Abstract
Objectives. The aim of the present study was to evaluate the effect of tramadol on isolated rat hearts subjected to global ischemia-reperfusion injury. Design. Langerdorff perfused isolated rat hearts were subjected to 60 min of global ischemia following 60 min of reperfusion. In group I and III hearts were received tramadol before the onset of ischemia. Following the ischemic period, group II and III hearts were received tramadol infusion. Group I and IV hearts were subjected to saline at the same time point. The myocardial postischemic recovery was compared using hemodynamic, coronary flow, biochemical parameters from coronary effluent, and oxidative stress markers from heart tissue homogenates. Results. There were significant differences between tramadol and saline used groups in hemodynamic parameters. GPx values of groups I and III were significantly lower than group IV (p<0.05). SOD values of groups I, II and III were higher than group IV (p<0.05). LDH values of groups I and II were significantly lower than groups III and IV (p<0.05). Conclusion. Tramadol provides a cardioprotective effect against myocardial ischemia-reperfusion in isolated rat heart.
Myocardial ischemia-reperfusion injury is defined as the adverse effects that ensure upon restoration of the circulation, which allows blood and nutrients to reach cells previously subjected to ischemia. Restoration of blood flow can be accompanied by the release of oxygen free radicals, the apperance of intracellular calcium overload, and alterations in cell metabolism -all situations that can give rise to functional or structural myocardial injury Citation1. Oxidative damage due to the presence of radical oxygen species is the essential step that initiates a wide range of intracellular stress signaling processes that culminate in excessive chemokine response, adhesion molecule upregulation and nitric oxide overproduction Citation2.
The optimal preservation methods for myocardium during ischemia-reperfusion have been the focus of many studies. Efficient analgesia may be the major objective in the cardiovascular risk patient following myocardial emergencies. Centrally acting analgesics are extensively used in patients with cardiovascular disorders Citation3. It has been proved that morphine has cardioprotective effects during ischemia-reperfusion Citation4, Citation5. Factors such as respiratory depression and histamine release are the disadvantages of morphine usage in postoperative period of open heart surgery Citation6. Tramadol is a centrally acting analgesic with negligible respiratory depressant action and very low tolerance and physical dependence liability. Müller et al. Citation3 reported in their study that tramadol exerts a positive inotropic action on the heart; in addition, it also causes slight hypertensive and positive chronotropic effects. These effects offer some advantages in cardiovascular risk patients due to its minor cardiovascular and respiratory side effects. This prompted us to explore the possibility that tramadol might protect against myocardial ischemia-reperfusion injury.
The aim of our study was to evaluate the effect of tramadol on isolated rat hearts subjected to global ischemia-reperfusion injury.
Material and methods
Animals
This study was approved by Osmangazi University Institutional Local Animal Care and Use Committee. Male Sprague-Dawley rats, 250–300 g body weight were used for the study. Animals were fed a standard rat chew. They were housed in temperature-controlled (20–25°C) cages with a 12 h dark and 12 h light cycle. All procedures were performed under sterilized conditions.
Heart perfusion
All rats were heparinized (300 mg/kg) and anesthetized with 50 mg/kg sodium pentobarbital administered by intraperitoneal injection. Each rat heart was rapidly excised through a median sternotomy and immersed into ice-cold heparinized saline solution during preparation. After the heart was cleaned of the surrounding fat and other tissues, aorta was immediately tied to stainless steel cannula of the modified Langerdorff perfusion apparatus. Aortic perfusion was initiated at a constant perfusion pressure of 80 mmHg with gassed (95% O2 and 5% CO2) Krebbs-Henseleit bicarbonate buffer (K-H) (in mmol/l: NaCl 118, KCl 4.7, CaCl2 2.0, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11.1) while pH was maintained at 7.4–7.5. A pulmonary arteriotomy was performed to allow free drenaige of coronary sinus effluent. The heart temperature was monitored using a thermistor probe placed in the right ventricle wall, and kept constant at 37°C during the stabilization and reperfusion periods and at 24°C during the ischemic period. The hearts were paced to 300 beats/min at 4 V using an external pacemaker (HSE Stimulator P, Type 201, Hugo Sachs Electronic, Germany). A water filled latex balloon was inserted in to the left ventricle cavity through a small incision in the left atrium and connected to a pressure transducer (Transpac II, Abbott, Bedford, MA, USA) by rigid polyethylene tubing. The balloon volume was adjusted to produce 10 mmHg of diastolic pressure. Hemodynamic data from the latex balloon were analyzed using a real-time data acquisition and analysis system (Biopack MP 30 system, Santa Barbara, CA, USA) and displayed on a monitor during the experiment. The hearts were excluded from the experiment if they displayed lower contractile function (+dP/dtmax<3000) or severe arrhythmia. All hearts then underwent a 20 min stabilization period.
Drug and experimental design
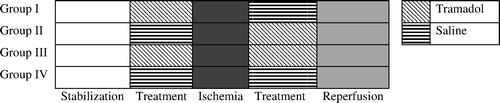
A schematic illustration of the experimental protocol is given in . After the stabilization period group I and III hearts received tramadol at the concentration of 1×10−4 M/l for 10 min. Meanwhile group II and IV were subjected to saline infusion for duration of 10 min over the same time period. Following the infusion period, all hearts in each group were then arrested with cold (+4°C) crystalloid cardioplegia solution (Plegisol, Abbott) and then subjected to 60 min of global ischemia at 24°C. Pacing was stopped during ischemia. Following the ischemic period, group II and III hearts were received tramadol infusion at the concentration of 1×10−4 M/l for 10 min. Group I and IV hearts were subjected to saline at the same time point. The hearts were then reperfused for a 60 min period with K-H solution at 37°C and paced at 300 beats/min.
Hemodynamic parameters
Left ventricular pressure waveforms (obtained from left ventricular latex balloons) were analyzed and peak systolic pressure (PSP) and the maximum rate of increase and decrease of left ventricular pressure (+dP/dtmax) were recorded at the end of the stabilization period and at 20 min intervals throughout the reperfusion period. Coronary effluent was collected in a reservoir for 1 min and measured as coronary flow (CF) after the stabilization period and at 20 min intervals during reperfusion.
Biochemical assay
The coronary effluent was collected throughout the stabilization and reperfusion periods separately, and samples were stored at −80°C. Ischemic damage was assessed using lactate dehidrogenase (LDH) level, creatine kinase (CK-MB) activity. LDH and CK-MB activities were determined in a Hitachi 917 automated analyzer by using commercial kits supplied from Roche Diagnostic (Manhaime Germany).
Evidence of oxidative stress was determined from heart tissue homogenates using glutathione peroxidase (GPx), malondialdehyde (MDA), and superoxide dismutase (SOD) activities. Following reperfusion the hearts were rapidly arrested by immersing in ice-cold K-H solution and stored at −80°C. The tissues were homogenized in 0.1 M phospate buffer (pH 7.4) with Ultra Turrax homogenizer (IKA T18 basic, Wilmington NC, USA). The homogenates were centrifuged at 5000 rpm at +4°C for 10 min, and the supernatants were removed and assayed for MDA, GPx, and SOD activities. Tissue GPx and SOD activities were measured with a Hitachi 917 autoanalyser using commercial kits (Randox Laboratories LTD. Antrim, UK). Tissue MDA levels were determined by thiobarbituric acid method of Okhawa et al. Citation7. All chemicals for MDA analysis were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Statistical analysis
Results were expressed as means±SD. Statistical analysis was performed using ANOVA (variance analysis for multiple comparisons). The post hoc Tukey multiple range test was used after ANOVA and p< 0.05 was accepted as statistically significant. Biochemical data were tested using the Kruskall-Wallis nonparametric test of difference and values are expressed as the median (25th, 75th percentiles).
Results
Hemodynamic parameters
There was no significant difference in hemodynamic parameters during stabilization and tramadol infusion periods (p > 0.05).
Peak Systolic Pressures (PSP)
PSP values of all groups were not statistically significant during stabilization period and 20 min from ischemia in each other (p > 0.05). According to ANOVA test after 40 and 60 min from ischemia the difference between PSP values in group II and III was significant (p < 0.05). PSP values of group II was significantly higher than other groups (p < 0.05). On the contrary, PSP values of group III was significantly lower than other groups at the same time points (p < 0.05) ().
Table I. Hemodynamic and coronary flow measurements of the groups in pre-ischemic and post-ischemic period. There were six rats in each group. Values are expressed as±standard deviation of the mean (±SD)
+dP/dtmax
The difference between +dP/dtmax values of all groups were not statistically significant during stabilization period (p > 0.05). According to ANOVA test after 20 and 40 min from ischemia the difference between +dP/dtmax values in group II and III was significant (p < 0.05). +dP/dtmax values of group II was significantly higher than other groups after 20 and 40 min from ischemia (p < 0.05). But, the values of group III was significantly lower than other groups at the same time points (p < 0.05). After 60 min from ischemia the difference between +dP/dtmax values of all groups were not statistically significant than each other (p > 0.05).
Coronary flow
Comparison of the coronary flow values of each group by using ANOVA test revealed no statistical significance at any time point in our study (p > 0.05).
Biochemical results
The biochemical results are presented in . MDA values were insignificantly higher in group IV (p > 0.05). GPx values of groups I and III were significantly lower than group IV (p < 0.05). GPx value of group II was also lower than group IV insignificantly (p > 0.05). SOD values of groups I, II and III were significantly higher than group IV (p < 0.05) (). CK-MB levels were similar in all groups (p > 0.05). LDH values of groups I and II were significantly lower than groups III and IV (p < 0.05)
Figure 2. Effects of Tramadol on oxidative stress indicators. Values are expressed as the median (25th, 75th percentiles). *p > 0.05, statistically significant than group IV.
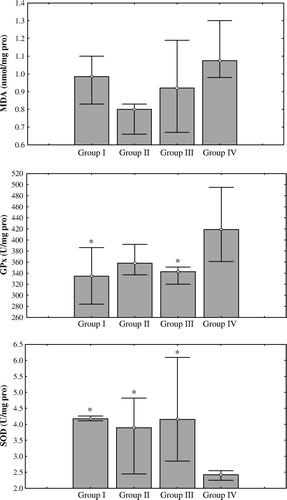
Table II. Biochemical results of the groups. Values are expressed as the median (25th, 75th percentiles)
Discussion
The results of this study suggest that tramadol a centrally acting analgesic agent, may have cardioprotective properties against ischemia-reperfusion injury. This condition is a complex, multifactorial process that results in the formation of reactive oxygen species (ROS). In the presence of high intracellular calcium, ROS promote the opening of the mitochondrial permeability transition pore, release of cytochrome c into the cytoplasm, and activation of the caspase cascade, resulting in apoptosis Citation8. Many cells can manifest an antioxidant enzyme system against the toxic action of ROS including superoxide dismutase (SOD), glutathione peroxidase and catalase. In ischemic conditions the capacity of antioxidant system is not sufficient to compensate for the increased toxic effects of ROS Citation9. During early period of reperfusion, the amount of ROS increases as a result of both an increased production of ROS and insufficient antioxidant mechanism Citation10. In myocardial tissue, augmentation in the formation of free nitrogen and ROS leads to ischemia-reperfusion injury and myocardial damage. In this regard, ROS scavengers and antioxidants may decrease the negative consequence of ischemia-reperfusion injury Citation9. In our study the antioxidant potential of tramadol was investigated using MDA, GPx and SOD contents in myocardial tissue. The MDA level is a complex marker of the tissue lipid peroxidation. The amount of MDA accumulation in tissue is an index of the extent of lipid peroxidation and oxidative stress Citation11. Finding lower levels of MDA in the groups receiving tramadol compared to the control group supports the hypothesis that tramadol shows oxidative stress reducing effects by scavenging peroxyl radicals. GPx activity is known to depend on reduced levels of glutathione, glutathione transferase and glutathione reductase. Activities of these enzymes play an essential role in the cellular defense against free radical Citation11. In our study, GPx levels of control group were higher than tramadol used groups. Data of SOD were supporting the probability of antioxidant effect of tramadol when it was used in preischemic period. The decreased levels of MDA content and elevated levels of SOD activity in tissues may be an evidence of a decreased lipid peroxidation and an increased anti-oxidative ability.
Nitric oxide (NO) is a potent vasodilator produced by the endothelium under basal conditions and in response to a variety of agonists Citation12. Strong evidence exists that many of the peripheral effects of opioids involve NO Citation13. Kaya et al. Citation12 demonstrated in their study that tramadol stimulates NO production by the activation of the nitric oxide synthase (NOS)-guanylate cyclase pathway and produces greater degree of vasodilatation in rabbit aorta. The up-regulation of NO causes relaxation of vascular smooth muscles, resulting in vasodilatation through cyclic GMP up-regulation in coronary artery Citation9. NO downregulates interactions between neutrophils and endothelium (e.g., tethering, triggering, gluing, and transmigration). These processes are essential for neutrophil accumulation at sites of inflammation and a prerequisite for tissue injury in, for example, ischemic myocardium. It has been reported that morphine provides a cardioprotective effect in patients with acute myocardial infarction Citation13, Citation14. Therefore, tramadol may also exert protective effect against ischemia-reperfusion injury by the same way.
Pretreatment of the hearts with tramadol provided a protection against postischemic myocardial injury, and this effect was not associated with any significant change in coronary flow in our study. Tramadol has a dual mechanism of action: weak µ-opioid receptor agonist and a reuptake inhibitor of serotonin and noradrenaline Citation15. The existence of κ- and δ-opioid receptors, but not µ-receptors, has been reported in rat atrial and ventricular tissue Citation16. There is evidence that κ- and δ-opioid receptors are involved in opioid-induced cardioprotection and an intracardiac µ-opioid receptor is not involved in this condition Citation17. So we can not attribute the effects of tramadol in ischemia-reperfusion injury to µ-opioid receptors.
The effect of tramadol on noradrenaline uptake may contribute to its cardioprotective action. [Dmt1]DALDA, a highly potent and long-acting µ-opioid receptor-binding peptide analgesic, inhibits norepinephrine uptake in a manner similar to tramadol and cardioprotective effects of this agent has been proved in many of the studies Citation8, Citation18. Similarly, tramadol may exert cardioprotective action by the inhibition of NE uptake into cardiac synaptosomes as [Dmt1]DALDA.
In conclusion, tramadol provides a cardioprotective effect against myocardial ischemia and reperfusion in isolated rat heart. Unlike other opioid agents, tramadol has the advantage of more reliable cardiovascular stability. Minor cardiovascular and respiratory side effects are making it an attractive agent for cardiac interventions that may put the patient at risk for cardiac ischemia. If these findings can be confirmed clinically, tramadol may offer several advantages over other pharmacological interventions.
References
- Ferez Santander SM, Marquez MF, Pena Duque MA, Ocaranza Sanchez R, De la Pena Almaguer E, Eid Lidt G. Myocardial reperfusion injury. Rev Esp Cardiol. 2004; 57: 9–21
- Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004; 9: 81–3
- Müller B, Wilsman K. Cardiac and hemodynamic effects of centrally acting analgesics tramadol and pentazocine in anaesthetized rabbits and isolated guinea-pig atria and papillary muscles. Drug Res. 1984; 34: 430–3
- Groban L, Vernon JC, Butterworth J. Intrathecal morphine reduces infarct size in a rat model of ischemia-reperfusion injury. Anesth Analg. 2004; 98: 903–9
- McPherson BC, Yao Z. Signal transduction of opioid-induced cardioprotection in ischemia-reperfusion. Anesthesiology. 2001; 94: 1082–8
- Ellmauer S, Dick W, Otto S, Muller H. Different opioids in patients at cardiovascular risk. Comparison of centrally and peripheral hemodynamic adverse effects. Anaesthesist. 1994; 43: 743–9
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351–8
- Song W, Shin J, Lee J, Kim H, Oh D, Edelberg JM, et al. A potent opiate agonist protects against myocardial stunning during myocardial ischemia and reperfusion in rats. Coron Artery Dis. 2005; 16: 407–10
- Dernek S, Ikizler M, Erkasap N, Ergun B, Koken T, Yilmaz K, et al. Cardioprotection with resveratrol pretreatment: Improved beneficial effects over standard treatment in rat hearts after global ischemia. Scand Cardiovasc J. 2004; 38: 245–54
- Zweier JL. Measurement of superoxide derived free radicals in the reperfused heart: Evidence for a free radical mechanism of reperfusion injury. J Biol Chem. 1988; 263: 1353–7
- Polat A, Emre MH. Effects of melatonin or acetylsalicylic acid on gastric oxidative stress after bile duct ligation in rats. J Gastroenerol. 2006; 41: 433–9
- Kaya T, Gursoy S, Karadas B, Sarac B, Kafali H, Soydan AS. High-concentration tramadol-induced vasodilatation in rabbit aorta is mediated by both endothelium-dependent and -independent mechanisms. Acta Pharmacol Sin. 2003; 4: 385–9
- Stefano GB. Autoimmunovascular regulation: Morphine and ancondamide and ancondamide stimulated nitric oxide release. J Neuroimmunol. 1998; 83: 70–6
- Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997; 80: 1532–5
- Close BR. Tramadol: Does it have a role in emergency medicine. Emerg Med Australas. 2005; 17: 73–83
- Zaugg M, Luchinetti E, Uecker M, Pasch T, Schaub MC. Anaesthetics and cardiac preconditioning. Part I. Signalling and cytoprotective mechanisms. Br J Anaesth. 2003; 91: 551–65
- Zhang Y, Irwin MB, Wong TM. Remifentanyl preconditioning protects against ischemic injury in the intact rat heart. Anesthesiology. 2004; 101: 918–23
- Wu D, Soong Y, Zhao GM, Szeto HH. A highly potent peptide analgesic that protects against ischemia-reperfusion-induced myocardial stunning. Am J Physiol Heart Circ Physiol. 2002; 283: 783–91