Abstract
Objectives. There has been a considerable change in the patient population referred for cardiac surgery in the last decade. More complex and marginal patients require optimized myocardial protection. An insufficient cardioplegic procedure results in anaerobic metabolism during cardiac arrest with subsequent lactate accumulation. Increased lactate level is regarded as a predictor for low cardiac output syndrome. In an acute porcine model we examined two standard cardioplegic methods. Myocardial microdialysis was used to investigate the metabolism during cardioplegic arrest and in the reperfusion period. Methods. Twelve domestic pigs were randomly chosen to receive either cold blood-or cold crystalloid cardioplegia. After midline sternotomy two microdialysis probes were implanted in two different regions of the heart. Cardiopulmonary bypass was initiated, aorta was clamped, and antegrade cardioplegia was delivered. These conditions were maintained for 90 min. Subsequent to myocardial reperfusion the animals were observed for 180 min. Microdialysis and plasma markers to characterize myocardial metabolism, and plasma markers for myocardial failure and necrosis were obtained every 30 min. Results. Lactate concentrations were significantly increased in the cold crystalloid cardioplegia group compared to the cold blood cardioplegia group, in tissue dialysate (p < 0.001) as well as in serum (p = 0.018). Pyruvate concentrations in the dialysate were significantly increased in the cold crystalloid cardioplegia group compared to the cold blood cardioplegia group (p = 0.008). There were no significant differences in dialysate concentrations of glycerol. Plasma markers for myocardial failure (Brain Naturetic Peptide) and for myocardial necrosis (Cardiac Troponin T) showed no differences between the groups. Conclusion. The results indicate that cold blood cardioplegia offers superior protection of the heart, in terms of more rapid normalization of myocardial metabolism. The microdialysis technique seems to have a high sensitivity and ability to detect even minor metabolic changes. This enhances the possibility of designing a myocardial protection, which might lower morbidity and mortality risk.
There has been a considerable change in the patient population referred for cardiac surgery in the last decade. Heart surgery is offered to an increasing number of children and adults with complex heart failure and comorbidity Citation1. Cardiac surgery for these demanding categories of patients requires increased ischemic periods during surgery. This has resulted in a renewed focus on the cardioplegic procedure.
The cardioplegic solutions currently used all have the same target, explicitly preserving the myocardial function, and avoiding iatrogenic injury induced by cardiopulmonary bypass itself, or by surgically imposed ischemia Citation2.
This aim has been achieved in several different ways including hypothermic crystalloid cardioplegic solutions Citation3, hypothermic Citation4 or normothermic Citation5 blood cardioplegia solutions Citation6, antegrade or retrograde administration Citation7, cardioplegic solutions with various additives like insulin/glucose Citation8, glutamate/aspartate Citation9, L-arginine, or quinaprilat Citation10.
Despite continued improvements in cardioplegic techniques, low cardiac output syndrome (LCOS) following cardiac surgery remains an ongoing problem in a number of patients. LCOS developed in 9.1% of 4 558 patients after CABG Citation11. LCOS was defined by Rao et al. as patients needing treatment with intra aortic balloon pump or inotropic support for more than 30 minutes after the patients had returned to the intensive care unit to maintain the systolic blood pressure higher than 90 mm Hg and a cardiac index greater than 2.2 L/m2/min. The mortality rate increased from 0.9% in the group without LCOS to 16.9% in the group with LCOS Citation11. Thus, there is a clear need for further understanding and improvement of the cardioplegic procedure, if this increased mortality is to be reduced.
The optimal outcome measures for studies focusing on cardioplegic procedures are survival and cardiac function. However, these outcome measures are difficult to use because of the multi factorial aspects of the individual diseases and treatment. Instead, a number of plasma or serum markers have been used to reflect the degree of myocardial damage during the procedures. Most frequently, creatinekinase and the cardiac isoenzyme (CK-MB) are used to detect a perioperative myocardial cell necrosis and to evaluate the degree of damage. However the most reliable marker for myocardial necrosis is the cardiac troponin T Citation12, the cardio specificity is high, and it even allows detection of minor ischemic incidents Citation13.
Brain natriuretic peptide (BNP) is a hormone, which is synthesized and released from the cardiac ventricles. BNP is released when the myocyte is stretched, which occurs at overloading and threatening insufficiency. As a hormone BNP causes an afterload reduction by natriuresis, diuresis and vasodilatation and thereby protects the heart. Increased plasma levels of BNP are seen in patients with congestive heart failure. There are many reports on BNP in regard to chronic heart failure, but fewer about BNP in acute heart failure Citation14. Beside serum markers for myocardial damage and cardiac failure, we aimed to analyze concentrations of different myocardial metabolites in a porcine model of cardioplegia. We used microdialysis to evaluate oxidative metabolism, and implanted the probes in two different regions of the myocardium. The technique has previously been evaluated and reported as a unique tool to peek into the interstitium of the myocyts Citation15. With microdialysis, glycerol was used as a marker for cell destruction, and pyruvate and lactate used as markers for the oxidative metabolism.
The aim of the study was to compare the protective effect of cold blood cardioplegia versus cold crystalloid cardioplegia on the myocardium. This was evaluated by using myocardial microdialysis for oxidative metabolism and the stated plasma markers for myocardial ischemic damage. The observation period included the first three hours of reperfusion and the ischemic period during aortic cross clamping.
Materials and methods
Interventions
Twelve female pigs from a domestic stock, with a median body weight at 40 (range 35–47 kg), were randomly chosen according to one of two different cardioplegic regimes: Group 1: Cardioplegia consisting of a mixture of circulating oxygenated blood and crystalloid St. Thomas 2 solution enriched with 60 mmol potassium-chloride/l delivered at a ratio of 4:1 at 5°C, Group 2: Crystalloid St. Thomas 2 solution delivered at 5°C. The St. Thomas no.2 cardioplegic solution contains the following: calcium chloride 335 mg/l, potassium chloride 1.5 g/l, magnesium chloride 3.3 g/l, sodium chloride 8.6 g/l and procaine 223 mg/l. The randomization method consisted of opaque, numbered envelopes. After cross clamping the aorta, cardioplegia was administered antegrade. In both groups, the cardioplegia was delivered in the aortic root, with a pressure between 60 and 80 mmHg. The initial dose was 600 ml. During the period of arrest, 150 ml of cardioplegia were administrated every 20 min, or at signs of insufficient myocardial arrest, (defined as macro-visual contractions of the myocardium).
Animals and anaesthesia
All experiments were performed in accordance with the Ethical Committee for Animal Experiments, The Royal Danish Justice department, Slotholmsgade 10 DK-1216 Copenhagen K, who also approved the experiment.
After fasting over night, the pigs were premedicated with 0.5 mg/kg midazolam (Roche, Switzerland) intramuscularly (i.m.). Anaesthesia was induced with Zoletil 50 Vet. (Vibac SA, France) i.m. 0.1 ml/kg. The pigs were intubated and ventilated with a minute volume at 100 ml/kg/min and an oxygen/air mixture of 0.33%. The acid-base balances were continuously monitored throughout the experiment in order to maintain normoventilation. For maintenance of the anaesthesia, propofol (Abott, Denmark) (10 mg/ml) 15 mg/kg/hr and 400 µg/hr fentanyl (Hameln Pharmaceuticals, Germany) intravenously (IV) were used. Before cannulation of the heart, 40 000 International Units (IU) of heparin (Leo, Sweden) were administrated. During cardiopulmonary bypass (CPB), the activated clotting time (ACT) was measured using a Hemochron coagulometer (Medtronic, USA) and kept above 480 s at all times. All IV administrations were given through an auricular vein. A bolus of amiodarone (Sonofi-Synthelabo, Sweden) 150 mg IV was administrated before the chest was opened to minimize the risk for arrhythmia. The pigs were sacrificed with pentobarbital (60 mg/kg) IV at the end of the study.
Monitoring
Mean arterial blood pressure (MAP), central venous pressure (CVP), electro cardiogram (ECG) (3 lead), and arterial and venous saturations were continuously monitored. Data was registered every 15 min. Furthermore, cardioplegic delivery pressure and flow, as well as main pump flow were recorded.
Surgical procedure
The right carotic artery and the internal jugular vein were exposed to allow caterization for blood pressure measurements by fluid filled pressure catheters (Edwards Life Science, USA). A Hewlett Packard bedside monitor (Stanford, USA) displayed the hemodynamic data.
After a midline sternotomy, the pericardium was opened to expose the heart. Two CMA 20 microdialysis probes (CMA/Microdialysis, Sweden) were inserted into the myocardium using an introducer with a splitting tube as a guide wire. After the probes were in position the introducer was removed, and they were fixed to the myocardium with a suture. One probe was placed in the anterior wall of the left ventricle, and the other in the inferior wall of the left ventricle.
A 29 Fr two-stage venous catheter (Medtronic, USA) was placed in the right atrium, and a 18 Fr arterial cannulla (Shererwood, UK) was placed in the ascending aorta. A DLP 9 Fr aortic root cannulla (Medtronic, USA) for cardioplegic delivery was inserted.
Cardio-pulmonary bypass
A heart-lung machine (Polystan, Denmark) with semiocclusive roller pumps was used. The extracorporeal circuit consisted of a hollow fiber membrane oxygenator with integrated venous/cardiotomy reservoir (Safe Maxi, Polystan) and 3/8‘‘ PVC tubing. Additionally for Group 2 a cardioplegic deliverance set with integrated heat exchanger (Terumo, Japan) was used.
The circuits were primed with lactated Ringer's solution and added 10.000 IU of heparin, 1 000 ml in the crystalloid cardioplegia group and 1 200 ml in the blood cardioplegia group. The 200 ml that differs between the groups are represented in the prime volume of the cardioplegia deliverance/heat-exchanger set.
The CPB was conducted at normothermia with a cardiac index between 2 and 2.4 l/m2/min. When full flow rate was established the aorta was cross clamped, cardioplegia was delivered, and the CPB was maintained at these conditions for 90 min. Prior to release of the aortic cross clamp, 150 mg amiodarone, 0.5 g calcium chloride (SAD, Denmark) and 8 mg pavulon (Organon, Holland) was administered.
When stabile hemodynamic conditions were re-established, the pig was weaned from CPB.
Microdialysis
Before manipulating the heart, baseline samples were collected from dialysate, plasma and arterial blood gas.
The sampling rate for microdialysate was every 30 min throughout the experiment. CMA/20 microdialysis probes with a 10 mm membrane were used. The molecular cut-off point for the dialysis membrane was 20 kDa. The probes were perfused with Ringer chloride at a speed of 0.6 µl/min. Because of the dead space in the outlet tube and the slow perfusion rate, there was a ten minute delay between the collected perfusate and the interventions on the heart. The sampling interval was 30 min. To drive forward the perfusion fluid a CMA/102 microdialysis pump with two glass syringes was used. The dialysate was analyzed at a CMA 600 analyzer (CMA/Microdialysis, Sweden) for lactate, pyruvate, and glycerol concentrations.
Plasma analyses
Arterial blood gasses were analyzed every hour during the experiment at an arterial blood laboratory (ABL 600 Radiometer, Denmark). The arterial samples were analyzed for pH, paO2, paCO2, hemoglobin/hematocrit, glucose, lactate, potassium, sodium, and calcium. Blood samples were obtained after cross clamp release, and every hour until sacrifice of the animal. After centrifuging (Varifuge 3.2 RS, Canada) the samples, the plasma was stored at −80°C until analyses were conducted. Plasma troponin T was measured with an Elecsys 2010 (Roche, Copenhagen, Denmark). Plasma pro BNP concentrations were measured with a radio immuno analysis that uses antibodies raised against the N-terminal decapeptide of porcine proBNP
Statistics
For data presentation “area under the curve” (AUC) was calculated Citation16, and Wilcoxon Rank-Sum test for differences in median made comparison between groups. A p-value at 0.05% or below was considered significant. Microsoft Excel 2000, Lap pilot CMA/microdialysis, and NCSS for Windows were used for statistical calculations and presentation.
Results
In the present study, twelve animals were randomly chosen and a schematic overview of the data is presented in . Two animals died during the procedure and they were replaced. The first animal from Group 1 suffered from surgical complications. The right atrium was penetrated when placing the venous catheter; the trauma was corrected and CPB initiated and completed. After weaning from CPB the animal suffered from malignant ventricular fibrillation despite antiarrhythmic treatment, and died after one hour of reperfusion. The second animal that died during the procedure also was in Group 1. After an uncomplicated CPB the aortic cross clamp was released, the animal developed ventricular fibrillation, which was converted into a 3rd degree A/V-block, and was subsequently asystolic. Despite pacing and medical treatment the animal died after 50 min of reperfusion. Throughout the study microdialysate was collected from the anterior and the inferior wall of the myocardium.
There are no significant differences between the groups with the baseline measurements ( and ) or after the arrest period ( and ). However, in both groups a moderate P-lactate increase is noted, and with reference to dialysate conditions values in Group 2 show a general decrease. Data for the reperfusion period are presented as median value of AUC, and range. AUC was calculated and interpreted as an expression of the metabolic picture that exposed in the reperfusion phase.
Table I. Baseline concentrations of P-lactate, P-troponin T and P proBNP after steady state conditions for plasma samples.
Table II. Baseline concentrations of lactate, pyrovate and glycerol after steady stateconditions for microdialysis.
Table III. Conditions after 90 min of ischemia, serum analysis.
Table IV. Conditions after 90 min of ischemia. Microdialysis analysis.
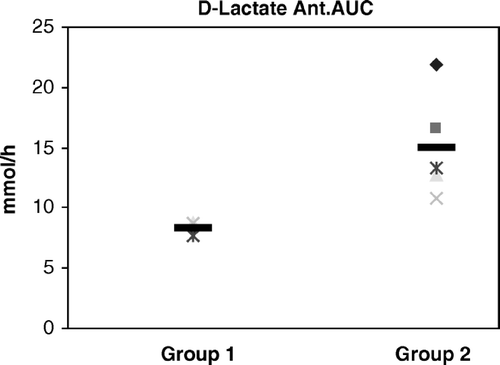
For D-Lactate in Group 1, anterior wall (Ant.), the results were 8.7 (7.2–8.8) mmol/hr and for the inferior wall (Inf.) 7.4 (7.1–8.1) mmol/hr (p = 0.78).
For D-lactate in Group 2 Ant. the results were 14.89 (10.8–21.4) mmol/hr and for Group 2 Inf. 14.1 (12.2–21.8) mmol/hr (p = 0.53). There are no differences within the groups. There were significant differences between the groups in the anterior wall (p = 0.0022), as well as in the inferior wall (p = 0.0011) ().
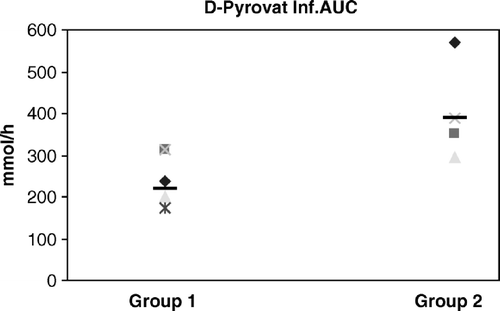
Results for D-Pyruvate, in Group 1 Ant. were 117.7 (89.9–124.6) µmol/hr. In Group 1 Inf. 218 (173.6–313.9) µmol/hr, (p = 0.0086). In Group 2 Ant. the result was 391.5 (301.5–738.7) µmol/hr and in Group 2 Inf. 388.6 (293.6–717.8) the differences are statistical significant between the groups, for the anterior wall (p = 0.0022), and for the inferior wall (p = 0.0086) ().
The ratio of lactate and pyruvate was calculated and showed no significant differences between the groups (p = 0.31).
D-Glycerol in Group 1 Ant. was 1057 (906–1 221) µmol/hr and in Group 1 Inf. 959 (588–1 781) µmol/hr (p = 0.60). In Group 2 Ant. 1245 (725–3 087) µmol/hr, and in Group 2 Inf. 1392 (886–3 165) µmol/hr (p = 0.35). In this comparison there were no statistically differences for the anterior wall (p = 0.33) and for the inferior wall (p = 0.12).
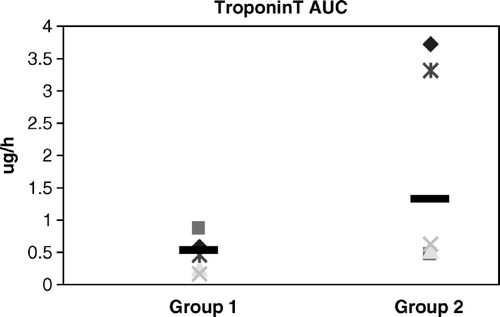
P-lactate showed a similar pattern as D-lactate. Results in Group 1 were 200 (128–336) mmol/hr. In Group 2 the result was 357 (186–363) mmol/hr (p = 0.018). In Group 1 P-proBNP was 155 (116–176) ρmol/hr and in Group 2 125 (36–171) ρmol/hr; there were no statistical differences between the groups. In Group 1 p-troponin T was 0.522 (0.239–0.781) µg/hr and in Group 2 it was 1.367 (0.626–3.312) µg/hr (p = 0.066) ().
Discussion
The aim of the study was to compare the cardio protective effect of two standard cardioplegic procedures in terms of plasma markers for myocardial damage, supplemented with myocardial microdialysis for changes in oxidative metabolism and possible cell damage. We primarily investigated the reperfusion period (three hours after release of the aortic cross clamping), and secondary the ischemic period during aortic cross clamping. The reperfusion period was the main interest because in this period serious clinical problems often are manifested.
Reperfusion injury is a well described clinical situation that occurs when the myocardium is revascularized after an ischemic period. The pathogenesis is still not fully revealed. Reperfusion injury can progress to ventricular dysfunction, myocardial stunning and arrhythmia, with severe consequences for the patients Citation17.
The quality, and thereby the protective ability of the cardioplegic procedure is essential. While the heart is arrested, and between the bolus injections of the cold cardioplegia, there is a natural and inevitable rewarming of the heart from the surroundings tissues, which often are mild hypo- or normothermic. When rewarmed, the cardiac metabolism increases. This metabolism is anaerobic due to discontinued oxygen supply and the heart is thereby suffering from hypoxia. If the myocardium is not properly protected, it may result in myocardial injury, which can lead to LCOS Citation11.
Normally, cardiac ischemia is monitored with ECG, and blood analysis for cardiac enzymes. These are well known and reliable methods. However, there is a problem with the lack of sensitivity and the delay from when the tests are taken until the results are available and sufficient action can be initiated.
The microdialysis technique is a sensitive and site specific procedure. The technique was used first by Delgado et al. Citation18 in the early seventies. A small probe with a semipermeable membrane is inserted into the tissue and perfused with a liquid that equilibrates with the surrounding fluid by simple diffusion. The method has shown to give a quick and reliable picture of ongoing metabolism in numerous of experiments. The microdialysis technique has mainly been used in detecting cerebral ischemia Citation19, but is presently used in other areas to characterize i.e. splanchnic Citation20 and myocardial metabolism Citation21.
Microdialysis seems to be a reliable method of monitoring metabolism and ischemia in the extracelluar Citation21, Citation22 and intravascular Citation23 space. The procedure gives a quick and precise overview of the particular tissue that is at observation. The technique even allows bedside monitoring and thereby it reduces the “turn around time”.
During ischemic conditions cardiac metabolism shift from aerobic to anaerobic glycolysis, which result in an accumulation and release of glycolytic intermediates such as lactate. Lactate is an often used marker to detect anaerobic metabolism. It reacts quickly to ischemic periods, and it is associated with higher incidents of postoperative morbidity and mortality Citation24, Citation25.
Our results shows that lactate in the reperfusion period was significantly higher in the group that was treated with cold crystalloid cardioplegia (Group 2) compared to the group treated with cold blood cardioplegia (Group 1). The levels of lactate were significantly increased in Group 2 in both the microdialysis- and plasma samples. Hyper lactatemia can be associated with other pathophysiological conditions i.e. liver diseases, but as the animals in our study all were healthy and randomly allocated to the groups, this confounder can most likely be ruled out.
When the citrate cycle is inhibited, as seen at conditions with impaired oxygen delivery and anaerobic metabolism, there will be an accumulation of pyruvate, before it is converted to lactate. The increased level of pyruvate shows an impaired function of the normal cell metabolism, and can be interpreted as a shift from aerobic to anaerobic metabolism.
We found a significant difference between the groups in the reperfusion period for D-pyruvate concentrations; results from Group 2 were elevated compared to Group 1.
Increased levels of glycerol express the degree of cell membrane destruction and are seen in conditions with severe ischemia and myocardial infarction. We did not identify any significant differences in the glycerol levels. This indicates that the myocardial protective effect with respect to both cardioplegic procedures does not lead to severe cell death within the first three hours.
The overall picture for microdialysis results indicates that cold blood cardioplegia gives a better protection of the heart compared to cold crystalloid cardioplegia, this reflects a more sufficient myocardial metabolism in the reperfusion period, and thereby a better recovery from the arrested period. The increased level of P-lactate indicates that the protection with cold blood cardioplegia might result in a better post ischemic pump function compared to the cold crystalloid group. The other plasma analyses did not reveal any significant differences. The observation time might have been too short to detect a difference in proBNP. Keisuke et al. Citation14 showed a BNP peak after 24 hours.
In regards to Troponin T, the p-value was close to the limit of significance (0.07) and can be considered as borderline significant. This suggests, in relation with our other results, that an increased number of animals in the study might reveal a difference. Troponin T increases in both groups. This result indicates that myocardial damage is present at some extent in both groups, regardless which strategy of myocardial protection one may choose. The condition of the heart, right after the induced ischemic period, might be better in Group 2. D-lactate is significant higher in the cold blood cardioplegia group. This metabolite marker suggests that the heart is better protected in Group 2 during the aorta cross clamping while cooled. From a macro visual point of view, it seems that cold crystalloid cardioplegia gives a more complete period of heart arrest with no signs of contractions. Minor contractions as seen during cold blood cardioplegia might explain the higher lactate levels after induced ischemia. The lower viscosity of the cold crystalloid cardioplegic solution could provide an increased delivery in the myocardium and thereby explain this situation. (However, the results show that the animals in the cold blood cardioplegia group had a better metabolic recovery).
Both of the animals that died received cold blood cardioplegia. The first animal suffered surgical complications, which were unrelated to the cardioplegic procedure. The second animal died of arrhythmia which might have been due to insufficient myocardial protection during ischemia. Since this episode did not reoccur we regard it as being inconclusive.
This study has its limitations due to the limited number of animals and the short observation period. With an expanded study size and increased observation time, it might have been possible to demonstrate significant differences in our plasma markers, especially Troponin T. If we were able to correlate our microdialysis results with the more known serum markers it would have improved the study.
Our findings correlate with other investigators who also showed that cold blood cardioplegia is preferable to cold crystalloid cardioplegia. In the future a more optimal cardioplegic procedure should be designed which would allow for improved myocardial protection. Most clinical investigations regarding cardioplegia have a substantial risk of multi factorial outcome confounders. The risk of introducing confounders in a standardized experimental model is reduced, since the two different cardioplegic methods are the only actions that differ between the groups. Therefore we emphasize that our model is a reliable and precise way of monitoring and evaluating myocardial protection.
We conclude that cold blood cardioplegia seems superior in regard to myocardial protection compared to cold crystalloid cardioplegia. The better protection is evident in the reperfusion period, where our results show that the animals that received blood cardioplegia had an improved metabolic recovery after the induced ischemic period. The microdialysis technique seems to have a high sensitivity, and ability to detect even minor changes. This enhances the possibilities of designing a myocardial protection that will improve myocardial metabolic recovery after aortic cross clamping.
We are obliged to the staff at the Department of Cardiothoracic Surgery and the Department of Thoracic Anesthesiology at the heart centre H:S Rigshospitalet Copenhagen, Denmark and to the foundation of “Købmand Sven Hansen og Hustru”. This work was presented as a poster at the SATS & SCANSECT annual meeting in 2004, Gothenburg, Sweden.
References
- Trivedi KR, Azakie A, Benson LN. Collaborative interventional and surgical strategies in the management of congenital heart lesions. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2001; 4: 185–207
- Vinten-Johansen J, Thourani VH. Myocardial protection: An overview. J Extra Corpor Technol 2000; 32(1)38–48
- Robinson LA, Schwarz GD, Goddard DB, Fleming WH, Galbraith TA. Myocardial protection for acquired heart disease surgery: Results of a national survey. Ann Thorac Surg 1995; 59(2)361–72
- Caputo M, Dihmis W, Birdi I, Reeves B, Suleiman MS, Angelini GD, et al. Cardiac troponin T and troponin I release during coronary artery surgery using cold crystalloid and cold blood cardioplegia. Eur J Cardiothorac Surg 1997; 12(2)254–60
- Hendrikx M, Jiang H, Gutermann H, Toelsie J, Renard D, Briers A, et al. Release of cardiac troponin I in antegrade crystalloid versus cold blood cardioplegia. J Thorac Cardiovasc Surg 1999; 118(3)452–9
- Astorri E, Fiorina P, Grattagliano C, Medici D, Pinelli S, Albertini D, et al. Cardiac Troponin T to evaluate myocardial protection via intermittent cold blood or continuous warm blood cardioplegia in coronary artery bypass grafting. J Cardiovasc Surg (Torino) 1998; 39(6)797–802
- Elwatidy AM, Fadalah MA, Bukhari EA, Aljubair KA, Syed A, Ashmeg AK, et al. Antegrade crystalloid cardioplegia vs antegrade/retrograde cold and tepid blood cardioplegia in CABG. Ann Thorac Surg 1999; 68(2)447–53
- Bruemmer-Smith S, Avidan MS, Harris B, Sudan S, Sherwood R, Desai JB, et al. Glucose, insulin and potassium for heart protection during cardiac surgery. Br J Anaesth 2002; 88(4)489–95
- Korvald C, Elvenes OP, Myrmel T, Sorlie DG. Cardiac dysfunction and inefficiency after substrate-enriched warm blood cardioplegia. Eur J Cardiothorac Surg 2001; 20(3)555–64
- Korn P, Kroner A, Schirnhofer J, Hallstrom S, Bernecker O, Mallinger R, et al. Quinaprilat during cardioplegic arrest in the rabbit to prevent ischemia-reperfusion injury. J Thorac Cardiovasc Surg 2002; 124(2)352–60
- Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg 1996; 112(1)38–51
- Ravn HB, Moldrup U, Ilkjaer LB, Chew M, Jensen L, Johnsen S, et al. A new model for evaluation of thrombosis and ischaemia/reperfusion injury. APMIS 2000; 108(5)373–9
- Ravnkilde J, Hørder M, Gerhardt W, Ljungdal L. Diagnostic performance and prognostic value of serum troponin T in suspected acute myocardial infarction. Scand J Clin Invest 1993; 53: 677–85
- Morimoto K, Mori T, Ishiguro S, Matsuda N, Hara Y, Kuroda H. Perioperative changes in plasma brain natriuretic peptide concentrations in patients undergoing cardiac surgery. Surg Today 1998; 28(1)23–9
- Jackson K, Farias M, Caffrey JL. Cardiac microdialysis a powerful tool. Cardiovasc Res 2000; 46(3)367–9
- Matthews JNS, Altman DG, Cambell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J 1990; 300: 230–5
- Shernan SK. Perioperative myocardial ischemia reperfusion injury. Anesthesiology Clin N Am 2003; 21: 456–85
- Delgado JMR, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake mokeys. Arch Int Pharmacodyn 1972; 198: 9–21
- Ungerstedt U. Microdialysis-principles and applications for studies in animal and man. J Intern Med 1991; 230(4)365–73
- Backstoem T, Liska J, Oldner A, Lockowant U, Fraco-Cereceeda A. Splanchic metabolism during gut ischemia, endotoxin and hemorrhagic shock evaluated by intravasal microdialysis. Shock 2004; 21(6)572–8
- Wikstoem BG, Ronquist G, Waldenstroem A. No further improvements of ischaemic myocardial metabolism by combining preconditioning with beta-blokade: An in vivo experimental study in the pig using a microdialysis technique. Acta Physiol Scand 1997; 159: 23–32
- Kuzmin AI, Tskitishvili OV, Serebryakova LI, Saprygina TV, Kapelko VI, Medvedev OS. Cardiac microdialysis measurement of extracellular adenine nucleotide breakdown products during regional ischemia and reperfusion in canine heart: Protective effect of propanolol against reperfusion injury. J Cardiovasc Pharmacol 1992; 20: 961–8
- Backstrom, T, Liska, J, Samuelson, S, Franco-Cereceda, A. Intravasal microdialysis as a monitoring tool in high-risk patients undergoing cardiac surgery. In. “Intravasal microdialysis as a novel technique to monitor metabolism in myocardial ischemia and critical illness”. Stockholm: University of Stockholm; 2003. ISBN: 91–7349–534–4.
- Demers P, Elkouri S, Martineau R, Couturier A, Cartier R. Outcome with blood lactate levels during cardiopulmonary bypass in adult cardiac surgery. Ann Thorac Surg 2000; 70: 2082–6
- Rao V, Ivanov J, Weisel RD, Cohen G, Borger MA, Mickle DAG. Lactate release during reperfusion predicts low cardiac output syndrome after coronary bypass surgery. Ann Thorac Surg 2001; 71: 1925–30