Abstract
Objective. Aortic atherosclerosis may cause cerebrovascular accidents in cardiac surgery. Aortic plaque distribution was analyzed in relation to surgical manipulation. Design. In 24 autopsy subjects the thoracic aorta was digitally analyzed by macro-anatomic mapping of plaques. Plaque density was compared in different anatomical segments. Hazards associated with surgical manipulation were blindly studied by superimposing cannulation and cross-clamp locations onto the maps. Results. Plaques were frequent. The anterior wall of the ascending/arch aorta had higher plaque density than its posterior side (p = 0.039). However, an anterior plaque predicted to 83% a concomitant plaque in the posterior wall. Plaque formation correlated with age (p = 0.004). The theoretical risk of interfering with a plaque during cannulation and/or clamp positioning was 46%. Conclusions. Plaque formation is a frequent and age-dependent problem. In the surgery-exposed aorta, the anterior wall had higher plaque density than the posterior side, although the two sides showed strong plaque coexistence. Furthermore, there was an unexpectedly high risk of plaque interference during cannulation and/or clamp maneuvers if blindly performed. The present results emphasize the importance of epiaortic scanning.
Aortic atherosclerosis is common in the elderly patient Citation1. In cardiac surgery the aorta is rather brutally handled by clamps and cannulation procedures. It is difficult to predict the aortic quality from its outside appearance. Digital palpation is the most common method although epiaortic ultrasound has increased and is now considered the golden standard. In comparison, palpation has been shown to only detect about one third of the plaques evidenced by ultrasound Citation2. Cerebrovascular accidents (CVA) caused by dislodged material from the aortic inside may ruin an otherwise successful operation. Aortic calcification is known to be a major predicting variable behind CVA after cardiac surgery Citation3–5. It has been experimentally demonstrated that aortic manipulation by aortic cross clamping and cannulation, but also intra-aortic filter handling, give rise to both macroscopic and microscopic debris Citation6, Citation7.
In this study we investigated the aortic quality of subjects undergoing autopsy. The thoracic aorta was cut open to review intimal plaque formation, being monitored by computerized image analysis. Anatomical difference in plaque distribution was compared in different anatomical segments (e.g., ascending/arch versus descending aorta, and anterior versus posterior walls). The aim was to identify plaque distribution in relation to surgically exposed manipulation.
Materials and methods
Subjects and aortic characteristics
Subjects (n = 24) were selected at random from the daily autopsy program. The subjects had many characteristics in common with routine cardiac-surgery patients (). Umeå University ethical committee (Dnr 01-142) approved the study.
Table I. Subject demographics, autopsy data, aortic characteristics and cause of death.
Experimental model and digital image analysis
A macro-anatomic mapping technique of plaque distribution was developed. The thoracic aorta was removed from the body and dissected free from connective tissue. According to a strictly defined protocol, the aorta was cut open along its major curvature, dividing all cervical branches in half. The specimen was unfolded and spread out on a polystyrene bed with its intimal side facing upwards. In between the underlying bed and aorta a transparent plastic sheet was positioned. The aortic outline and the location of side-branches were traced by penetrating the plastic sheet using a needle tip. Needles of another tip and print-off character mapped the plaque location onto the plastic sheet. Plaques were classified by visual appearance and palpation into hard-calcified material (ulcerative and non-ulcerative) and by wall thickness of well-defined outline, similar to what is considered during surgical conditions. The smallest recorded plaque had an area of 9.5 mm2, which exemplifies the size limit. Plaque inclusion was thus based on macroscopic appearance only, giving a print-off map of 1:1 scale.
The maps were developed outside the autopsy room by tracing each type of needle puncture by using a marker pen. The plastic sheet with marked aorta was positioned on a lighted stage and the transmitted contours were copied onto two separate maps for aortic outline and plaque distribution, respectively. This routine was employed in order to blind the observer to plaque distribution when cross clamp and cannulation sites were superimposed onto the maps.
The map with aortic outline was subdivided in two steps; separating the descending part from the ascendens including arch, with reference to the left-subclavian artery branch. Each segment was further subdivided into its anterior versus posterior walls. The subdivision lines were transferred to the separate map holding plaque distribution. The two maps were recorded by digital image analysis. The analysis was performed in a step-wise order, first measuring the entire aorta, the segmental subdivision of ascendens/arch and descendens, and finally, anterior and posterior wall of each segment, respectively. Plaques that overlapped segments were digitally cut to consider only the part that belonged to the recorded segment or wall. Note that the apparent number of recorded plaques increased as they were split up to segmental and wall levels, which must be considered in . The image analysis was based on hard-contrast tracing without need for elaborate techniques. The geometric scale was calibrated. Measurements encountered; area, long-axis diameter, and shape factor (0-1 of which 1 equals a perfect circle). Due to the irregular shape of plaques, and for interpretation, the area was recalculated into its corresponding circular diameter.
Table II. Plaque characteristics of thoracic aorta with subdivision into anatomic regions.
Geometric data were transferred to an Excel spreadsheet for further analysis and calculations. The main parameter was the relative area of accumulated plaques in relation to the outline area of the aorta, segment, or wall, respectively. This parameter is a measure of the magnitude of plaque occurrence, referred to as ‘plaque density’. Plaque density is insensitive to the cutting of plaque during aortic subdivision. Plaque density was reviewed in relation to aortic regions but also to subject characteristics and to aortic observations at autopsy.
The risk associated with aortic cross-clamp and cannulation in relation to plaques was evaluated in a blinded fashion. The interference surface of a standard 70-mm aortic cross clamp (Pilling Co, Fort Washington, Pa, USA) and cannulation tip (24F, Baxter, Irvine, Ca, USA) was superimposed onto the aortic map. The positioning was guided by the brachiocephalic trunk to mimic the clamp and cannula locations on a live aorta under surgical conditions. An experienced cardiac surgeon, blinded to the location of plaques, conducted this step. The plaque distribution, being stored on a separate map, was then superimposed onto the marked clamp and cannulation sites, and their plaque interference was recorded.
Statistical analysis
Due to skewed data distribution and few observations, non-parametric statistics were used for group comparison, including Wilcoxon signed ranks test, Mann-Whitney U-test, and Spearman's rank correlation. Linear regression analysis of least-square method was used to calculate slope values and statistical dependency of continuous variables. ANCOVA was used to investigate confounding relationships. A p-value above 0.05 was considered non-significant. Statistica (StatSoft Tulsa, OK, USA) version 6.1 was used throughout.
Results
Aorta characteristics
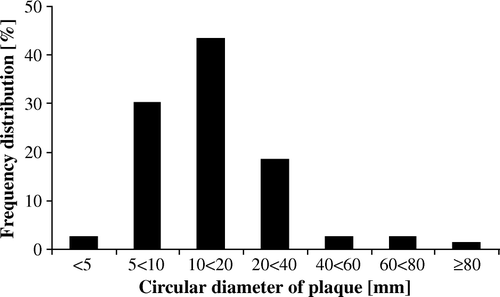
All of the 24 included autopsy cases had plaque formation of various degrees. When measured by image analysis, three of 24 subjects had a plaque density exceeding 50%, while the majority of patients had less than 20%. Three subjects had an insignificant plaque density of less than 2.5% (). In the pooled frequency distribution of plaque circular-diameter is presented. See for details of plaque geometry on subject level.
Distribution of plaques in different aortic regions
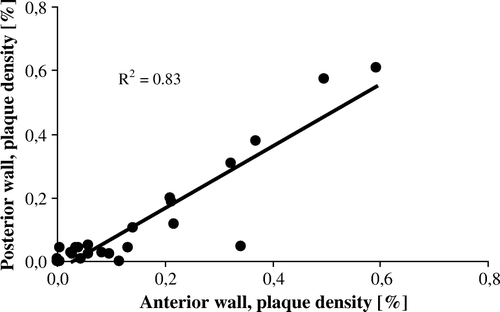
The highest numeric plaque density was observed for the posterior wall of the descending aorta although not being significantly different from the other aortic segments. Of significance was that the anterior wall of the ascending/arch aorta had a more pronounced plaque density compared with its posterior wall (p = 0.039). Although numerically different, their plaque density showed a significant co-variation between anterior and posterior sides (p < 0.001, R2=0.83, ).
Confounding variables to aortic plaque density
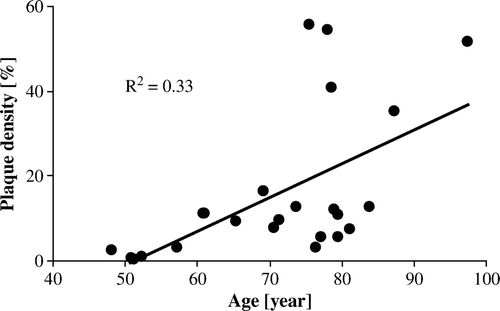
There was a significant positive correlation between plaque density and age (p = 0.004, of entire thoracic aorta, , ). Each 10-year increment of advanced age caused the plaque density to increase by on average 8% (). Presence of coronary disease was associated with a vast 571% increase in plaque density of the descending aorta (p = 0.008). Although numerically increased this relationship was not as pronounced or becoming significant for the ascending aorta (). Valvular disease showed a similar pattern, with reference to the entire thoracic aorta. A wider aorta, as measured in its ascending part, was significantly associated with increased plaque density in its descending segment. Aortic wall thickness showed no relationship to plaque density, nor did cardiac weight (). The described parameters were found to hide several confounding relationships. When entire-aorta plaque density was tested as dependent variable by an ANCOVA method, advanced age remained a significant predictor that explained the other correlations.
Table III. Magnitude of plaque density in relation to subject characteristics.
Risk of interference with a plaque during aortic cross clamping and/or cannulation
When blindly performed, the aortic cross clamp and/or cannula tip interfered with one or more plaques in 45.8% of the procedures (). There was an equal risk of 16.7% to interfere with a plaque by applying the cross clamp or introducing the cannula. In 12.5% of the procedures, both the clamp and cannula interfered with the same or separate plaques. Of the observed seven cross-clamp hits, five cases showed interference with more than one plaque.
Table IV. Risk of plaque interference by clamp and/or cannula.
Discussion
Atherosclerotic degeneration of the aorta is common. Cardiac-surgery techniques depend on the aortic condition, in particular with reference to the use of cardiopulmonary bypass Citation8. In the present study using aortic intimal visualization and palpation in autopsy subjects, all cases had various degree of plaque formation in the thoracic aorta. In cardiac surgery epiaortic scanning is a valuable tool to obtain a view of the aortic inside Citation4. This method has suggested the anterior wall of the ascending aorta to be more affected by plaque formation than the posterior wall Citation4. There was a proposed 12% to 8% difference in plaque magnitude between these walls, measured for the distal portion of the ascending aorta near the brachiocephalic trunk Citation4. However, an opposite relationship was suggested by Tobler and Edwards Citation9, although their study was based on plaque counts only and with no statistics performed. Our findings support the former of these two studies, suggesting a corresponding and significant 15% to 12% relationship between the anterior and posterior walls, respectively. Although the numeric plaque difference was modest, the finding may have a clinical interest in that the anterior wall is exposed to cannulation. However, of equal interest was our finding of a strong correlation in plaque density between the anterior and posterior aortic walls. A high R-square value emphasized that a presence of plaque in the anterior wall to 83% predicts the coexistence of a plaque also in its posterior part. The knowledge may have clinical implications when cross clamping is performed relying on palpation only, with the backside being more difficult to reach by hand. Moreover, our model tested a clinical procedure when the cross clamp and cannula were positioned without prior mapping of plaque distribution. The risk of interference with a plaque during these combined procedures was near 46%.
Our study confirmed that aortic plaque formation was strongly associated with age. Per decade of advanced age the plaque density increased by 5% and 10%, for the ascending/arch and descending aorta, respectively. Presence of coronary and/or valvular disease showed significance versus plaque formation in various aortic segments, and aortic diameter was positively correlated with plaque density. However, a co-variation was found for these variables, being explained by advanced age.
Our model had obvious limitations. A geometric error was created at unfolding of the torus-shaped aortic arch into a two-dimensional map. Nevertheless, this error equally affected the anterior and posterior walls of the aorta. Moreover, post-mortem changes must be considered to have affected the intimal wall of the aorta even though the plaques appeared intact to the observer. Due to time consuming experiments and ethical restrictions, the study cohort contained few observations and therefore with limited statistical power. For the same reason was the subdivision of the aorta limited to include only four segments as further subdivision would have diluted the results beyond statistical reach. However, this experimental model has not been previously conducted and may provide useful information in relation to existing knowledge based on epiaortic ultrasound.
It is concluded that plaque formation in the aorta is frequent and age dependent. The anterior wall of the ascending/arch aorta was more affected by plaques than its posterior wall, although with a pronounced coexistence between sides. The theoretical risk of plaque interference at cannulation and/or clamp maneuvers was prominent, a finding that strongly supports the clinical use of epiaortic scanning in cardiac surgery.
The authors would like to thank Mrs Anne-Marie Österdahl, Mr Dan Nylund, Dr Eva Lundin and Dr Karin Sixtensdotter Graffmo, Department of Clinical Pathology, Umeå University Hospital. This work was supported by Swedish Society for Medical Research, funds of the Medical Faculty, Umeå University Hospital, the Heart Foundation of Northern Sweden, and the Swedish Stroke Foundation.
References
- Ahonen J, Salmenpera M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand. 2004; 48: 4–19
- Ura M, Sakata R, Nakayama Y, Goto T. Ultrasonographic demonstration of manipulation-related aortic injuries after cardiac surgery. J Am Coll Cardiol. 2000; 35: 1303–10
- Vaage J, Jensen U, Ericsson A. Neurologic injury in cardiac surgery: Aortic atherosclerosis emerges as the single most important risk factor Scand Cardiovasc J. 2000; 34: 550–7
- van der Linden J, Hadjinikolaou L, Bergman P, Lindblom D. Postoperative stroke in cardiac surgery is related to the location and extent of atherosclerotic disease in the ascending aorta. J Am Coll Cardiol. 2001; 38: 131–5
- Boivie P, Edström C, Engström KG. Side differences in cerebrovascular accidents after cardiac surgery: A statistical analysis of neurologic symptoms and possible implications for anatomic mechanisms of aortic particle embolization. J Thorac Cardiovasc Surg. 2005; 129: 591–8
- Boivie P, Hansson M, Engström KG. Embolic material generated by multiple aortic crossclamping: A perfusion model with human cadaveric aorta. J Thorac Cardiovasc Surg. 2003; 125: 1451–60
- Boivie P, Hansson M, Engström KG. Intraluminal aortic manipulation by means of intra-aortic filter, cannulation, and external clamp maneuvers evaluated versus dislodged embolic material. J Thorac Cardiovasc Surg. 2006; 131: 283–9
- Kokotsakit J, Lazopoulos G, Milonakis M, Athanasiadis G, Romana K, Skouteli E, et al. Right axillary artery cannulation for surgical management of the hostile ascending aorta. Tex Heart Inst J. 2005; 32: 189–93
- Tobler HG, Edwards JE. Frequency and location of atherosclerotic plaques in the ascending aorta. J Thorac Cardiovasc Surg. 1988; 96: 304–6