Abstract
Objective. Ischaemic preconditioning protects the myocardium from ischaemic injury and may also protect the vascular endothelium from the deleterious effects of ischaemia and reperfusion. We examined the possibility that ischaemic preconditioning might preserve the integrity of the coronary microcirculation following ischaemia and reperfusion. Methods. Isolated rat hearts were perfused in Langendorff mode for 30 minutes and then subjected to 30 minutes of global ischaemia with or without ischaemic preconditioning (three×three minute cycles). Some hearts underwent an additional 60 minutes of reperfusion. At the end of each protocol, microvascular corrosion casts were made by methylmethacrylate injection. Results. Median left ventricular capillary density [interquartile range] after ischaemia was slightly but not significantly better with preconditioning at 6.8 [4.0–14.7]×10−2 mm3.mg−1vs. 5.2 [2.6–7.1]×10−2 mm3.mg−1 (p =0.13). After 60 min of reperfusion, capillary density in preconditioned left ventricles was 20.7 [10.7–22.8]×10−2 mm3.mg−1vs. 16.0 [10.2–23.0]×10−2 mm3.mg−1 for untreated ventricles (p =0.47). Coronary blood flow and heart rate were unchanged from before ischaemia. Conclusions. Ischaemia for 30 minutes induced global left ventricular capillary loss which was unmodified by preconditioning. We did not demonstrate vascular preconditioning using this model.
Ischaemic preconditioning has been shown to protect the myocardial cell from ischaemia and reperfusion damage Citation1. It is not clear whether the protection also holds true for the coronary microvasculature Citation2.
Treatment of myocardial ischaemia is largely focused on recanalisation of the occluded epicardial coronary artery. Whilst addressing large vessel pathology, the insult suffered by the myocardial microvasculature has largely been ignored Citation3, Citation4, despite evidence that microvascular reperfusion injury leads to persistent ST-segment elevation following primary angioplasty Citation5, and interference both with the reactive hyperaemic response and collateral flow Citation6–9. Any ischaemic insult, including cardioplegic arrest and reperfusion, may cause severe microvascular dysfunction Citation10–12.
The cardiac capillary lumen is narrowed after ischaemia and reperfusion which has been shown to be due to the presence of endothelial contractile apparatus; experiments, in which the endothelial cell's actin-myosin system was poisoned by phalloidin, demonstrated that ischaemia-reperfusion damage to the capillary network was attenuated as compared to untreated vessels Citation13. Further work examined the contribution of Rho-associated kinases in the contractile process which also showed protective effects from inhibition of ROCK1 by Y-27632 Citation14.
Evidence for the protective effect of ischaemic preconditioning on capillaries has been demonstrated in the vascularly isolated canine gracilis muscle; and was demonstrated to be due to activation of the ATP-sensitive potassium channels Citation15. It has also been shown that ischaemic preconditioning altered reactive hyperaemia by decreasing total hyperaemic flow and reducing the time to peak hyperaemic flow. While the former effect was attributed to a reduction in myocardial metabolism through the activation of the A1 receptors, the latter was likely to be due to an increased endothelial release of nitric oxide, suggesting that, in addition to a protective effect on the myocardium, ischaemic preconditioning also exerts a direct effect on the responsiveness of the coronary vasculature (vascular preconditioning) Citation16. These studies suggest that ischaemic preconditioning may be responsible for preserving vascular integrity and function. We investigated whether the same phenomenon could preserve capillary density in an isolated crystalloid-perfused rat heart model?
Methods
All procedures were carried out according with the guidelines of the Animals (Scientific Procedures) Act UK 1986. Adult male Sprague-Dawley rats weighing 350–500 g were anaesthetised with 2% halothane administered in 1:1 oxygen and nitric oxide. 1 000 U of sodium heparin was injected into a femoral vein. The hearts were quickly excised via a bilateral thoracotomy and the heart arrested by immediate immersion in Krebs-Henseleit buffer solution at 4°C; it was then transferred to the perfusion apparatus.
The aorta was cannulated and perfusion started with Krebs-Henseleit bicarbonate buffer containing (NaCl 118 mM, KCl 3.8 mM, KH2PO4 1.2 mM, NaHCO3 25 mM, MgSO4 1 mM, CaCl2 1.8 mM, glucose 10 mM) perfusion at 37°C and pH 7.4 at a constant pressure of 100 cm H2O according to the Langendorff technique. The pulmonary artery was incised to vent the right heart.
The heart rate (beats.min−1) was counted manually and coronary blood flow (ml.min−1) was measured by timed collection of effluent from the heart chamber. These variables were measured at 15 minute intervals during each period of perfusion.
An ischaemic period of 30 minutes was chosen based on our previous work Citation12, Citation17.
The preconditioning cycle was as follows: five minutes of ischaemia followed by five minutes of reperfusion; for these experiments three cycles were used.
Experimental protocol
After satisfactory perfusion in Langendorff mode for 30 minutes, the hearts in the different experimental groups underwent the following interventions before injection of microvascular cast material:
Group 1 (n = 6): 30 minutes of global warm ischaemia.
Group 2 (n = 6): 30 minutes of global warm ischaemia with 60 minutes of Langendorff reperfusion.
Group 3 (n = 6): Three ischaemic preconditioning cycles followed by 30 minutes of global warm ischaemia (as for group 1)
Group 4 (n = 6): Three ischaemic preconditioning cycles, 30 minutes of global warm ischaemia, then 60 minutes of Langendorff reperfusion (as for group 2).
Exclusion criteria
Once perfusion was established, hearts which neither resumed normal sinus rhythm nor good contraction were excluded from the series.
Myocardial Capillary density measurement
We followed our established practice Citation17 and, at the end of the experimental protocol, Batsons™ No. 17 polymer (Polysciences Inc, Warrington, PA) with added methylmethacrylate (Merck Ltd, Poole, Dorset, UK) was immediately injected into the aortic root at a pressure of 60–70 mm Hg until the solution was seen to be escaping from the right atrium. The cast material was then allowed to polymerise at room temperature for 2 hours. A cylinder of left ventricle (still containing intraluminal cast material) was dissected free of the right ventricle and the epicardial vessels and any cast material inside the ventricle was removed. Because coronary blood volume represents only about 12% of left ventricular mass, and 90% of the blood is in the capillaries, in order to prevent potential inaccuracies induced by rapid drying or surface tension effects due to excess surface water on the preparation, which in turn would make a large difference to such a small wet specimen, only the dry weights were considered for calculations.
The resulting preparation was oven-dried at 28°C for 48 hours and weighed:
(i) dry weight of Left Ventricle including microvascular cast. The myocardium was then macerated using alternate baths of 15% KOH and distilled water, until all organic matter was removed. The resultant cast of the myocardial capillaries, was washed, dried at 28°C for a further 48 hours and then weighed;
(ii) dry weight of microvascular cast. Subtracting (ii) from (i) we derived the left ventricular dry weight. Using the polymer density (1.051 g.ml−1), the volume of myocardial capillary per mg of left ventricular muscle was calculated.
The volume of cast material per mg of left ventricle is therefore equivalent to the perfused capillary density (mm3mg−1) of the left ventricle Citation17.
Scanning electron microscopy of capillary casts
Corrosion casts of the left ventricles were divided vertically into three parts and mounted on a conducting plate and coated with gold using a sputter coater (VG Microtech, East Grinstead, West Sussex, UK). Using an Environmental Scanning Electron Microscope (Phillips, Eindhoven, Netherlands) representative 2000× magnification electron micrographs were made for qualitative estimation of capillary anatomy and topography.
Power calculations
Previous studies indicated that large differences in the myocardial capillary density will be seen thus giving relatively small scale studies a high statistical power Citation12–14, Citation17. In order to assess the statistical analytical power of the study we first considered our previous experimental results in which the post-cardioplegic effect led to 100% of subjects falling below the control range with a maximum coefficient of variation of 9% (p < 0.0001). If a 25% difference was to be considered to be of practical importance and, because two groups are in use, a p-value of 0.025 was sought, by reference to the t-distribution, groups of six animals (successful experiments) would show significant differences at a power of 80%.
Statistical analysis
Summary data are shown either as mean±1 standard deviation or median [interquartile range]. Direct comparisons were made by unpaired t-tests. For all group comparisons, Kruskal-Wallis (one-level) distribution-free analysis of variance, using the Dwass-Steel-Critchlow-Fligner procedure for multiple comparisons, was performed with appropriate corrections for multiple comparisons or ties. The statistical software was StatsDirect (StatsDirect, Cheshire UK).
Results
Heart rate
The heart rate was consistent between groups as well as within the Groups indicating a stable preparation with only a very slight (non-significant) slowing after 60 minutes of reperfusion in both control and preconditioned hearts (see ).
Table I. Heart Rate (beats.min−1)–mean±SD.
Coronary blood flow
It can be seen that between group differences did not exist before ischaemia. In the reperfused hearts, mean coronary blood flow at 60 minutes of reperfusion was very similar to that seen before ischaemia (see ). Preconditioning appeared to have no effect.
Table II. Coronary flow rates (ml.min−1)–median [interquartile range].
Capillary density
The capillary density (see ), measured after 30 minutes of ischaemia (group 1), was significantly lower than that seen after 60 minutes of reperfusion which improved capillary density after the ischaemic insult. In hearts, which had undergone the same protocol plus ischaemic preconditioning, capillary density after ischaemia was slightly but not significantly higher. In preconditioned hearts which had been reperfused, the capillary density was no different from that seen in reperfused but unpreconditioned hearts.
Table III. Post-ischaemic Capillary density (mm3mg−1) – median [interquartile range].
Hence preconditioning the hearts before ischaemia did not appear to protect against capillary loss. Disappointingly, neither did the addition of preconditioning before reperfusion improve capillary density after ischaemia.
Capillary morphology
Electron micrographs of the capillary casts are shown in . After Langendorff perfusion the capillaries () are all patent and fairly densely arranged although there are some irregularities; the luminal surface is quite smooth. In contrast casts from those hearts which underwent ischaemia for 30 minutes. It can be seen that all show gaps in the architecture, stumps of capillaries and a roughened endothelial surface.
Figure 1. Langendorff perfusion for 30 minutes followed by ischaemia for 30 minutes; not reperfused. Note the irregularity of the luminal diameter as well as gaps in the architecture and flattening of the capillary lumen consistent with that seen in previous studies Citation14, Citation17.
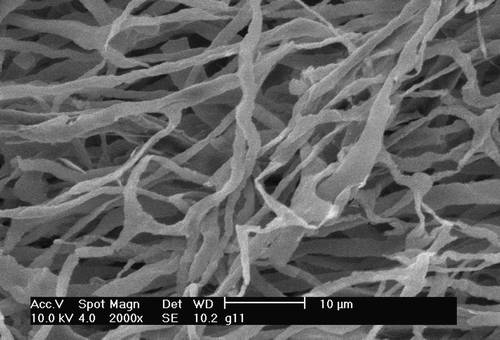
Figure 2. 30 minutes pre-ischaemic Langendorff mode perfusion followed by warm ischaemia for 30 minutes and then Langendorff mode reperfusion for 60 minutes. Note the more regular luminal diameters although the surface of the cast material is somewhat roughened. Gaps in the capillary architecture are still apparent.

Figure 3. 30 minutes of pre-ischaemic Langendorff mode perfusion, then three ischaemic preconditioning cycles, followed by 30 minutes of warm ischaemia. Not reperfused. Note similarity of findings to , indicating a lack of protective effect.
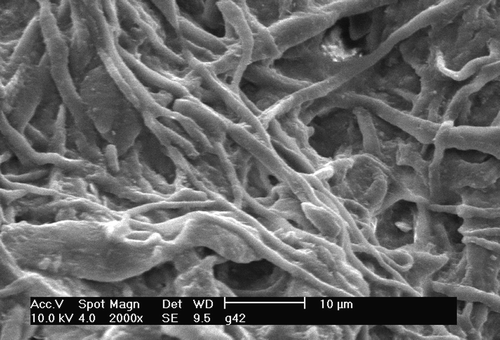
Figure 4. Langendorff perfusion for 30 minutes followed by three ischaemic preconditioning cycles. Then: 30 minutes of warm ischaemia (as in ) followed by reperfusion in Langendorff mode for 60 minutes. A few similarities are seen to like the roughened endothelial surface. Architectural defects persist although the capillaries are less flattened. This is consistent with previous studies Citation14, Citation17.

Discussion
The main finding of this study was that ischaemic preconditioning did not protect against the capillary loss associated with ischaemia. This remained so whether the hearts were reperfused or not.
After ischaemia and reperfusion there is usually a selective impairment of endothelium-dependent relaxation in isolated coronary arteries and indeed evidence for microvascular stunning has been demonstrated Citation18. However, in the intact coronary circulation, there is a general loss of vasodilator reserve as responses to both endothelium-dependent and endothelium-independent agonists are attenuated. Ischaemic preconditioning is a widely studied phenomenon since the introduction of the concept Citation1. It is well established that preconditioned myocardium is protected against ischaemia and reperfusion Citation19: ischaemic preconditioning is able to prevent ischaemic damage to the myocardium but the vasculature may be less well protected as reperfusion is enhanced but the vasodilator reserve continues to be limited which indicates damage to the classical resistance vessels Citation2, although others have shown that ischaemic preconditioning can protect the vascular endothelium Citation10. Either way it is not clear whether this protection extends to the microvasculature. It is also not known if coronary artery preconditioning is intrinsic, i.e., originating in the vessels themselves, or is extrinsic, i.e., the mechanism is mediated through the myocardial cells. Our present study suggests that ischaemic preconditioning does not protect the microvasculature from the insults caused by ischaemia and reperfusion, either singly or together. We are unable to confirm either theory.
In vivo blood flow in capillaries has been hard to measure directly. Therefore the morphology of the microvasculature has been extensively studied. Vascular corrosion casting has been used for about 40 years to produce replicas of normal and abnormal vasculature and microvasculature of various tissues and organs. In combination with scanning electron microscopy, the primary application of corrosion casting has been to describe the morphology and anatomical distribution of blood vessels in these tissues. Casts of the microcirculation should also contain quantitative information about that vasculature Citation20. The technique of microvascular corrosion casting has also provided a qualitative means of assessing capillary morphology in many species as well as information about adjacent endothelial cells Citation21–24. We are aware that the overall architecture of the specimen may be difficult to determine Citation25 but local details are easy to see. Unfortunately the quantitative assessment of the cast is also fraught with difficulties such as orientation of counting systems and this has impeded its widespread use as an investigative tool Citation25. We therefore followed the approach of Hossler Citation20, Citation23, who compared the weight of myocardial casts before and after maceration of myocardial tissue, and found that it was an accurate determinant of the myocardial intravascular volume. Additionally vascular casts reflect the shape, size and distribution of the vessels in question and record a replica of the endothelial surface of that vessel; this is useful in assessing quality of the endothelial surface of the capillary. We acknowledge that dynamic contrasts cannot be made using the system and also that unpaired comparisons must be made although previous experience has confirmed that the method is robust Citation12, Citation17.
Another criticism of this study may be that we did not measure muscle necrosis. There are two reasons for this: first we removed all the muscle to show the capillary cast architecture; second it takes 24 hours for all the myocytes to die within an infarcted area and thus be detected Citation26. We are unable to speculate about the effects of preconditioning on regional ischaemia; this model uses capillary casting which does not lend itself to the technique because we examine the entire vasculature. Whether the vasculature protects the myocardium or vice versa remains unanswered by the present study, although the presence of contractile elements in the endothelial cell would favour the former.
The release of vasoconstrictor(s) and plugging of capillaries with leukocytes may cause the impairment of vasodilatation of the coronary circulation as well as a reduction in basal blood flow seen with the no-reflow phenomenon Citation27; however previous investigations Citation12 have not been able to confirm this as a mechanism of capillary loss. We have used corrosion casting to examine capillary behavior during cardioplegic arrest, both in the pig with whole blood perfusion, and in the isolated perfused rat heart, perfused with resuspended erythrocytes. Despite a loss of 30% of the control capillary density, overall coronary flow remained at pre-ischaemic levels Citation17, Citation12. Other experiments with thrombosis have demonstrated normal resting flow with diminution of the reactive hyperaemic response Citation6–8. The persistence of pre-ischaemic coronary blood flow in the present experiments suggests that the model behaved in a fashion which was consistent with capillary loss irrespective of the cause of ischaemia and independent of the lack of cellular components in the perfusate.
Conclusion
In the isolated crystalloid perfused rat heart, ischaemic preconditioning neither prevented microvascular loss following ischaemia nor improved capillary density on reperfusion. Microvascular preconditioning cannot be observed in this crystalloid-perfused model although myocardial function appeared unaltered.
Acknowledgements
This work was supported by Ross Hall Hospital and The Coronary Thrombosis Trust.
References
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74: 1124–36
- Bauer B, Simkhovich BZ, Kloner RA, Przyklenk K. Does preconditioning protect the coronary vasculature from subsequent ischemia/reperfusion injury?. Circulation. 1993; 88: 659–72
- Gavin JB, Maxwell L, Edgar SG. Microvascular involvement in cardiac pathology. J Mol Cell Cardiol. 1998; 30: 2531–40
- Glazier JJ. Attenuation of reperfusion microvascular ischemia by aqueous oxygen: Experimental and clinical observations. Am Heart J. 2005; 149: 580–4
- Claeys MJ, Bosmans J, Veenstra L, Jorens P, De R, Vrints CJ. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction: Importance of microvascular reperfusion injury on clinical outcome. Circulation. 1999; 99: 1972–7
- Belcher PR, Vergroesen I, Drake-Holland AJ, Noble MI. The pressure-flow relation in the canine coronary artery: Combined effects of critical stenosis and intracoronary thrombosis. Cardiovasc Res. 1995; 30: 807–14
- Mansaray M, Belcher PR, Vergroesen I, Wright ZM, Hynd JW, Drake-Holland AJ, et al. Downstream resistance effects of intracoronary thrombosis in the stenosed canine coronary artery. Cardiovasc Res. 1999; 42: 193–200
- Mansaray M, Hynd JW, Vergroesen I, Belcher PR, Drake-Holland AJ, Noble MI. Measurement of coronary collateral flow and resistance in the presence of an open critical stenosis, and the response to intra-arterial thrombosis. Cardiovasc Res. 2000; 47: 359–66
- Mansaray M, Hynd JW, Vergroesen I, Belcher PR, Drake-Holland AJ, Noble MI. Thrombosis in one coronary artery causes generalized coronary vasoconstriction in a dog model of unstable angina. Clin Sci (Lond) 2001; 100: 405–10
- Laude K, Beauchamp P, Thuillez C, Richard V. Endothelial protective effects of preconditioning. Cardiovasc Res. 2002; 55: 466–73
- Lin PJ, Chang CH, Yao PC, Liu HP, Hsieh HC, Tsai KT. Endothelium-dependent contraction of canine coronary artery is enhanced by crystalloid cardioplegic solution. J Thorac Cardiovasc Surg. 1995; 109: 99–105
- Pathi VL, McPhaden AR, Morrison J, Belcher PR, Fenner JW, Martin W, et al. The effects of cardioplegic arrest and reperfusion on the microvasculature of the heart. Eur J Cardiothorac Surg. 1997; 11: 350–7
- Glyn MC, Ward BJ. Contraction in cardiac endothelial cells contributes to changes in capillary dimensions following ischaemia and reperfusion. Cardiovasc Res. 2000; 48: 346–56
- Glyn MC, Lawrenson JG, Ward BJ. A Rho-associated kinase mitigates reperfusion-induced change in the shape of cardiac capillary endothelial cells in situ. Cardiovasc Res. 2003; 57: 195–206
- Jerome SN, Akimitsu T, Gute DC, Korthuis RJ. Ischemic preconditioning attenuates capillary no-reflow induced by prolonged ischemia and reperfusion. Am J Physiol. 1995; 268: H2063–H2067
- Gattullo D, Linden RJ, Losano G, Pagliaro P, Westerhof N. Ischaemic preconditioning changes the pattern of coronary reactive hyperaemia in the goat: Role of adenosine and nitric oxide. Cardiovasc Res. 1999; 42: 57–64
- Chaudhry MA, Belcher PR, Day SP, Muriithi EW, Wheatley DJ. Erythrocyte-containing versus crystalloid cardioplegia in the rat: Effects on myocardial capillaries. Ann Thorac Surg. 2003; 75: 890–8
- Bolli R, Triana JF, Jeroudi MO. Prolonged impairment of coronary vasodilation after reversible ischemia. Evidence for microvascular “stunning”. Circ Res. 1990; 67: 332–43
- Hearse DJ, Maxwell L, Saldanha C, Gavin JB. The myocardial vasculature during ischemia and reperfusion: A target for injury and protection. J Mol Cell Cardiol. 1993; 25: 759–800
- Hossler FE, Douglas JE. Vascular corrosion casting: Review of advantages and limitations in the application of some simple quantitative methods. Microsc Microanal. 2001; 7: 253–64
- Anderson BG, Anderson WD. Microvasculature of the canine heart demonstrated by scanning electron microscopy. Am J Anat. 1980; 158: 217–27
- Anderson WD, Anderson BG, Seguin RJ. Microvasculature of the bear heart demonstrated by scanning electron microscopy. Acta Anat (Basel) 1988; 131: 305–13
- Hossler, FE, Douglas, JE, Douglas, LE. Anatomy and morphometry of myocardial capillaries studied with vascular corrosion casting and scanning electron microscopy: A method for rat heart. Scan Electron Microsc. 1986;Pt4:1469–75.
- Irino, S, Ono, T, Shimohara, Y. Microvascular architecture of the rabbit ventricular walls: A scanning electron microscopic study of corrosion casts. Scan Electron Microsc. 1982;Pt4:1785–92.
- Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts–technique and applications: Updated review. Scanning Microsc. 1990; 4: 889–940
- Axford-Gatley RA, Wilson GJ. The “border zone” in myocardial infarction. An ultrastructural study in the dog using an electron-dense blood flow marker. Am J Pathol. 1988; 131: 452–64
- Kloner RA, Ganote CE, Jennings RB, Reimer KA. Demonstration of the “no-reflow” phenomenon in the dog heart after temporary ischemia. Recent Adv Stud Cardiac Struct Metab. 1975; 10: 463–74