC-1α-TFAM pathway. Int Rev Neurobiol. 145:177–209. doi:10.1016/bs.irn.2019.04.002.
- Chen D, Zhang Y. 2023. Clinical effect of tangmanning decoction combined with lipoic acid on diabetic peripheral neuropathy. Chinese J Clin Rational Drug Use. 16:82–85. Chinese.
- Chen H, Yang J, Liang M, Zhang Y, Zhao Z. 2017. Observation on intervention effect of Mudan granule on sciatic nerve in diabetic rats. Chinese J Diabetes. 25:359–362. Chinese.
- Chen X. 2018. Clinical study on Danggui Sini decoction in treating diabetic peripheral neuropathy with cold-dampness and spleen-trapped type. Chinese Med Nat Prod. 33:756–759. Chinese.
- Cheng D, Xia H, Huang F. 2020. Clinical observation on treatment of diabetic peripheral neuropathy with traditional Chinese medicine decoction. Chin Continuing Med Edu. 12:161–163. Chinese.
- Cheng S. 2020. Based on NF-κB signaling pathway, explore the protective mechanism of Danggui Sini decoction on peripheral neuropathy in diabetic rats [Master’s thesis]. Changchun Univ Chinese Med.
- Cheng S, Zhou X, Li X, Wu D, Qian T, Zhang W. 2019. Based on NF-κB signaling pathway, explore the protective mechanism of Danggui Sini decoction on peripheral neuropathy in diabetic rats. J Changchun Univ Chinese Med. 35:128–131. Chinese.
- Chiorcea-Paquim AM. 2023. Electrochemistry of flavonoids: a comprehensive review. Int J Mol Sci. 24(21):15667. doi:10.3390/ijms242115667.
- Chowdhury SK, Smith DR, Fernyhough P. 2013. The role of aberrant mitochondrial bioenergetics in diabetic neuropathy. Neurobiol Dis. 51:56–65. doi:10.1016/j.nbd.2012.03.016.
- Čižmárová B, Hubková B, Tomečková V, Birková A. 2023. Flavonoids as promising natural compounds in the prevention and treatment of selected skin diseases. Int J Mol Sci. 24(7):6324. doi:10.3390/ijms24076324.
- Cui H, Zhu W, Zhou J, Xiao Q, Huang J, Shen X, Yang W. 2018. Clinical observation on treatment of diabetic peripheral neuropathy with compound Xiongshao capsule. Chin Pharm. 29:223–228.
- Dong F. 2015. Therapeutic effect of puerarin injection on diabetic peripheral neuropathy and its effect on hemorheology. J Clin Med Pract. 19:114–115. Chinese.
- Dong J, Li H, Bai Y, Wu C. 2019. Muscone ameliorates diabetic peripheral neuropathy by activating the Akt/mTOR signalling pathway. J Pharm Pharmacol. 71(11):1706–1713. doi:10.1111/jphp.13157.
- Du X. 2018. Clinical study on Tongxinluo capsule in treating diabetic peripheral neuropathy. Diabetes New World. 21:177–178 + 187. Chinese.
- DCCT Research Group. 1995. Effect of intensive diabetes treatment on nerve conduction in the diabetes control and complications trial. Ann Neurol. 38(6):869–880. doi:10.1002/ana.410380607.
- Eid SA, Rumora AE, Beirowski B, Bennett DL, Hur J, Savelieff MG, Feldman EL. 2023. New perspectives in diabetic neuropathy. Neuron. 111(17):2623–2641. doi:10.1016/j.neuron.2023.05.003.
- Eseberri I, Trepiana J, Léniz A, Gómez-García I, Carr-Ugarte H, González M, Portillo MP. 2022. Variability in the beneficial effects of phenolic compounds: a review. Nutrients. 14(9):1925. doi:10.3390/nu14091925.
- Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. 2020. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 18(2):117–124. doi:10.2174/1570161117666190502103733.
- Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. 2019. Diabetic neuropathy. Nat Rev Dis Primers. 5(1):41. doi:10.1038/s41572-019-0092-1.
- Gao Y. 2022. Syndrome differentiation and treatment of diabetic peripheral neuropathy and effect of Huangqi Guizhi Wuwu decoction on PI3K/AKT and oxidative stress of Schwann cells in high glucose environment [Master’s thesis]. Ningxia Med Univ.
- Geisler S, Holmström KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W. 2010. The Pink1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy. 6(7):871–878. doi:10.4161/auto.6.7.13286.
- Gong G, Qin Y, Huang W, Zhou S, Wu X, Yang X, Zhao Y, Li D. 2010. Protective effects of diosgenin in the hyperlipidemic rat model and in human vascular endothelial cells against hydrogen peroxide-induced apoptosis. Chem Biol Interact. 184(3):366–375. doi:10.1016/j.cbi.2010.02.005.
- Gong W, Li J, Chen Z, Huang J, Chen Q, Cai W, Liu P, Huang H. 2017. Polydatin promotes the Nrf2-ARE antioxidative pathway by activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys. Free Radic Biol Med. 106:393–405. doi:10.1016/j.freeradbiomed.2017.03.003.
- Gong X, Gui Z, Ye X, Li X. 2023. Jatrorrhizine ameliorates Schwann cell myelination via inhibiting HDAC3 ability to recruit Atxn2l for regulating the NRG1-ErbB2-PI3K-AKT pathway in diabetic peripheral neuropathy mice. Phytother Res. 37(2):645–657. doi:10.1002/ptr.7641.
- Güçlü-Ustündağ O, Mazza G. 2007. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 47(3):231–258. doi:10.1080/10408390600698197.
- Guo C, Huang Q, Wang Y, Yao Y, Li J, Chen J, Wu M, Zhang Z, E M, Qi H, et al. 2023. Therapeutic application of natural products: NAD+ metabolism as a potential target. Phytomedicine. 114:154768. doi:10.1016/j.phymed.2023.154768.
- Hao GM, Liu YG, Wu Y, Xing W, Guo SZ, Wang Y, Wang ZL, Li C, Lv TT, Wang HL, et al. 2017. The protective effect of the active components of ERPC on diabetic peripheral neuropathy in rats. J Ethnopharmacol. 202:162–171. doi:10.1016/j.jep.2017.03.015.
- He H. 2021. Study on the mechanism of Tangtong recipe regulating autophagy in the treatment of diabetic peripheral neuropathy [Master’s thesis]. Chin Academy Chinese Med Sciences.
- He L, Huan P, Xu J, Chen Y, Zhang L, Wang J, Wang L, Jin Z. 2022. Hedysarum polysaccharide alleviates oxidative stress to protect against diabetic peripheral neuropathy via modulation of the Keap1/Nrf2 signaling pathway. J Chem Neuroanat. 126:102182. doi:10.1016/j.jchemneu.2022.102182.
- He Y, Huang Y, Guan H, Wang X, Luo J, JW, Shi Y. 2019. Effect and mechanism of compound Longqi decoction on peripheral neuropathy in type 2 diabetic rats. Chin Archi Tradit Chin Med. 37:2694–2698. Chinese.
- Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. 2021. Quercetin and metabolic syndrome: a review. Phytother Res. 35(10):5352–5364. doi:10.1002/ptr.7144.
- Hu M, Jiang W, Ye C, Hu T, Yu Q, Meng M, Sun L, Liang J, Chen Y. 2023. Honokiol attenuates high glucose-induced peripheral neuropathy via inhibiting ferroptosis and activating AMPK/SIRT1/PGC-1α pathway in Schwann cells. Phytother Res. 37(12):5787–5802. doi:10.1002/ptr.7984.
- Hu Y, Chen C, Liang Z, Liu T, Hu X, Wang G, Hu J, Xie X, Liu Z. 2023. Compound Qiying Granules alleviates diabetic peripheral neuropathy by inhibiting endoplasmic reticulum stress and apoptosis. Mol Med. 29(1):98. doi:10.1186/s10020-023-00698-3.
- Huang S, Liu H, Wang X, Yuan J, Du L. 2024. Research progress of treating diabetic peripheral neuropathy with traditional Chinese medicine based on mitochondrial quality control. Chin J Experimental Tradi Med Formulae. 30:255–263. Chinese.
- Hussain Z, Thu HE, Amjad MW, Hussain F, Ahmed TA, Khan S. 2017. Exploring recent developments to improve antioxidant, anti-inflammatory and antimicrobial efficacy of curcumin: a review of new trends and future perspectives. Mater Sci Eng C Mater Biol Appl. 77:1316–1326. doi:10.1016/j.msec.2017.03.226.
- Iqbal Z, Azmi S, Yadav R, Ferdousi M, Kumar M, Cuthbertson DJ, Lim J, Malik RA, Alam U. 2018. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 40(6):828–849. doi:10.1016/j.clinthera.2018.04.001.
- Ji H, Zhang X, Du Y, Liu H, Li S, Li L. 2012. Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res Bull. 87(1):50–59. doi:10.1016/j.brainresbull.2011.09.021.
- Jiang W, Hu T, Ye C, Hu M, Yu Q, Sun L, Liang J, Chen Y. 2023. Formononetin attenuates high glucose-induced neurotoxicity by negatively regulating oxidative stress and mitochondrial dysfunction in Schwann cells via activation of sirt3. Food Chem Toxicol. 182:114156. doi:10.1016/j.fct.2023.114156.
- Jiang X, Liu W, Deng J, Lan L, Xue X, Zhang C, Cai G, Luo X, Liu J. 2013. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med. 60:292–299. doi:10.1016/j.freeradbiomed.2013.02.030.
- Jiao D, Liu Y, Hou T, Xu H, Wang X, Shi Q, Wang Y, Xing Q, Liang Q. 2021. Notoginsenoside R1 (NG-R1) promoted lymphatic drainage function to ameliorating rheumatoid arthritis in TNF-Tg mice by suppressing NF-κB signaling pathway. Front Pharmacol. 12:730579. doi:10.3389/fphar.2021.730579.
- Jin M, Wang C, Xu Y, Zhang Z, Wu X, Ye R, Zhang Q, Han D. 2022. Pharmacological effects of salidroside on central nervous system diseases. Biomed Pharmacother. 156:113746. doi:10.1016/j.biopha.2022.113746.
- Koushki M, Amiri-Dashatan N, Ahmadi N, Abbaszadeh HA, Rezaei-Tavirani M. 2018. Resveratrol: a miraculous natural compound for diseases treatment. Food Sci Nutr. 6(8):2473–2490. doi:10.1002/fsn3.855.
- Kumar A, Sharma SS. 2010. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 394(2):360–365. doi:10.1016/j.bbrc.2010.03.014.
- Kunnumakkara AB, Bordoloi D, Harsha C, Banik K, Gupta SC, Aggarwal BB. 2017. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin Sci (Lond). 131(15):1781–1799. doi:10.1042/CS20160935.
- Law BY, Mo JF, Wong VK. 2014. Autophagic effects of chaihu (dried roots of Bupleurum chinense DC or Bupleurum scorzoneraefolium Wild). Chin Med. 9(1):21. doi:10.1186/1749-8546-9-21.
- Leng J, Li X, Tian H, Liu C, Guo Y, Zhang S, Chu Y, Li J, Wang Y, Zhang L. 2020. Neuroprotective effect of diosgenin in a mouse model of diabetic peripheral neuropathy involves the Nrf2/HO-1 pathway. BMC Complement Med Ther. 20(1):126. doi:10.1186/s12906-020-02930-7.
- Li J, Li Y, Yuan X, Yao D, Gao Z, Niu Z, Wang Z, Zhang Y. 2023. The effective constituent puerarin, from Pueraria lobata, inhibits the proliferation and inflammation of vascular smooth muscle in atherosclerosis through the miR-29b-3p/IGF1 pathway. Pharm Biol. 61(1):1–11. doi:10.1080/13880209.2022.2099430.
- Li M. 2021. Clinical effect of Buyanghuanwu decoction on diabetic peripheral neuropathy with qi deficiency and blood stasis. Chin Health Standard Manage. 12:127–129. Chinese.
- Li M, Zhang Y. 2023. Discussion on the new target of traditional Chinese medicine in preventing and treating nervous system diseases from mitochondrial quality control. Chinese Med Nat Prod. 38:726–732. Chinese.
- Li PA, Hou X, Hao S. 2017. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 95(10):2025–2029. doi:10.1002/jnr.24042.
- Li R, Guo Y, Zhang Y, Zhang X, Zhu L, Yan T. 2019. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-Κb and MAPK signaling pathways. Int J Mol Sci. 20(5):1103. doi:10.3390/ijms20051103.
- Li X, Liu S, Qu L, Chen Y, Yuan C, Qin A, Liang J, Huang Q, Jiang M, Zou W. 2021. Dioscin and diosgenin: insights into their potential protective effects in cardiac diseases. J Ethnopharmacol. 274:114018. doi:10.1016/j.jep.2021.114018.
- Li X, Yao W, Yang X. 2019. Effects of Tangluoning on sciatic nerve function and endoplasmic reticulum stress IRE1α-XBP-1-CHOP pathway in diabetic peripheral neuropathy rats. J Tradit Chinese Med Univ Hunan. 39:841–847. Chinese.
- Lin W, Popko B. 2009. Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci. 12(4):379–385. doi:10.1038/nn.2273.
- Lin YR, Chen HH, Ko CH, Chan MH. 2006. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur J Pharmacol. 537(1-3):64–69. doi:10.1016/j.ejphar.2006.03.035.
- Liu G, Zhang Y, Qin L, Zhang C, Zhang Q, Bai H, Zhao Y, Liu T, Mu X. 2023. Tangbikang granules regulates the AMPK/PGC-1α/SIRT3 signal pathway and inhibits oxidative stress of sciatic nerve in diabetic rats. Chin J Experimental Tradi Med Formulae. 30:75–82. Chinese.
- Liu M. 2020. Clinical observation of Qiguitangtongning granule on patients with type 2 diabetic peripheral neuropathy and its influence on the expression of perk and chop in model rats [Master’s thesis]. Anhui Univ Chinese Med.
- Liu Q. 2021. Target-effect metabonomics study of Liuwei Luobi granule in improving diabetic peripheral neuropathy in db/db mice [dissertation]. Chin Academy Chinese Med Sciences.
- Liu Q, Chen Y, Wang B, Chen Y, Li B, Guan S, Du K, Liu X, Yu Y, Liu J, et al. 2023. Arginine biosynthesis pathway found to play a key role in the neuroprotective effect of Liu-Wei-Luo-Bi (LWLB) granules in diabetic db/db mice with peripheral neuropathy using an untargeted metabolomics strategy. Diabetes Metab Syndr Obes. 16:4065–4080. doi:10.2147/DMSO.S423388.
- Liu QS, Pang ZR, Liu R, He GR, Cui J, Yin XY. 2011. Effective compounds group of Mongolian prescriptions BAIMAI-SAN protect against peripheral neuropathy in lower limbs of rats through neuro protective effect. J Ethnopharmacol. 135(3):786–791. doi:10.1016/j.jep.2011.04.026.
- Liu SY, Chen L, Li XC, Hu QK, He LJ. 2018. Lycium barbarum polysaccharide protects diabetic peripheral neuropathy by enhancing autophagy via mTOR/p70S6K inhibition in streptozotocin-induced diabetic rats. J Chem Neuroanat. 89:37–42. doi:10.1016/j.jchemneu.2017.12.011.
- Lu Q, Chen B, Liang Q, Wu L, Luo L, Li A, Ouyang W, Wen Z, Liu Y, Lu J, et al. 2022. Xiaoketongbi formula vs. pregabalin for painful diabetic neuropathy: a single-center, randomized, single-blind, double-dummy, and parallel controlled clinical trial. J Diabetes. 14(8):551–561. doi:10.1111/1753-0407.13306.
- Lu Y, Yao J, Gong C, Wang B, Zhou P, Zhou S, Yao X. 2018. Gentiopicroside ameliorates diabetic peripheral neuropathy by modulating PPAR-γ/AMPK/ACC signaling pathway. Cell Physiol Biochem. 50(2):585–596. doi:10.1159/000494174.
- Ma J, Yu H, Liu J, Chen Y, Wang Q, Xiang L. 2016. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci Lett. 610:139–143. doi:10.1016/j.neulet.2015.11.005.
- Magliano DJ, Boyko EJ, committee IDFDAtes. 2021. IDF diabetes atlas. IDF diabetes atlas. Brussels: international Diabetes Federation © International Diabetes Federation.
- Memarzia A, Khazdair MR, Behrouz S, Gholamnezhad Z, Jafarnezhad M, Saadat S, Boskabady MH. 2021. Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. Biofactors. 47(3):311–350. doi:10.1002/biof.1716.
- Mu C. 2016. Analysis of therapeutic effect of Jiangtang Tongmailing capsule on diabetic peripheral neuropathy. Heilongjiang J Tradit Chinese Med. 45:13–14. Chinese.
- Nadile M, Retsidou MI, Gioti K, Beloukas A, Tsiani E. 2022. Resveratrol against cervical cancer: evidence from in vitro and in vivo studies. Nutrients. 14(24):5273. doi:10.3390/nu14245273.
- O’Brien PD, Hinder LM, Sakowski SA, Feldman EL. 2014. Er stress in diabetic peripheral neuropathy: a new therapeutic target. Antioxid Redox Signal. 21(4):621–633. doi:10.1089/ars.2013.5807.
- Peng X, Liang B, Song R, Wang H, Chen L, Hou J, Liu J, Ren L, Yuan Q. 2023. Effect of modified Taohong Siwu decoction on diabetic peripheral neuropathy. Jilin J Tradit Chinese Med. 43:170–173. Chinese.
- Price R, Smith D, Franklin G, Gronseth G, Pignone M, David WS, Armon C, Perkins BA, Bril V, Rae-Grant A, et al. 2022. Oral and topical treatment of painful diabetic polyneuropathy: practice guideline update summary: report of the AAN guideline subcommittee. Neurology. 98(1):31–43. doi:10.1212/WNL.0000000000013038.
- Qi Y, Yu S. 2015a. Effect of Mudan granule on kv7.3 gene expression in rats with painful diabetic peripheral neuropathy. Chin Archi Tradit Chin Med. 33:552–554. Chinese.
- Qi Y, Yu S. 2015b. Effect of Mudan granule on oxidative stress of painful diabetic peripheral neuropathy. Lishizhen Med Mat Med Res. 26:1561–1563. Chinese.
- Qu L, Liang X, Gu B, Liu W. 2014. Quercetin alleviates high glucose-induced Schwann cell damage by autophagy. Neural Regen Res. 9(12):1195–1203. doi:10.4103/1673-5374.135328.
- Rauf A, Olatunde A, Imran M, Alhumaydhi FA, Aljohani ASM, Khan SA, Uddin MS, Mitra S, Emran TB, Khayrullin M, et al. 2021. Honokiol: a review of its pharmacological potential and therapeutic insights. Phytomedicine. 90:153647. doi:10.1016/j.phymed.2021.153647.
- Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H. 2005. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care. 28(9):2130–2135. doi:10.2337/diacare.28.9.2130.
- Roy M, Liang L, Xiao X, Feng P, Ye M, Liu J. 2018. Lycorine: a prospective natural lead for anticancer drug discovery. Biomed Pharmacother. 107:615–624. doi:10.1016/j.biopha.2018.07.147.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, Metzger BE, Nathan DM, Kirkman MS. 2023. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 46(10):e151–e199. doi:10.2337/dci23-0036.
- Shi H. 2019. Clinical effect of Huangqi Danshen Tongluo recipe on diabetic peripheral neuropathy. Clin Res Prac. 4:114–115. Chinese.
- Song W, Jiang W, Wang C, Xie J, Liang X, Sun Y, Gong L, Liu W, Qu L. 2019. Jinmaitong, a traditional Chinese compound prescription, ameliorates the streptozocin-induced diabetic peripheral neuropathy rats by increasing sciatic nerve IGF-1 and IGF-1R expression. Front Pharmacol. 10:255. doi:10.3389/fphar.2019.00255.
- Song W, Sun Y, Liang XC, Zhang Q, Xie J, Wang C, Liu W. 2021. Jinmaitong ameliorates diabetes-induced peripheral neuropathy in rats through Wnt/β-catenin signaling pathway. J Ethnopharmacol. 266:113461. doi:10.1016/j.jep.2020.113461.
- Sun J, Liu X. 2020. Clinical efficacy and mechanism of Panax notoginseng saponins capsule in the treatment of diabetic peripheral neuropathy. Shaanxi Med J. 49:1496–1498 + 1506. Chinese.
- Sun Q, Wang C, Yan B, Shi X, Shi Y, Qu L, Liang X. 2019. Jinmaitong ameliorates diabetic peripheral neuropathy through suppressing TXNIP/NLRP3 inflammasome activation in the streptozotocin-induced diabetic rat model. Diabetes Metab Syndr Obes. 12:2145–2155. doi:10.2147/DMSO.S223842.
- Tan Y, Cheng H, Su C, Chen P, Yang X. 2022. PI3k/AKT signaling pathway ameliorates oxidative stress-induced apoptosis upon manganese exposure in pc12 cells. Biol Trace Elem Res. 200(2):749–760. doi:10.1007/s12011-021-02687-1.
- Tanrıkulu-Küçük S, Başaran-Küçükgergin C, Seyithanoğlu M, Doğru-Abbasoğlu S, Koçak H, Beyhan-Özdaş Ş, Öner-İyidoğan Y. 2019. Effect of dietary curcumin and capsaicin on testicular and hepatic oxidant-antioxidant status in rats fed a high-fat diet. Appl Physiol Nutr Metab. 44(7):774–782. doi:10.1139/apnm-2018-0622.
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, et al. 2010. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 33(10):2285–2293. doi:10.2337/dc10-1303.
- Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, Rajbhandari S, Sharma S, Rayman G, Gouni R, et al. 2022. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (option-dm): a multicentre, double-blind, randomised crossover trial. Lancet. 400(10353):680–690. doi:10.1016/S0140-6736(22)01472-6.
- Thomas PK. 1997. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes. 46 Suppl 2: s 54–57. doi:10.2337/diab.46.2.s54.
- Tian X, Chen X, Jiang Q, Sun Q, Liu T, Hong Y, Zhang Y, Jiang Y, Shao M, Yang R, et al. 2022. Notoginsenoside r1 ameliorates cardiac lipotoxicity through AMPK signaling pathway. Front Pharmacol. 13:864326. doi:10.3389/fphar.2022.864326.
- Tian Y, Deng X, Su D. 2020. Research progress of Danggui Sini decoction in treating diabetic peripheral neuropathy. Clin J Tradit Chinese Med. 32:1392–1395. Chinese.
- Wang H, Lu B. 2017. Treatment of 80 cases of diabetic peripheral neuropathy with Qidan Dihuang decoction. World Latest Med Inf. 17:129. Chinese.
- Wang J, Wang L, Lou GH, Zeng HR, Hu J, Huang QW, Peng W, Yang XB. 2019. Coptidis rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm Biol. 57(1):193–225. doi:10.1080/13880209.2019.1577466.
- Wang L, Wen H, Cai L. 2018. Effect of puerarin injection on diabetic peripheral neuropathy and its influence on electromyography and hemorheology. World Chinese Med. 13:1929–1932. Chinese.
- Wang Q, Li W, Zhang X, Chung SL, Dai J, Jin Z. 2023. Tauroursodeoxycholic acid protects Schwann cells from high glucose-induced cytotoxicity by targeting NLRP3 to regulate cell migration and pyroptosis. Biotechnol Appl Biochem. 71(1):28–37. doi:10.1002/bab.2518.
- Wang QQ, Zhai C, Wahafu A, Zhu YT, Liu YH, Sun LQ. 2019. Salvianolic acid B inhibits the development of diabetic peripheral neuropathy by suppressing autophagy and apoptosis. J Pharm Pharmacol. 71(3):417–428. doi:10.1111/jphp.13044.
- Wang S, Zhang S, Wang S, Gao P, Dai L. 2020. A comprehensive review on Pueraria: insights on its chemistry and medicinal value. Biomed Pharmacother. 131:110734. doi:10.1016/j.biopha.2020.110734.
- Wang SX, Hu LM, Gao XM, Guo H, Fan GW. 2010. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res. 35(7):1029–1037. doi:10.1007/s11064-010-0151-1.
- Wang W, Hao Y, Li F. 2019. Notoginsenoside R1 alleviates high glucose-evoked damage in RSC96 cells through down-regulation of miR-503. Artif Cells Nanomed Biotechnol. 47(1):3947–3954. doi:10.1080/21691401.2019.1671434.
- Wang Y, Chen Z, Ye R, He Y, Li Y, Qiu X. 2013. Protective effect of jiaweibugan decoction against diabetic peripheral neuropathy. Neural Regen Res. 8(12):1113–1121. doi:10.3969/j.issn.1673-5374.2013.12.006.
- Wang Y, Huang X, Feng M. 2023. Effects of Chaihu Shugan powder on transient receptor potential vanillin Subtype 1/Calcitonin Gene Related peptide pathway and electrophysiological changes of sciatic nerve in diabetic peripheral neuropathy rats. Anhui Med Pharm J. 27:1312–1317 + 1485. Chinese.
- Wei M, Tian Y, Huang D, Zhu X, Feng C, Wang H, Li M. 2019. Effect of Tangtongfang on the expression of PARP and NF-κB in sciatic nerve of rats with diabetic peripheral neuropathy. Chin Archi Tradit Chin Med. 37:1844–1848. Chinese.
- Wei X, Zheng Y, Ai Y, Li B. 2022. Regulatory effects of astragaloside IV on hyperglycemia-induced mitophagy in Schwann cells. Evid Based Complement Alternat Med. 2022:7864308–7864308. doi:10.1155/2022/7864308.
- Wen Y. 2017. Clinical analysis of Danshen Gegen formula granule in treating diabetic peripheral neuropathy. Electronic J Clin Med Literature. 4:5887–5890. Chinese.
- Wu J, Fu Y, Wu YX, Wu ZX, Wang ZH, Li P. 2021. Lycorine ameliorates isoproterenol-induced cardiac dysfunction mainly via inhibiting inflammation, fibrosis, oxidative stress and apoptosis. Bioengineered. 12(1):5583–5594. doi:10.1080/21655979.2021.1967019.
- Wu Q. 2021. Clinical efficacy and safety analysis of Tangshen’an decoction in treating diabetic peripheral neuropathy. Chinese Med Modern Distance Education Chin. 19:32–34. Chinese.
- Wu Q, Yan G, Zeng D, Zhang M, Li L, Lu J. 2019. Clinical observation on 30 cases of diabetic peripheral neuropathy treated by Jiangtang Huoluo pill combined with fumigation and washing with traditional Chinese medicine. Hunan J Tradit Chinese Med. 35:1–3 + 11. Chinese.
- Wu Y, Xue B, Li X, Liu H. 2012. Puerarin prevents high glucose-induced apoptosis of Schwann cells by inhibiting oxidative stress. Neural Regen Res. 7(33):2583–2591. doi:10.3969/j.issn.1673-5374.2012.33.003.
- Xiang Q, Liu Y, Chen L. 2023. Saikosaponin D (SSD) alleviates diabetic peripheral neuropathy by regulating the AQP1/RHOA/ROCK signaling in streptozotocin-induced diabetic rats. Acta Diabetol. 60(6):805–815. doi:10.1007/s00592-023-02060-9.
- Xiao H, Sun X, Lin Z, Yang Y, Zhang M, Xu Z, Liu P, Liu Z, Huang H. 2022. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm Sin B. 12(6):2887–2904. doi:10.1016/j.apsb.2021.12.023.
- Xiao H, Sun X, Liu R, Chen Z, Lin Z, Yang Y, Zhang M, Liu P, Quan S, Huang H. 2020. Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol Res. 151:104559. doi:10.1016/j.phrs.2019.104559.
- Xie J, Song W, Liang X, Zhang Q, Shi Y, Liu W, Shi X. 2020a. Jinmaitong ameliorates diabetic peripheral neuropathy in streptozotocin-induced diabetic rats by modulating gut microbiota and neuregulin 1. Aging (Albany NY). 12(17):17436–17458. doi:10.18632/aging.103750.
- Xie J, Song W, Liang X, Zhang Q, Shi Y, Liu W, Shi X. 2020b. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed Pharmacother. 127:110147. doi:10.1016/j.biopha.2020.110147.
- Xu C. 2020. Protective effect and mechanism of salvianolic acid A on diabetic peripheral neuropathy [master’s thesis]. Harbin Univ Commerce.
- Xu JW, Xu X, Ling Y, Wang YC, Huang YJ, Yang JZ, Wang JY, Shen X. 2023. Vincamine as an agonist of G-protein-coupled receptor 40 effectively ameliorates diabetic peripheral neuropathy in mice. Acta Pharmacol Sin. 44(12):2388–2403. doi:10.1038/s41401-023-01135-1.
- Xu LN, Yin LH, Jin Y, Qi Y, Han X, Xu YW, Liu KX, Zhao YY, Peng JY. 2020. Effect and possible mechanisms of dioscin on ameliorating metabolic glycolipid metabolic disorder in type-2-diabetes. Phytomedicine. 67:153139. doi:10.1016/j.phymed.2019.153139.
- Xu X, Cui L, Zhang L, Yang L, Zhuo Y, Li C. 2023. Saikosaponin D modulates the polarization of tumor-associated macrophages by deactivating the PI3k/AKT/mTOR pathway in murine models of pancreatic cancer. Int Immunopharmacol. 122:110579. doi:10.1016/j.intimp.2023.110579.
- Xue H, Li P, Luo Y, Wu C, Liu Y, Qin X, Huang X, Sun C. 2019. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 54:240–247. doi:10.1016/j.phymed.2018.10.031.
- Yang H. 2017a. Effect of Danggui Sini decoction on hemorheology and nerve conduction velocity in elderly diabetic peripheral neuropathy with blood deficiency and cold coagulation. Modern J Integrated Tradit Chinese Western Med. 26:1755–1757. Chinese.
- Yang J, Wei Y, Zhao T, Li X, Zhao X, Ouyang X, Zhou L, Zhan X, Qian M, Wang J, et al. 2022. Magnolol effectively ameliorates diabetic peripheral neuropathy in mice. Phytomedicine. 107:154434. doi:10.1016/j.phymed.2022.154434.
- Yang L. 2017b. Clinical observation on 100 cases of diabetic peripheral neuropathy treated with Chinese medicine of supplementing qi and promoting blood circulation. Guide Chin Med. 15:205–206. Chinese.
- Yang W, Yu Y, Xu X, Ren H, Liang J, Zhang L. 2015. Therapeutic effect of Mudan granule on experimental diabetic peripheral neuropathy in rats. Chinese J Diabetes. 23:1099–1102. Chinese.
- Yang X, Li X, Zhu Y, Gao Y, Xu L. 2022. Paeoniflorin upregulates mitochondrial thioredoxin of Schwann cells to improve diabetic peripheral neuropathy indicated by 4D label-free quantitative proteomics. Oxid Med Cell Longev. 2022:4775645. doi:10.1155/2022/4775645.
- Yang X, Yao W, Liu H, Gao Y, Liu R, Xu L. 2017. Tangluoning, a traditional Chinese medicine, attenuates in vivo and in vitro diabetic peripheral neuropathy through modulation of PERK/Nrf2 pathway. Sci Rep. 7(1):1014. doi:10.1038/s41598-017-00936-9.
- Yang X, Yao W, Shi H, Liu H, Li Y, Gao Y, Liu R, Xu L. 2016. Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis. J Ethnopharmacol. 185:361–369. doi:10.1016/j.jep.2016.03.031.
- Yang X, Zhang L, Li T, Sheng L, Guo W. 2019. Clinical efficacy of Qiteng Tongluo decoction in the treatment of type 2 diabetic peripheral neuropathy. Chin J Integrative Med Cardio-Cerebrovasc Dis. 17:4069–4072. Chinese.
- Yang XW, Liu FQ, Guo JJ, Yao WJ, Li QQ, Liu TH, Xu LP. 2015. Antioxidation and anti-inflammatory activity of tang bi kang in rats with diabetic peripheral neuropathy. BMC Complement Altern Med. 15(1):66. doi:10.1186/s12906-015-0600-0.
- Yang Y. 2016. Evaluation and analysis of puerarin injection in treating diabetic peripheral neuropathy. Chin Pract Med. 11:158–159. Chinese.
- Yao L. 2018. Application of Mudan granule in diabetic peripheral neuropathy with qi deficiency and blood stasis syndrome. J Clin Med Pract. 22:104–106. Chinese.
- Yerra VG, Kalvala AK, Sherkhane B, Areti A, Kumar A. 2018. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology. 131:256–270. doi:10.1016/j.neuropharm.2017.12.029.
- Yi W, Zhang F, Wang Y, Sun H, Hu Y, Wu S, Liu T. 2019. Clinical observation on 54 cases of diabetic peripheral neuropathy with phlegm and blood stasis syndrome treated by Mongolian medicine Zhenbao pill. J Tradit Chinese Med. 60:42–46. Chinese.
- Yin Y, Qu H, Yang Q, Fang Z, Gao R. 2021. Astragaloside IV alleviates Schwann cell injury in diabetic peripheral neuropathy by regulating microRNA-155-mediated autophagy. Phytomedicine. 92:153749. doi:10.1016/j.phymed.2021.153749.
- Yovera-Aldana M, Velásquez-Rimachi V, Huerta-Rosario A, More-Yupanqui MD, Osores-Flores M, Espinoza R, Gil-Olivares F, Quispe-Nolazco C, Quea-Vélez F, Morán-Mariños C, et al. 2021. Prevalence and incidence of diabetic peripheral neuropathy in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS One. 16(5):e0251642. doi:10.1371/journal.pone.0251642.
- Yu D. 2016. Treatment of diabetic neuropathy with qi deficiency and blood stasis by Danggui Sini decoction combined with acupoint application. Diabetes New World. 19:81–82. Chinese.
- Yu H, Lin L, Zhang Z, Zhang H, Hu H. 2020. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 5(1):209. doi:10.1038/s41392-020-00312-6.
- Yu MX, Lei B, Song X, Huang YM, Ma XQ, Hao CX, Yang WH, Pan ML. 2021. Compound Xiongshao capsule ameliorates streptozotocin-induced diabetic peripheral neuropathy in rats via inhibiting apoptosis, oxidative-nitrosative stress and advanced glycation end products. J Ethnopharmacol. 268:113560. doi:10.1016/j.jep.2020.113560.
- Yu R, Zhang Y, Zhang C, Zhang Q, Mu X, Bai H, Zuo X, Liu G, Liu T, Qin L. 2023. Effects of Tangbikang on oxidative stress, mitochondrial energy metabolism and AMPK/Sirt3 pathway of sciatic nerve in diabetic model rats. J Tradit Chinese Med. 64:1140–1148. Chinese.
- Yu S. 2022. To explore the clinical effect of compound Danshen dripping pills on improving early peripheral neuropathy in type 2 diabetes mellitus. Clin J Chinese Med. 14:77–80. Chinese.
- Yuan Q, Zhang X, Wei W, Zhao J, Wu Y, Zhao S, Zhu L, Wang P, Hao J. 2022. Lycorine improves peripheral nerve function by promoting Schwann cell autophagy via AMPK pathway activation and MMP9 downregulation in diabetic peripheral neuropathy. Pharmacol Res. 175:105985. doi:10.1016/j.phrs.2021.105985.
- Zan Y, Kuai CX, Qiu ZX, Huang F. 2017. Berberine ameliorates diabetic neuropathy: TRPV1 modulation by PKC pathway. Am J Chin Med. 45(8):1709–1723. doi:10.1142/S0192415X17500926.
- Zeng X. 2020. Therapeutic effect of puerarin injection on type 2 diabetes mellitus complicated with peripheral neuropathy. Shenzhen J Integrated Tradit Chinese Western Med. 30:28–29. Chinese.
- Zhang H. 2018. Clinical effect of Fuyuan Huoxue decoction on diabetic peripheral neuropathy. World Latest Med Inf. 18:131–137. Chinese.
- Zhang H. 2020. Clinical evaluation of tangmanning, a compound preparation of traditional Chinese medicine, in the treatment of diabetic peripheral neuropathy. Guide Chin Med. 18:130–131. Chinese.
- Zhang K, Peng P, Huang J, Chen M, Liu F, Zhu C, Lu Q, Wang M, Lin C. 2024. Integrating plasma metabolomics and gut microbiome to reveal the mechanisms of Huangqi Guizhi Wuwu decoction intervene diabetic peripheral neuropathy. J Ethnopharmacol. 319(Pt 3):117301. doi:10.1016/j.jep.2023.117301.
- Zhang L. 2017. Clinical observation on puerarin injection in the treatment of 62 cases of diabetic peripheral neuropathy. J Aerospace Med. 28:410–411. Chinese.
- Zhang L. 2019. Effect of Mudan granule on the syndrome of qi deficiency and blood stasis in diabetic peripheral neuropathy. Systems Med. 4:132–134. Chinese.
- Zhang L, Qi Y, Zhang L. 2022. Based on Sirt1/PGC1α/TFAM pathway, the mechanism of Yiqi Huoxue Tongluo recipe in treating diabetic peripheral neuropathy was discussed. Chin J Tradit Chinese Med Pharm. 37:5395–5399. Chinese.
- Zhang W, Yu H, Lin Q, Liu X, Cheng Y, Deng B. 2021. Anti-inflammatory effect of resveratrol attenuates the severity of diabetic neuropathy by activating the Nrf2 pathway. Aging (Albany NY). 13(7):10659–10671. doi:10.18632/aging.202830.
- Zhang WX, Lin ZQ, Sun AL, Shi YY, Hong QX, Zhao GF. 2022. Curcumin ameliorates the experimental diabetic peripheral neuropathy through promotion of NGF expression in rats. Chem Biodivers. 19:e202200029.
- Zhang X, Dong H, Feng J, Cui L, Guo F, Zhao L, Li Y, Su R. 2019. Effect of Wenyang Tongluo collaterals on the expression of serum nerve growth factor in diabetic peripheral neuropathy. Chinese Remedies Clinics. 19:739–740. Chinese.
- Zhang X, Dong H, Feng J, Guo G. 2019. Clinical observation on the treatment of diabetic peripheral neuropathy with Fuyang Tongluo decoction. China’s Naturopathy. 27:44–46. Chinese.
- Zhang Y, Long H, Wang X, Cao W, Wu L, Liu T, Zhou J. 2023a. Effect of Huangqi Guizhi Wuwu decoction on apoptosis-related bax and caspase-12 of sciatic nerve cells in diabetic rats. Chin J Experimental Tradi Med Formulae. 29:58–64. Chinese.
- Zhang Y, Long H, Wang X, Cao W, Wu L, Liu T, Zhou J. 2023b. Effect of Huangqi Guizhi Wuwu decoction on IRE1α/CHOP pathway of endoplasmic reticulum stress in sciatic nerve of diabetic rats. Chin J Experimental Tradi Med Formulae. 29:43–51. Chinese.
- Zhang Y, Tang F. 2012. Advance in latest studies on pharmacological effects of magnolol. Zhongguo Zhong Yao Za Zhi. 37(23):3526–3530. Chinese.
- Zhang Y, Zhang M. 2018. Clinical effect of Fuyuan Huoxue decoction on diabetic peripheral neuropathy. World Latest Med Inf. 18:161. Chinese.
- Zhao B, Zhang Q, Liang X, Xie J, Sun Q. 2021. Quercetin reduces inflammation in a rat model of diabetic peripheral neuropathy by regulating the TLR4/myd88/NF-κB signalling pathway. Eur J Pharmacol. 912:174607. doi:10.1016/j.ejphar.2021.174607.
- Zhao G. 2021. Clinical effect of Mongolian medicine Zhenbao pill in treating diabetic peripheral neuropathy with phlegm and blood stasis syndrome. J Med Pharm Chinese Minorities. 27:14–16. Chinese.
- Zhao T, Zhou Z, Zhao S, Wan H, Li H, Hou J, Wang J, Qian M, Shen X. 2023. Vincamine as an agonist of G protein-coupled receptor 40 effectively ameliorates pulmonary fibrosis in mice. Phytomedicine. 118:154919. doi:10.1016/j.phymed.2023.154919.
- Zheng T, Wang Q, Bian F, Zhao Y, Ma W, Zhang Y, Lu W, Lei P, Zhang L, Hao X, et al. 2021. Salidroside alleviates diabetic neuropathic pain through regulation of the AMPK-NLRP3 inflammasome axis. Toxicol Appl Pharmacol. 416:115468. doi:10.1016/j.taap.2021.115468.
- Zhou M, Yao W. 2021. Effect of Mudan granule on TLR4-related pathway in diabetic peripheral neuropathy rats. West J Tradit Chinese Med. 34:52–56. Chinese.
- Zhou P, Shi W, He XY, Du QY, Wang F, Guo J. 2021. Saikosaponin D: review on the antitumour effects, toxicity and pharmacokinetics. Pharm Biol. 59(1):1480–1489. doi:10.1080/13880209.2021.1992448.
- Zhou P, Xie W, Meng X, Zhai Y, Dong X, Zhang X, Sun G, Sun X. 2019. Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells. 8(3):213. doi:10.3390/cells8030213.
- Zhou W. 2019. Effect of Yiqi Huoxue Tongluo decoction on diabetic peripheral neuropathy. J Med Theory Pract. 32:3806–3807. Chinese.
- Zhou Y. 2017. Study on the clinical effect of Chinese medicine for warming yang, activating blood circulation and dredging collaterals on diabetic peripheral neuropathy. Guide Chin Med. 15:192. Chinese.
- Zhou YX, Zhang H, Peng C. 2014. Puerarin: a review of pharmacological effects. Phytother Res. 28(7):961–975. doi:10.1002/ptr.5083.
- Zhu J, Yang X, Li X, Han S, Zhu Y, Xu L. 2021. Tang Luo Ning, a traditional Chinese compound prescription, ameliorates Schwannopathy of diabetic peripheral neuropathy rats by regulating mitochondrial dynamics in vivo and in vitro. Front Pharmacol. 12:650448. doi:10.3389/fphar.2021.650448.
- Ziegler J, Facchini PJ. 2008. Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol. 59(1):735–769. doi:10.1146/annurev.arplant.59.032607.092730.
