Abstract
Objectives. To investigate the diagnostic approach in Nordic hospitals receiving patients suspected of acute myocardial infarction (MI), especially focusing on implementation of the recently proposed criteria by the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) for the definition of MI. Design. A survey with questionnaires of the diagnostic approach was conducted among all relevant departments (220) in the Nordic countries. Results. Seventy-six percent (167) of hospitals responded. Troponins I and T (TnI and TnT) and creatinine kinase monobasic fraction (mass concentration) (CKMBmass) covered 93 and 65% of hospitals, respectively. Of troponin users, 34% indicated use of TnI vs 66% using TnT. Sporadic use of AST, CK, LD and myoglobin was reported. There was a tendency to lower cut-off levels in Sweden and Finland. Among troponin assays, there was considerable heterogeneity regarding cut-off levels. Conclusions. The Nordic countries are approaching ESC/ACC consensus on cardiac markers. Compared with previous national surveys (1995–1999), there is a shift towards the use of troponins. However, differences in cut-off levels of troponin emphasize the need for harmonization of assays.
Introduction
The diagnostic criteria of myocardial infarction (MI) have previously been shown to vary considerably between countries and even between hospitals in the same regional setting Citation1–4. Today, the diagnosis of acute coronary syndrome (ACS) is applied in patients suffering from unstable angina, non-ST-segment elevation MI and ST-segment elevation MI. Until year 2000 the most commonly used criteria for MI were those published by the World Health Organization (WHO) in 1979 Citation5. According to these criteria, MI was defined by a combination of: typical symptoms (e.g. chest discomfort), enzyme rise and a typical electrocardiographic pattern.
Recent years have seen rapid progress in the field of biochemical cardiac markers. Markers now provide a very large degree of sensitivity and specificity. Today, there is solid documentation for the use of creatinine kinase monobasic fraction (mass concentration) (CKMBmass) and cardiac troponins (TnI or TnT) Citation6–8. Use of one or both markers with a modern assay provides the treating physician with a powerful instrument to detect myocardial necrosis, which again facilitates correct treatment of the patient Citation9.
In year 2000 a consensus document on the diagnosis of MI was published by the European Society of Cardiology (ESC) and the American College of Cardiology (ACC) Citation10. The definition of MI was considered from several points of view, although the major changes, as compared with the WHO definition, were based on cardiac markers Citation11. Several authors have investigated or commented on the impact of the proposed changes Citation12,13. As expected, there seems to be an increase in the number of patients receiving the diagnosis of MI, if the consensus statement is followed. Regarding the implementation of the ESC/ACC consensus report, Hasdai et al. found that only 63% of patients underwent examination for troponin levels during the Euro Heart Survey in 2000/2001 Citation14. There are no recent data on the implementation of the ESC/ACC consensus report from the Nordic countries.
The Nordic countries (Denmark, Finland, Iceland, Norway and Sweden) exhibit rather similar features regarding healthcare systems. The population is roughly 25 million in total. Although there are marked geographical differences in population density (e.g. Denmark 120 persons per square km; Finland 16 persons per square km), healthcare systems are similar and well organized.
Hypothesis and objective
The objective of the present survey was to investigate the diagnostic approach in various Nordic hospitals receiving patients suspected of myocardial infarction, especially focusing on the implementation of the ESC/ACC criteria for the definition of MI.
Material and methods
A survey regarding the use of cardiac markers in the diagnosis of MI was conducted throughout Denmark, Finland, Iceland, Norway and Sweden. All departments receiving patients suspected of ACS received a questionnaire. Questionnaires were sent to the Head of Department – or if a department of internal medicine – the responsible consultant for cardiology. The survey was conducted from 1 March 2004. Departments that did not respond to the questionnaire received up to three reminders by mail and e-mail. Inclusion was finally closed on 31 August 2004. As the present survey did not include patient sensitive material, no approval from Ethical Committees was needed.
Questionnaires were distributed from investigators in each country and were returned to the Department of Cardiology, Aalborg Hospital – Aarhus University Hospital for analysis.
The questionnaire included questions in the following categories: number of patients admitted for ACS per year; use of cardiac markers in different time settings from onset of symptoms; cut-off values for cardiac markers; manufacturer of assays and laboratory equipment; percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) performed at the hospital and diagnosis of periprocedural MI; use of other markers for risk stratification in ACS.
Statistics
All data are presented as descriptive findings. No statistical analysis was performed.
Results
A total of 220 questionnaires were distributed of which 167 (76%) were returned for evaluation. The distribution of respondents is shown in . A rough estimate of 113 000 patients suspected of ACS are admitted to the responding departments each year (Denmark: 33 000; Finland: 4000; Iceland: 1700; Norway: 32 800; Sweden: 41 500). Mean number of admissions were 676 patients per hospital (range: 20 patients (Iceland)–5000 patients (Sweden)). The majority of hospitals (98%) returned questionnaires that were complete. Two percent returned questionnaires, where one or more questions remained unanswered. These cases concerned two hospitals that reported their preferred markers but failed to report cut-off values and which assays were used. Seven hospitals (4%) indicated that they did not have a suitable marker for the period 0–6 h from onset of symptoms. This was particularly the case in hospitals with small to medium in-take of patients.
Table I. Nordic hospitals reporting use of different markers.
Demographics of cardiac markers
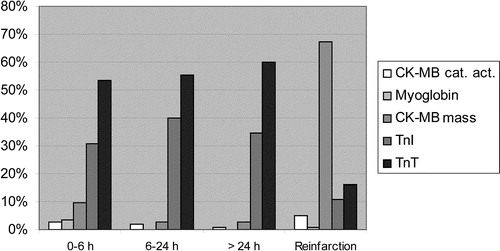
The survey showed that eight different markers of necrosis were used in the region. Numbers and percentages of responding hospitals reporting use of different markers are shown in .
Nine different assays for measurement of CKMBmass were used in the region: the most prevalent assay was the immunoassay manufactured by Roche Diagnostics (58%).
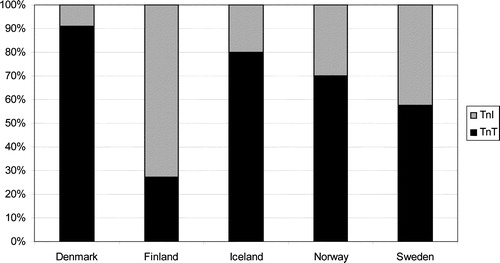
Ninety-three percent of hospitals examined ACS patients for elevations of troponins. TnI was used in 31% of hospitals as compared with 62% for TnT. National differences among hospitals stating use of troponins are illustrated in .
Fifty-two hospitals (31%) reported the use of TnI. Among these, nine different assays were identified: Abbott AxSYM; Bayer Centaur; Beckman Access (second-generation TnI); Dade-Behring Stratus CS; Dade-Behring Dimension RxL (second-generation TnI); Diagnostic Products Corporation Immulite 2000; Innotrac Aio! (second-generation TnI); Biocard TnI; Ortho-Clinical Diagnostics Vitros ECI. The most prevalent assays were Beckman Access (30%) and Abbott AxSYM (24%).
Only one manufacturer of TnT assays is represented in the Nordic countries (Roche Diagnostics). TnT is analyzed by a third-generation immunoassay either on a point-of-care instrument (Cardiac Reader) (13%) or on standard laboratory equipment (Roche Elecsys 1010/2010 or Modular) (87%). CK, AST and LD were only used sporadically in 0.6–6% of hospitals. Only six hospitals (3.6%) reported the use of myoglobin.
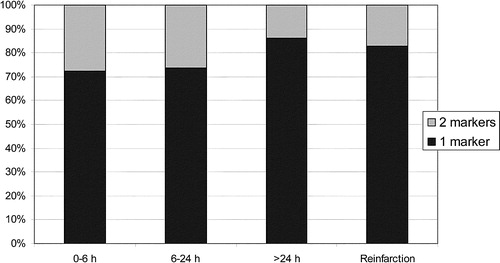
Also, hospitals reported their preferred marker in different clinical settings. The distribution of a one-marker vs a two-marker strategy over the clinical course of time is shown in . In Danish hospitals, there was a lower prevalence of a one-marker strategy (52%) in the first 6 h from onset of symptoms, but otherwise there were no major differences. In hospitals indicating a one-marker strategy, TnT was the preferred marker at all times, except in the case of reinfarction (). CKMBmass was the preferred marker in this setting. In hospitals indicating a two-marker strategy, the most frequent combination of markers at 0–6 h after onset of symptoms was TnT and CKMBmass (62%) as compared to TnI and CKMBmass (19%).
Cut-off values for MI
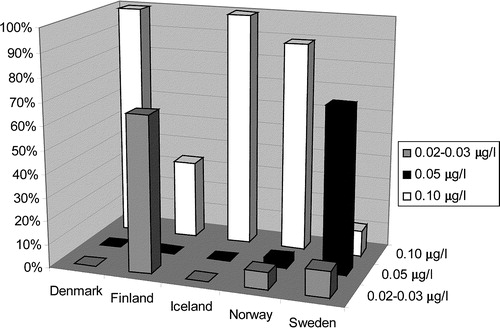
Differences in cut-off values for the same markers were reported. Cut-off values for TnT in the Nordic countries can be divided into three categories: 0.02–0.03, 0.05 and 0.10 µg/l. The distribution of hospitals employing the different cut-off levels was 7, 19 and 74%, respectively. However, all users of TnT in Denmark reported a cut-off value of 0.1 µg/l, whereas 81% of TnT users in Sweden and 67% in Finland reported a cut-off of 0.05 µg/l or less. The complex distribution of cut-off levels for TnT is illustrated in . The reported cut-off values for TnI were very heterogeneous and, in some instances, included a wide range of different values for the same assay, e.g. Abbott AxSYM (0.4, 0.5, 0.8, 1 and 2 µg/l).
Concerning CKMBmass, there is a similar trend with lower cut-off values in Sweden and Finland than the rest of the Nordic countries.
Procedure-related MI after PCI and CABG
Thirty-five (21%) of the responding hospitals performed PCI, whereas 24 (14%) hospitals performed CABG. Thirty-two hospitals (91%) and 22 (92%), respectively, reported diagnosing periprocedural MI. The used markers (alone or in combination) after PCI were CKMBmass (80%), troponins (23%) and CKMBactivity (3%). After CABG, preferred markers were CKMBmass (54%), troponins (23%), CKMBactivity (14%) and AST (14%).
After PCI, only a minority of hospitals used cut-off levels which were similar to the ones used in routine detection of ACS. In the case of CKMBmass and CKMBactivity, most hospitals used cut-off levels which show some diversity but usually are 3×Upper Limit of Normal (ULN). Four different assays exhibiting different cut-off values were used for measuring TnI, i.e. 0.06 (Innotrac Aio!), 0.30 (Bayer Centaur), 0.4 (Dade-Behring Stratus CS) and 70.5 µg/l (DPC Immulite 2000). Only one assay for TnT (Roche) was used for the detection of MI after PCI with a cut-off level of 0.10 µg/l.
After CABG, cut-off levels also show diversity. As an example of this, cut-off levels for CKMBmass were reported in the range of 5–80 µg/l (n=13), and the range for TnT was 0.10–2.5 µg/l (n=3). Only two hospitals measured TnI with two different assays, i.e. Innotrac Aio! and DPC Immulite 2000 (0.06 and 70.5 µg/l, respectively).
Discussion
Our survey documents a clear shift towards the use of TnI and TnT as compared with national surveys in Norway (1997), Sweden (1998) and Denmark (1999) Citation1–4. Results from earlier surveys conducted in the region are shown in .
Table II. Nordic countries: percentages of hospitals using a one- or two-marker strategy in detection of acute coronary syndrome.
There are, however, important national differences in a region which otherwise exhibits very similar features. These fall into two categories. First, differences in the choice of markers. Secondly, differences in cut-off levels between the same markers.
As compared with the Euro Heart Survey from 2000/2001, the prevalence of troponins has risen further from 63 to 93% Citation14. In the present survey, the choice of troponins exhibits some variety, where TnT is the most commonly used troponin. In Sweden, however, there is an almost even distribution between TnI and TnT, and in Finland the majority of hospitals use TnI. As opposed to troponins, the distribution of CKMBmass between countries is rather even. However, CKMBactivity still plays a role especially in Denmark, where it is used in 32% of hospitals as compared to 13.3% of all hospitals in the Nordic region. In all cases, CKMBactivity was used as a second marker accompanied by either TnI or TnT.
Cut-off levels
We also show that there are local differences in cut-off levels for troponins and CKMBmass. Only one assay for TnT is used in the region as opposed to nine different assays for TnI. There is a wide array of different cut-off levels for TnI. Obviously, this constitutes a problem for standardization of TnI. However, standardization of TnI does not seem a realistic goal because of vast differences in the antibodies used in different assays Citation15.
In recent years, more evidence has been gathered that even very low levels of troponins are markers of a poor prognosis Citation16. In this survey, the differences in cut-off levels could represent the beginning of a gradual movement towards lower cut-off values and therapeutic thresholds. However, the lower cut-off values were almost exclusively seen in Sweden and Finland.
Disturbingly, however, we find some low cut-off levels that are associated with increased imprecision. The ESC/ACC Biochemistry panel has recommended that ULN be defined as the 99th percentile (three standard deviations above the mean of the normal range) and that the coefficient of variability (CV) be ≤10% Citation11,17. As an example, some hospitals reported a cut-off level of 0.4 µg/l for the Abbott AxSYM TnI assay. However, at this level the CV is 20%, which consequently increases the likelihood of false positive test results Citation18. Also, 0.4 µg/l is below the recommended 99th percentile. On the other hand, some cut-off levels for other assays were reported to be well above the levels where the assays exhibit a CV ≤ 10%. One example is the Beckmann Access TnI assay, where some hospitals indicated a cut-off level of 0.5 µg/l. This value is 16×the value that exhibits a CV of 10% Citation19. Consequently, a cut-off level of this magnitude increases the likelihood of false negative test results. When run on the Elecsys system, the Roche TnT assay has a CV ≤ 10% at 0.035 µg/l, which is one-third of the cut-off level for MI as determined by ROC analysis Citation17. In support of using this level for the diagnosis of MI, Lindahl et al. found that any detectable rise in TnT levels was associated with a worse prognosis, as these patients have a higher probability of thrombus formation and significant coronary stenosis Citation16. Several hospitals in our survey indicated that they used a cut-off level for MI at 0.10 µg/l equal to the ROC analysis. Apple et al. have published an admirable tabulation of assays and their performance, including the concentration with CV ≤ 10%, MI cut-off values determined by ROC analysis, lower limits of detection and 99th percentiles Citation17. The observed differences in cut-off levels of TnT between countries in the Nordic Survey as well as the article by Apple et al. Citation18 illustrate that consensus is needed on the matter in order to choose the right cut-off value for AMI (99th percentile, based on ROC analysis, level of 10% CV or maybe a fourth option).
Periprocedural MI
When performing PCI and CABG, minor elevations of cardiac markers are likely to occur Citation20. In PCI patients the risk of adverse outcomes seems to rise in a continuous fashion with higher values of peak CKMB Citation21. This effect is particularly pronounced in patients with peak CKMB 10×ULN, but it is also significant at CKMB levels 3–5×ULN. Some authors have found that a rise in troponins after PCI predicts adverse outcomes Citation22, whereas others have not been able to find this association Citation21.
The picture is even less clear regarding CABG patients where there seems to be a threshold phenomenon at CKMB 10×ULN for predicting mortality Citation20. Traditionally, the development of Q-waves after CABG has been considered the most reliable criterion for MI. However, this has been challenged by some authors, who have not found any association between new Q-waves and release of necrosis markers indicative of major myocardial tissue damage Citation23. Moreover, only appearance of necrosis markers together with Q-waves is associated with adverse events, whereas development of isolated Q-waves does not alter the postoperative course Citation24.
There is no consensus on exact levels of markers that define clinically meaningful damage to the myocardium. In this survey, we show that the preferred marker for this purpose is CKMBmass; however, cut-off levels are very heterogeneous, indicating that some confusion exists in this area. Also, caution must be applied when interpreting cut-off levels and ULN in different publications. Some authors define the ULN as the cut-off for spontaneous MI, whereas others define ULN as the upper limit of a reference population. This inevitably adds to the confusion.
Need for harmonization
The consensus document, published in 2000 by the ESC and ACC, proposed several changes in the diagnosis of MI Citation10. More than ever before, biochemical markers (troponins and CKMBmass) were considered pivotal in making the diagnosis of MI. In order to create further global consensus on diagnostic criteria, a Global Task Force for Revision of the ESC/ACC MI Consensus Document has been established in 2003 under the guidance of ESC, ACC, American Heart Association (AHA), World Heart Foundation (WHF) and WHO. The task force includes six working groups on various aspects of the diagnosis of MI, of which one will evaluate the biochemical diagnosis of MI.
The abundance of different markers, assays and cut-off levels documented in this survey emphasizes the need for a global standard on cardiac markers. Problems are not only confined to the clinical setting, but are also very apparent when reviewing potential new cardiac markers in clinical trials, where endpoints are often defined by certain levels of cardiac markers Citation25. Consequently, lack of harmonization of cut-off levels and assays is likely to hamper conclusions.
The many different assays are indeed part of the problem. However, variations in cut-off levels for the same assays illustrate that not only manufacturers are to blame. Clinicians and biochemists are at great length responsible for the lack of harmonization between institutions.
In an attempt to move towards harmonization of markers, the International Federation of Clinical Chemistry (IFCC) has recently published a tabulation of assays and their performances at lower levels Citation26. It is to be hoped that the Global Task Force will propose more exact cut-off levels or even a new common standard for cardiac markers in order to improve clarity in interpretation. A possible way forward could be a normalized ratio, a measure which is widely accepted and validated among clinicians as well as laboratory specialists in anticoagulation treatment. Another way of harmonizing assays could be through the creation of a website under the auspices of the IFCC and the cardiological societies of Europe and the USA. On such a website, a complete tabulation of assays and their performances at different levels could be continuously updated after approval from scientific societies – much along the lines of the tabulation of Apple et al. as previously mentioned Citation17. It would thus be clear to the users at any time, how cut-off levels were to be managed.
Furthermore, a review comparing different assays and their capability of diagnosing MI is needed. Any of these initiatives could improve diagnostic quality and would thus enable clinicians and researchers to draw correct conclusions.
Conclusion
We show, for the first time, that the use of cardiac markers in the Nordic countries has evolved considerably towards the standards proposed by ESC/ACC in the consensus document from year 2000. However, differences exist in the region regarding the choice of cardiac markers and assays. Also, cut-off values are very heterogeneous, pointing towards lower levels in Sweden and Finland. This emphasizes the need for a global harmonization of cardiac markers and more clear recommendations of their use in order to improve decision making in daily clinical work as well as in the setting of a clinical trial.
The authors wish to thank all colleagues at Nordic Hospitals who responded to the questionnaire. Also, Dr Kristian Thygesen is thanked for constructive criticism.
References
- Abedini, S., Nielsen, OW., Sajadieh, A., Sigurd, BM. [Diagnosis of acute myocardial infarction in Denmark]. Ugeskr Laeger. 1999;16:5165–8.
- Agert, G., Nilsson, G. [What is a myocardial infarction? Technical diagnosis is varying between different hospitals. Quality comparisons require uniformity]. Lakartidningen. 1995;92:381–5.
- Fretland, S., Kruger, O. [Differences in diagnostic criteria for acute myocardial ischemia at Norwegian hospitals]. Tidsskr Nor Laegeforen.1997;117:817–9.
- Jernberg, T., Lindahl, B., Wallentin, L. [Uniform diagnosis of myocardial infarction. Rapid development at Swedish hospitals]. Lakartidningen.1998;95:515–20.
- Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation. 1979;59:607–9.
- Hamm CW, Ravkilde J, Gerhardt W, Jorgensen P, Peheim E, Ljungdahl L, et al. The prognostic value of serum troponin T in unstable angina. N Engl J Med 1992; 327: 146–50
- Ravkilde J, Nissen H, Horder M, Thygesen K. Independent prognostic value of serum creatine kinase isoenzyme MB mass, cardiac troponin T and myosin light chain levels in suspected acute myocardial infarction. Analysis of 28 months of follow-up in 196 patients. J Am Coll Cardiol 1995; 25: 574–81
- Stubbs P, Collinson P, Moseley D, Greenwood T, Noble M. Prognostic significance of admission troponin T concentrations in patients with myocardial infarction. Circulation 1996; 94: 1291–7
- Wallentin L, Lagerqvist B, Husted S, Kontny F, Stahle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: The FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet 2000; 356: 9–16
- Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J.2000;21:1502–13.
- Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It's time for a change to a troponin standard. Circulation 2000; 102: 1216–20
- Kontos MC, Fritz LM, Anderson FP, Tatum JL, Ornato JP, Jesse RL. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am Heart J 2003; 146: 446–52
- Tunstall-Pedoe H. Comment on the ESC/ACC redefinition of myocardial infarction by a consensus dissenter. Eur Heart J 2001; 22: 613–6
- Hasdai D, Behar S, Boyko V, Danchin N, Bassand JP, Battler A. Cardiac biomarkers and acute coronary syndromes – the Euro Heart Survey of Acute Coronary Syndromes Experience. Eur Heart J 2003; 24: 1189–94
- Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000; 102: 1221–6
- Lindahl B, Diderholm E, Lagerqvist B, Venge P, Wallentin L. Mechanisms behind the prognostic value of troponin T in unstable coronary artery disease: A FRISC II substudy. J Am Coll Cardiol 2001; 38: 979–86
- Apple FS, Wu AH, Jaffe AS. European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: How to use existing assays clinically and for clinical trials. Am Heart J 2002; 144: 981–6
- Venge P, Lagerqvist B, Diderholm E, Lindahl B, Wallentin L. Clinical performance of three cardiac troponin assays in patients with unstable coronary artery disease (a FRISC II substudy). Am J Cardiol 2002; 89: 1035–41
- Venge P, Lindahl B, Wallentin L. New generation cardiac troponin I assay for the access immunoassay system. Clin Chem 2001; 47: 959–61
- Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J Am Coll Cardiol 2002; 40: 1961–7
- Kini AS, Lee P, Marmur JD, Agarwal A, Duffy ME, Kim MC, et al. Correlation of postpercutaneous coronary intervention creatine kinase-MB and troponin I elevation in predicting mid-term mortality. Am J Cardiol 2004; 93: 18–23
- Nageh T, Sherwood RA, Harris BM, Byrne JA, Thomas MR. Cardiac troponin T and I and creatine kinase-MB as markers of myocardial injury and predictors of outcome following percutaneous coronary intervention. Int J Cardiol 2003; 92: 285–93
- Svedjeholm R, Dahlin LG, Lundberg C, Szabo Z, Kagedal B, Nylander E, et al. Are electrocardiographic Q-wave criteria reliable for diagnosis of perioperative myocardial infarction after coronary surgery?. Eur J Cardiothorac Surg 1998; 13: 655–61
- Crescenzi G, Bove T, Pappalardo F, Scandroglio AM, Landoni G, Aletti G, et al. Clinical significance of a new Q wave after cardiac surgery. Eur J Cardiothorac Surg 2004; 25: 1001–5
- Jaffe AS, Katus H. Acute coronary syndrome biomarkers: The need for more adequate reporting. Circulation 2004; 110: 104–6
- Panteghini M, Pagani F, Yeo KT, Apple FS, Christenson RH, Dati F, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem 2003; 50: 327–32