Abstract
Objective. Cardiopulmonary bypass (CPB) is associated with fluid overload. We examined how a continuous infusion of hypertonic saline/dextran (HSD) influenced fluid shifts during CPB. Materials and methods. Fourteen animals were randomized to a control-group (CT-group) or a hypertonic saline/dextran-group (HSD-group). Ringer's solution was used as CPB-prime and as maintenance fluid at a rate of 5 ml/kg/h. In the HSD group, 1 ml/kg/h of the maintenance fluid was substituted with HSD. After 60 min of normothermic CPB, hypothermic CPB was initiated and continued for 90 min. Fluid was added to the CPB-circuit as needed to maintain a constant level in the venous reservoir. Fluid balance, plasma volume, total tissue water (TTW), intracranial pressure (ICP) and fluid extravasation rates (FER) were measured/calculated. Results. In the HSD-group the fluid need was reduced with 60% during CPB compared with the CT-group. FER was 0.38(0.06) ml/kg/min in the HSD-group and 0.74 (0.16) ml/kg/min in the CT-group. TTW was significantly lower in the heart and some of the visceral organs in the HSD-group. In this group ICP remained stable during CPB, whereas an increase was observed in the CT-group (p <0.01). Conclusions. A continuous infusion of HSD reduced the fluid extravasation rate and total fluid gain during CPB. TTW was reduced in the heart and some visceral organs. During CPB ICP remained normal in the HSD-group, whereas an increase was present in the CT-group. No adverse effects were observed.
Cardiopulmonary bypass (CPB) is associated with fluid overload partly related to the quality and volume of the priming- and cardioplegic solutions. Priming with crystalloid solutions is standard procedure in most centers. However, the initial hemodilution triggers an extravasation of fluid from the intravascular to the interstitial compartment Citation1. As a consequence supplemental fluid is regularly needed to maintain fluid levels in the venous reservoir during the initial phase of CPB. The use of hypothermia is an additional factor contributing to increased fluid extravasation Citation2, Citation3. The fluid overload results in tissue edema and occasionally organ dysfunction affecting heart Citation4, respiratory system Citation5 and gastrointestinal tract Citation6. Cererbral edema following normothermic as well as hypothermic CPB has also been reported Citation7, Citation8.
The well known clinical problem with tissue edema has brought more emphasis on intra-operative and post-operative strategies to limit extravascular fluid accumulation. Such strategies include the use of natural and artificial colloidal priming solutions and the use of modified ultrafiltration procedures during/after CPB. A variety of colloids are available, including fresh frozen plasma, human serum albumin, gelatins, dextran and hydroxyethyl starch.
Several studies have shown beneficial effects of hyperoncotic additives to the CPB prime in reducing fluid overload during CPB Citation9, Citation10. Hypertonic saline/hyperoncotic solutions were developed for use in rescue situations as a bolus given within few minutes. These preparations have been given as a bolus pre- per- as well as post-CPB to reduce fluid overload during cardiac surgery Citation11–13. Bolus administration of such solutions results in rapid and significant fluid shifts which can be unfavorable in heart patients with cardiac insufficiency. In this study we evaluated the effect of a hypertonic saline/dextran (HSD) infusion administered at a rate of 1 ml/kg/h to a total dose of 4 mg/kg, during normothermic followed by hypothermic CPB.
Materials and methods
The animal handling and procedures described were approved and in accordance with recommendations given by Norwegian Animal Research Authority. All animals were acclimatized in the laboratory animal housing area for at least 3 days prior to the experiments.
Fourteen immature domestic pigs (Norwegian landrace-Yorkshire hybrid) weighing 25–32 kg were randomized into two study groups, the HSD-group (hypertonic saline/dextran) (n = 7) and CT-group (the control-group) (n = 7).
General anesthesia and surgical preparation
Premedication, induction and maintenance of anesthesia was done as previously described Citation14.
With the animals in prone position a 0.5 cm burr hole was made 1 cm lateral to the midline suture and 0.5 cm cranial to the coronal suture. The dura mater was incised with surgical diathermy. A Codman MicroSensor ICP Transducer (Codman, Johnson & Johnson professional Inc, Raynham, MA, USA) was introduced 15 mm into the brain parenchyma and connected to a Codman ICP ExpressTM Monitor for intracranial pressure (ICP) monitoring (Johnson & Johnson professional Inc, Raynham, MA, USA).
With the animal in supine position intravascular catheters were placed in the femoral artery for recording of the mean arterial pressure (MAP) and for blood sampling and in the femoral vein for fluid substitution. A suprapubic urine bladder catheter was placed for monitoring of diuresis.
A midline sternotomy was then performed. Before cannulation heparin 6 mg/kg was given i.v. and supplemented with 3 mg/kg prior to initiation of CPB. CPB was carried out via a 32 F, 35 cm single venous return cannula placed in the right atrium and an 18F aortic arch cannula connected to standard equipment for open heart surgery (Quadrox, hollow fibre membrane oxygenator with venous hardshell cardiotomy reservoir, VHK 4200, Jostra, AG, Hirrlingen, Germany). The machine reservoir was filled to a level of 300 ml and maintained at that level during the bypass period. Initial prime volume was 1115 ml of acetated Ringer's solution. Pump flow was set to 2.7 l/min/m2. Flow pattern was non-pulsatile. The CPB head pressures were in the range of 200–250 mmHg. During CPB the height difference between the machine reservoir and the right atrium was fixed (73±3 cm). Left ventricle was vented by use of a 17 Fr. vent catheter. Free venous drainage was ensured continuously by visual inspection and by monitoring of the central venous pressure (CVP) in the right atrium. A 5 Fr pulmonary artery catheter was introduced until wedging position in the pulmonary artery (Edwards Lifesciences, Irvine, Ca, USA).
Surgery was normally completed within 30 min. Thereafter the animals were allowed 60 min stabilization before initiation of CPB. During this time baseline laboratory parameters were measured together with plasma volume.
Experimental protocol
In the HSD-group 4 ml/kg/h of acetated Ringer's solution combined with 1 ml/kg/h of hypertonic saline 7.5% and dextran 6% ( Rescueflow® “BioPhausia”, Stockholm, Sweden) was given. The infusions started after induction of anesthesia and continued until termination of CPB. Prior to HSD administration, dextran 1 (Promiten®) 20 ml was given.
The animals of the CT-group received a continuous infusion of acetated Ringer's solution, 5 ml/kg/hour, throughout the same period.
After 60 min of stabilization, normothermic CPB was initiated and continued for 60 min followed by 90 min of hypothermic CPB. A decrease in the core temperature from 38°C to 28°C was achieved within 20 minutes.
The fluid level of the machine reservoir was measured continuously during CPB and changes from the 300 ml level were recorded at 5 min intervals. Such changes were used as an indicator of fluid loss (or gain) from the intravascular compartment or changes in vascular tone. Whenever the blood level in the venous reservoir decreased, acetated Ringer's solution was added to the reservoir to restore the 300 ml level.
Measurements
Hemodynamics and diuresis
Heart rate, MAP, Cardiac output (CO), CVP and diuresis were obtained as earlier described Citation2. Cerebral perfusion pressure (CPP) was calculated as: MAP-ICP.
Blood parameters
Blood samples ( Hematocrit (Hct), serum-albumin, serum-osmolality, serum-electrolyte concentrations, and acid-base parameters) drawn from the arterial line, were analyzed as previously described Citation2.
Plasma volume determination
Plasma volume was measured using carbon monoxide as label 30 min prior to start of CPB (baseline value) Citation15. Subsequent plasma volumes were calculated at 30 min intervals from determination of Hct values and blood losses. To assess the in vivo PV during CPB, the calculated PV were corrected according to the following equation:
Net fluid balance and fluid extravasation rate
Net fluid balance (NFB), i.e. all fluid additions minus diuresis and blood losses during CPB, was calculated at 30 min intervals. Blood losses during surgical preparation were substituted by use of Ringer's solution in volumes three times the blood loss volume. During CPB blood loss into the open chest was returned to circulation via the machine suction. NFB was used together with changes in plasma volume (▵PV) to calculate the fluid extravasation rate (FER) according to the formulae:
Colloid osmotic pressure
Colloid osmotic pressures were measured in plasma (COPp) and interstitial fluid (COPi) with a colloid osmometer using a semi-permeable membrane with a cut off level at 10.000 D (PM-10, Millipore Corporation, Bedford MA, USA) and acetated Ringer's solution in the reference chamber. The osmometer was designed to accept 5 µl sample volumes Citation16.
Fluid for determination of the interstitial colloid osmotic pressure was sampled by means of multifilamentous nylon wicks sewn subcutaneously into thoracoabdominal skin folds and left in situ for 90 min Citation17. At the end of the implantation period, the wicks were pulled out swiftly and placed in centrifuge tubes containing mineral oil. Wick fluid from three implantation periods, before, during normothermic and hypothermic CPB was analyzed.
Serum albumin mass
Total intravascular albumin masses (gram), were calculated as the product of measured plasma volume (within animal, tubings and reservoir) and the serum-albumin concentrations in g/l. Extravasation/intravasation of albumin was calculated as the change in albumin mass from one 30 min period to the next.
Total Tissue Water (TTW)
The pigs were killed with an intravenous injection of 20 ml of saturated KCl solution. Immediately after this injection, tissue samples (3 parallel pieces) were taken from right and left side of the heart, lung, liver, both kidneys, stomach, pancreas, ileum, colon, left quadriceps muscle, abdominal skin, and brain, and placed in pre-weighed vials, reweighed and placed in a drying chamber at 70°C. The vials were weighed repeatedly until a stable weight was obtained. TTW was recorded as percent water content.
Statistical methods
Statistical analysis was performed by use of SPSS, version 13.0 for windows. The results are presented as mean with standard deviation (SD) in parentheses. Repeated measure analysis of variance with one grouping variable was used to test the relationship of outcome variables at different times. If a significant between-group P-value was found, post-hoc-t-tests were performed to compare data before CPB and at the end of normothermic and hypothermic CPB. On finding a significant within-group P-value, a post-hoc paired-t-test was used to compare the changes at the end of normothermic and hypothermic CPB.
An independent t-test or a Mann-Whitney Test was used for two-group comparison of water content in the different organs. Significance level was accepted as p < 0.05. The p-value was adjusted according to the number of comparisons.
Results
All animals studied were comparable with respect to age, weight and sex. Values were 73.4 (9.7) vs. 73.1 (10.1) days, 28.3 (1.3) vs. 27.7 (1.4) kg and M/F: 3/4 vs. 5/2 in the HSD-group and CT-group, respectively.
Hemodynamics, plasma volume, colloid osmotic pressures, and fluid balance
All animals remained cardiovascular stable with beating hearts throughout the experiments. The MAP values are presented in . MAP increased significantly during hypothermic CPB in both groups with no between-group differences.
Table I. The values are presented as mean with SD in parentheses.
Plasma volume (PV) decreased significantly in both study groups after 60 min of normothermic CPB, but returned to pre-bypass level during hypothermic CPB. No between-group differences were detected ().
COPp decreased significantly in both groups during the experiments and between-group differences were obtained at the end of normothermic as well as hypothermic CPB, with higher values in the HSD-group.
COPi decreased significantly in the CT-group during hypothermic CPB as compared with pre-bypass values, while COPi in the HSD-group remained unchanged during the experiments ().
There were no significant differences between the groups with respect to diuresis and blood loss during the study period.
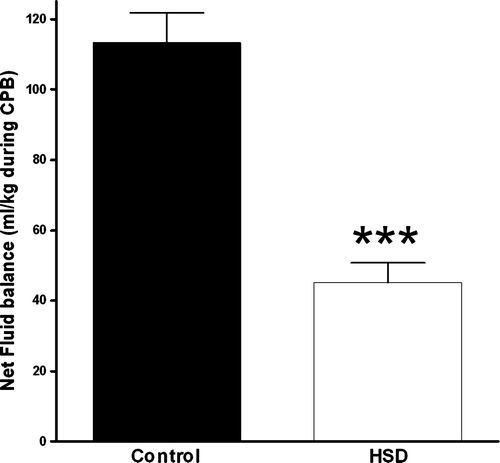
The total fluid gain (ml/kg) following 150 min of CPB was significantly reduced from 116.0 (23.1) ml/kg, in CT-group to 48.8 (14.6) ml/kg when HSD was administered (p < 0.01) ().
NFB () was effectively reduced from 0.84 (0.27) ml/kg/min in the CT-group to 0.44 (0.21) ml/kg/min in the HSD-group during normothermic CPB (p < 0.01). The between-group difference in NFB was even more pronounced during hypothermic CPB, with NFB values of 0.69 (0.12) ml/kg/min and 0.21 (0.09) ml/kg/min in the CT-group and HSD-group, respectively (p < 0.001).
FER increased after start of CPB from 0.20 (0.07) ml/kg/min to 1.75 (0.53) ml/kg/min in the CT-group and from 0.16 (0.03) ml/kg/min to 1.34 (0.28) ml/kg /min in the HSD-group. Although FER tended to be lower in the HSD-group, no significant between-group difference was observed during the first 30 min of CPB ().
Figure 2. FER during pre-bypass (0), during normotermic CPB (30–60) and during hypotermic CPB (90–120–150). * : p < 0.05 compared to CT-group; ** : p < 0.01 compared to CT-group.
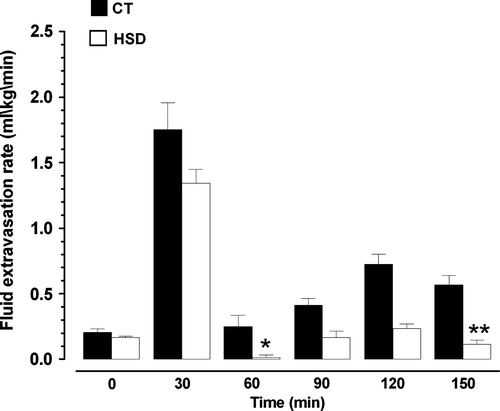
In the period from 30–60 min of normothermic CPB, FER stabilized at a lower level with a significant between-group difference (p < 0.05) (). During hypothermic CPB the between-group differences in FER were even more pronounced after 90 min with FER of 0.56 (0.20) ml/kg/min and 0.12 (0.04) ml/kg/min in the CT-group and HSD-group, respectively (p < 0.01).
Laboratory parameters
Hct fell significantly in both study groups after start of CPB (). Serum-osmolality, serum-sodium and serum-chloride levels were significantly higher in the HSD group. However, the values remained within normal range throughout the experiments, with maximal serum-sodium and serum-chloride concentration below 150 and 114 mmol/l in the HSD-group (). Acid-base parameters were similar in both groups, and the values remained within normal ranges throughout the study ().
Table II. The values are presented as mean with SD in parentheses.
Intracranial pressure (ICP) cerebral perfusion pressure (CPP)
As displayed in , ICP increased significantly during CPB in the CT-group, but remained stable in the HSD-group.
Cerebral perfusion pressure is displayed in . No significant between-group differences were present during the study period.
Total tissue water content (TTW)
TTW in different organs are presented in . Generally, tissue edema tended to be lower in most organs in the HSD-group, but between-group differences reached the significance level only in the left heart, and some visceral organs as compared with the CT-group. There was no difference in TTW in cerebral tissue.
Table III. Total tissue water content (in per cent) following CPB.
Discussion
The main message of the present study is briefly that a continuous infusion of HSD even at a lower infusion rate (1 ml/kg/hour) effectively reduces the total fluid load during CPB through lowering of FER (fluid extravasation) from the intravascular to the interstitial compartment. Consequently, myocardial edema and edema of some visceral organ can be significantly reduced as indicated in the HSD group as compared with the CT group at termination of CPB.
Initial hemodilution with an abrupt lowering of COPp leads to an extravasation of fluid from the intravascular to the interstitial compartment Citation1. As a consequence, supplemental fluid is regularly needed to maintain the fluid level in the venous reservoir during CPB.
As observed in our study the initial hemodilution induced a substantial leakage of fluid to the extravascular compartment in both groups. Thus, a low-dose infusion of HSD was not able to fully counteract the effects of the prime volume on COPp and FER. However, within 30 min of normothermic CPB, FER was significantly lowered in the HSD-group and remained lower compared with the CT group throughout the CPB period. Thus, it seems that the protective effect of HSD infusion against fluid accumulation would be of particular interest during complex procedures with prolonged CPB and hypothermia.
Hyperosmolar-hyperoncotic solutions were originally developed for trauma care and the dosage recommended was 4 ml/kg given within 2–5 min. Oliveira and coworkers Citation18 administered HSD 15 min prior to hypothermic CPB in a volume sufficient to double PCWP. HSD patients run into a slightly negative fluid balance, while the control patients run into a large positive fluid balance. Bueno et al., Citation12 infused 4 ml/kg HSD during 20 min before CPB. They observed a near zero fluid balance, higher cardiac index postoperatively, better PaO2/ FiO2 relation and shorter time to extubation in the HSD group. Although the effects on fluid homeostasis of 4 ml/kg of hypertonic saline/hyperoncotic preparations given within minutes have been studied in cardiac patients, such treatment could be potentially harmful in vulnerable cardiac patients in normovolemic or even hypervolemic state. In these patients the use of a continuous infusion at a lower infusion rate as in this study, probably would be more favorable.
HSD has also been administered after termination of surgery Citation19. That resulted in more rapid mobilization of fluid excess and improved gas exchange and cardiac index in the postoperative period.
Increased organ tissue edema may negatively affect organ function in the postoperative period. Even a small increase in myocardial tissue water content will affect both systolic and diastolic function. As demonstrated by Laine and Allen Citation20 reported a 40% reduction in myocardial compliance when myocardial tissue water content was increased by 2–3%. Thus, the slight, but significant, reduction of myocardial tissue water content observed in our study could theoretically contribute to improved myocardial function the post-CPB.
Intracranial pressure (ICP) is a major determinant of cerebral perfusion pressure, but monitoring ICP during routine CPB is not feasible and clinical data are scarce. Lundar and co-workers Citation21 introduced epidural pressure transducers in patients undergoing cardiac surgery with CPB and demonstrated the presence of elevated ICP in some cases. McDaniel et al. Citation11, found a significant increase in ICP from a level of 12 to 21 mmHg during CPB with isotonic prime solution in a porcine model. In the present study ICP increased gradually during CPB in the CT-group, whereas ICP remained stable in the HSD-group. These findings are in accordance with McDaniel et al. who also found normal and stable ICP values throughout 120 min of CPB when HSD was used as prime. The clinical implication of these results could be that infusion of HSD during CPB improves cerebral perfusion pressure during conditions of low mean arterial pressure.
Despite an increase of ICP in the present CT-group, no between-group differences were observed in cerebral water content. Elevation of ICP may be caused by increased content of interstitial or intracellular fluid, increased volume of blood or cerebrospinal fluid. The data does not allow any conclusion regarding the respective roles of these factors.
The use of hypertonic saline has been associated with hyperchloremic metabolic acidosis. In our study s-chloride concentration increased to a maximum level of 114 mmol/l in the HSD-group, and pH remained at all times above 7.40 and BE above 0 mmol/l in both groups. Hypertonic saline solutions are also associated with hypernatremia and may limit their use. In the present study s-sodium concentration remained below 150 mmol/l at all times and should thus not represent a problem.
Conclusion
A continuous infusion of 1 ml/kg/hour of a mixture of hypertonic saline/ dextran reduced the total fluid gain by 60% during CPB, and resulted in less tissue edema in the heart and some visceral organs. During CPB ICP remained stable in the HSD-group, whereas a significant increase was observed in the CT-group. The administered dose was not associated with any adverse effects.
Acknowledgements
The Board of the Faculty of Medicine, University of Bergen has authorized the “Locus for Circulatory Research” as an officially recognized research group within the faculty. We greatly acknowledge this support.
Venny L. Kvalheim is at present a research candidate supported by the Section for Cardiothoracic Surgery, Department for Surgical Sciences and Department of Heart Disease, Haukeland University Hospital, Bergen, Norway.
This study was financially supported by the Western Norwegian Societies of Cardiology, the Frank Mohn Foundation, Bergen, Norway and the Western Norway Regional Health Authority, Stavanger, Norway.
References
- Farstad M, Haugen O, Rynning SE, Onarheim H, Husby P. Fluid shift is moderate and short-lived during acute crystalloid hemodilution and normothermic cardiopulmonary bypass in piglets. Acta Anaesthesiol Scand. 2005; 49: 949–55
- Farstad M, Heltne JK, Rynning SE, Lund T, Mongstad A, Husby P. Fluid extravasation during cardiopulmonary bypass in piglets–effects of hypothermia and different cooling protocols. Acta Anaesthesiol Scand. 2003; 47: 397–406
- Farstad M, Kvalheim VL, Husby P. Cold-induced fluid extravasation during cardiopulmonary bypass in piglets can be counteracted by use of iso-oncotic prime. J Thorac Cardiovasc Surg. 2005; 130: 287–94
- Mehlhorn U, Geissler HJ, Laine GA, Allen SJ. Myocardial fluid balance. Eur J Cardiothorac Surg. 2001; 20: 1220–30
- Boldt J, von Bormann B, Kling D, Scheld H, Hempelmann G. The influence of extracorporeal circulation on extravascular lung water in coronary surgery patients. Thorac Cardiovasc Surg. 1986; 34: 110–5
- Weinstein PD, Doerfler ME. Systemic complications of fluid resuscitation. Crit Care Clin. 1992; 8: 439–48
- Harris DN, Oatridge A, Dob D, Smith PL, Taylor KM, Bydder GM. Cerebral swelling after normothermic cardiopulmonary bypass. Anesthesiology. 1998; 88: 340–5
- Harris DN, Bailey SM, Smith PL, Taylor KM, Oatridge A, Bydder GM. Brain swelling in first hour after coronary artery bypass surgery. Lancet 1993; 342: 586–7
- Himpe D. Colloids versus crystalloids as priming solutions for cardiopulmonary bypass: A meta-analysis of prospective, randomised clinical trials. Acta Anaesthesiol Belg. 2003; 54: 207–15
- Eising GP, Niemeyer M, Gunther T, Tassani P, Pfaunda M, Lange R. Does a hyperoncotic cardiopulmonary bypass prime affect extravascular lung water and cardiopulmonary function in patients undergoing coronary artery bypass surgery?. Eur J Cardiothorac Surg. 2001; 20: 282–9
- McDaniel LB, Nguyen T, Zwischenberger JB, Vertrees R, Uchida T, Kramer GC. Hypertonic saline dextran prime reduces increased intracranial pressure during cardiopulmonary bypass in pigs. Anesth Analg. 1994; 78: 435–41
- Bueno R, Resende AC, Melo R, Neto VA, Stalf NA. Effects of hypertonic saline-dextran solution in cardiac valve surgery with cardiopulmonary bypass. Ann Thorac Surg. 2004; 77: 604–11
- Farstad M, Haugen O, Kvalheim VL, Hammersborg SM, Rynning SE, Husby P. Reduced fluid gain during cardiopulmonary bypass in piglets using a continuous infusion of a hyperosmolar/hyperoncotic solution. Acta Anaesthesiol Scand. 2006; 50: 855–62
- Husby P, Heltne JK, Koller ME, Birkeland S, Westby J, Fosse R, Lund T. Midazolam-fentanyl-isoflurane anaesthesia is suitable for haemodynamic and fluid balance studies in pigs. Lab Anim. 1998; 32: 316–23
- Heltne JK, Farstad M, Lund T, Matre K, Rynning SE, Husby P. Determination of plasma volume in anaesthetized piglets using the carbon monoxide (CO) method. Lab Anim. 2002; 36: 344–50
- Aukland K, Johnsen HM. A colloid osmometer for small fluid samples. Acta Physiol Scand. 1974; 90: 485–90
- Heltne JK, Husby P, Koller ME, Lund T. Sampling of interstitial fluid and measurement of colloid osmotic pressure (COPi) in pigs: Evaluation of the wick method. Lab Anim. 1998; 32: 439–45
- Oliveira, SA, Bueno, RM, Souza, JM, Senra, PF, Rocha-e-Silva. Effects of hypertonic saline dextran on the postoperative evolution of Jehovah's Witness patients submitted to cardiac surgery with cardiopulmonary bypass. Shock. 1995;3:391–4.
- Tollofsrud S, Noddeland H. Hypertonic saline and dextran after coronary artery surgery mobilises fluid excess and improves cardiorespiratory functions. Acta Anaesthesiol Scand. 1998; 42: 154–61
- Laine GA, Allen SJ. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991; 68: 1713–21
- Lundar T, Froysaker T, Lindegaard KF, Wiberg J, Lindberg H, Nornes H. Some observations on cerebral perfusion during cardiopulmonary bypass. Ann Thorac Surg. 1985; 39: 318–23