Abstract
Objectives. Determine if pre-emptive daily insulin glargine surpasses regular insulin when needed for glycaemic control after cardiac surgery. Design. Prospective, randomized study of 43 patients (scheduled for coronary artery bypass grafting) with preoperatively diagnosed diabetes (DM) or pre-DM. Lantus group received insulin glargine daily from start of surgery while Actrapid group received regular insulin (sliding scale) when needed (plasma glucose (P-glu) >10 mmol/l). Primary endpoint was percent of pre- and post-prandial P-glu values within Target Intervals: Pre-prandial P-glu: 4.5–7 mmol/l; post-prandial P-glu: 4.5–9 mmol/l. Study period 1–4 days after surgery. Tissue glucose was also measured continuously. Results. More than twice as many P-glu values were within Target Interval for Lantus patients as compared with Actrapid patients (p <0.001). One of 504 timed measurements was <4 mmol/l. Area under the curve for glucose >7 mmol/l was reduced by 61% by Lantus (p <0.001). Conclusion. The routine protocol with pre-emptive glargine insulin studied here provides a major improvement in glycaemic control with a minimal incidence of hypoglycaemia and without an excessive increase in nursing burden.
Under-treatment of hyperglycaemia the first days after coronary artery bypass grafting (CABG) is due to under-diagnosis of diabetes mellitus (DM) preoperatively and a lack of blood glucose testing in the majority of patients with postoperative dysglycaemia. Furthermore, adequate blood glucose control is nurse-intensive, bothersome for the patient, considered of secondary importance, and fraught with an exaggerated fear of hypoglycaemia. The Diabetes in Hospitals expert committee has summarized the problem and has emphasized that treatment of in-hospital hyperglycaemia is important and basic to good medical care Citation1.
While just over one of five patients referred for CABG are reported as diabetic, testing at admission has shown that only one of five is normoglycemic preoperatively Citation2. Virtually all patients, including the fully normal, develop in addition surgical stress-induced hyperglycaemia.
Mortality after CABG is strongly associated with pre-operative glycaemic state, with the highest plasma glucose concentration (P-glu) observed during cardiopulmonary bypass and with the average glucose concentrations during each of the first few days after surgery Citation3–6. Furnary has additionally shown that the average value of all glucose measurements during 72 hours from start of surgery is the glucose parameter most strongly associated with mortality, deep wound infections, and length of hospital stay. After the third post-operative day there is no apparent association Citation6.
The purpose of this randomized pilot study is to evaluate an insulin treatment protocol on the surgical wards which can be used routinely in the large majority of patients with post-operative hyperglycaemia after CABG surgery. The study investigates the effectiveness of pre-emptive treatment using insulin glargine once daily compared with using sliding scale regular insulin when required. The primary endpoint is the proportion of timed plasma glucose (P-glu) measurements which were within set target intervals during the first 4 days after leaving intensive care unit (ICU) in patients with preoperatively diagnosed impaired glucose tolerance or DM. Continuous tissue glucose concentrations were also measured.
Patients and methods
Forty-eight patients undergoing primary CABG were studied after permission from the local ethics committee and informed, signed consent. Only patients with diabetes mellitus or impaired glucose intolerance detected during the preoperative work-up were included. Glycaemic state was determined by oral glucose tolerance test (OGTT) and/or fasting plasma glucose (fP-glu) on 2 different days. Exclusion criteria were renal failure, known diabetes and other endocrinological diseases. Patients were randomised (sealed envelope method) to two groups with different protocols for treating post-operative hyperglycaemia (Lantus and Actrapid) on the surgical wards after release from the ICU.
All operations were performed on cardiopulmonary bypass at 35–36oC and ICU care was standardized. No routines were changed for this study except one preoperative glargine insulin dose in the Lantus group, as described below. Patients were anaesthetized with propofol and fentanyl, maintained with sevoflurane except during cardiopulmonary bypass when they received propofol infusion. An infusion of regular insulin (Actrapid, NovoNordisk AB, Sweden) is routinely started pre-emptively for all patients at the start of surgery to prevent hyperglycaemia. Insulin infusion rate is adjusted intuitively by the attending nursing staff according to blood glucose sampling every 30–40 minutes during surgery and every 1–2 hours in the ICU (goal 4.5–7 mmol/l). No glucose infusion was given during surgery but an infusion (glucose 100 mg/kg-hr or 0.4 kcal/kg-hr) was started upon arrival to the ICU. Both insulin and glucose infusions were ended at 6 o'clock the morning after surgery and a “fasting” P-glu concentration is taken before breakfast 2 hours later. Patients are routinely discharged on the first morning after surgery to the surgical wards where patient eating including grazing is unrestricted.
Glucose measurements
Capillary P-glu (Hemocue 201 + P-Glucose System; HemoCue AB, Ängelholm, Sweden) is measured 8 times daily; immediately before each of three meals, 90 minutes after each meal is begun, and at hours 23:00 and 03:00.
In addition, a CGMS Gold Continuous Glucose Monitor (Medtronic MiniMed Inc, Northridge, CA, USA) is attached to the patient just after anaesthetic induction. These monitors are calibrated 3–4 times daily and the data downloaded for computer analysis after the fifth day. Calculated values include average glucose, maximum glucose each day, percent of day for which P-glu > 7 mmol/l, and Area under the Curve (AUC) P-glu > 7 mmol/l.
Target intervals
Pre-prandial P-glu: 4.5–7 mmol/l. Post-prandial P-glu = 90 minutes after meal start: 4.5–9 mmol/l.
Treatment protocols
Group Lantus
Patients received 0.5 E/kg glargine insulin sc (Lantus® Sanofi-Aventis AB, Sweden) at the start of surgery. On the morning of Post-operative Day 1 (POD-1) while still in the ICU, patients receive 0.25–0.3 E/kg Lantus insulin sc depending on insulin requirements after surgery, the P-glu values at 6 o'clock and 8 o'clock.
On the mornings of POD's 2-4, Lantus is given at 8 o'clock with dose adjusted according to the 03 and 06 o'clock fasting P-glu values with the target interval of 4.5–7 mmol/l. Only Lantus insulin is given, but if pre-prandial P-glu ≥ 10 mmol/l rescue Actrapid is given with dose as below for Actrapid group.
Group Actrapid
Patients received regular insulin sc (Actrapid®, NovoNordisk AB, Sweden) by ward nurses according to a protocol when their pre-prandial P-glu exceeds 10 mmol/l. The dose Actrapid given was 8 international units (IU) if P-glu ≥ 10 mmol/l, 10 IU if P-glu ≥ 12 mmol/l, 12 IU if P-glu ≥ 14 mmol/l and 14 IU if P-glu ≥ 16 mmol/l.
Definitions and calculations
Diabetes mellitus was defined as either P-Glu ≥ 11.1 mmol/l at 2 hours after OGTT or fP-Glu ≥ 7.0 mmol/l on two separate days. Prediabetes is defined as either glucose intolerance (7.8 ≤ P-Glu < 11.1 mmol/l 2 hours after OGTT) and/or impaired fasting glucose (5.6 ≤ fP-Glu < 7.0 mmol/l). Hypoglycaemia is defined as P-glu < 4 mmol/l.
The primary endpoint is the proportion of measured pre- and post-prandial capillary P-glu values which are within the Target Intervals (see above). Power calculation showed that about 16 patients per treatment group had to be included to detect the difference 0.6 versus 0.4 in the primary endpoint with 80% power. Allowing for a non parametric test and about 20% dropout at least 20 patients per treatment group, need to be included in the study. The CGMS Glucose Monitor data provided average daily P-glu, daily peak P-glu, percentage of time each day when P-glu > 7 mmol/l, and area under the curve P-glu when P-glu > 7 mmol/l.
Statistics
Differences between continuous variables in the two patient groups were evaluated using the Student's t-test or in case of non-normal distributions by the Mann-Whitney U test. Data are expressed as mean and standard deviation (SD). Analysis of variance was applied to compare repeated measures in the two patient groups. A two-sided p-value less than 0.05 was considered significant.
Results
Surgery was uneventful for all patients but three patients in the Lantus group and two in the Actrapid group were dropped from the study. Two patients required extended intensive care and/or extended glucose drip, one returned to intensive care, and one patient developed a stroke on POD- 2 and received extended glucose drip. Patient characteristics are shown in . No differences before or during surgery were found except slightly higher BMI in Actrapid group.
Table I. Baseline characteristics of the 43 patients who completed the 4 days comparison of treatment with daily Lantus or Actrapid (Controls).
Day of surgery and ICU (POD-0)
Total insulin requirements during the day of surgery and ICU did not differ for the Lantus and Actrapid groups (93±23 IU) and (90±31 IU), respectively and are about 1 IU/kg. The preoperative Lantus dose (42±5 IU or 0.51±0.04 IU/kg) covered 45% of that day's insulin requirements. The average P-glu during POD-0 was lower for the Lantus group (5.6 vs. 6.7 mmol/l (p = 0.006)) as was the percent of day > 7 mmol/l (9% vs. 43% (p = 0.015)). No hypoglycaemia occurred during intensive care.
POD's 1-4
Glycaemic control data are shown in for both the capillary and the continuous glucose measurements. shows that P-glu is lower for the Lantus group (p < 0.001). Lantus was dosed for the morning after surgery at the lower Dose Protocol range (0.25 IU/kg) for the first 15 patients resulting in 10/15 patients with fP-glu above target on POD-2 and required an increased Lantus dose on POD-2. The higher dose (0.3 IU/kg) was used in the last five patients and resulted in all five within target range on POD-2. The lowest P-glu for any Lantus patient on POD-1 was 4.7 mmol/l. No patient had fP-glu < 5 mmol/l on POD-2. On the basis of fP-glu on POD-2, the appropriate Lantus dose on POD-1 was estimated to be 0.34 IU/kg rather than the 28±0.04 IU/kg that was given.
Figure 1. Capillary plasma glucose concentration (P-glucose) from the morning of the first post-operative day (POD) until lunch of POD-4 for patients treated with daily Lantus or sliding scale regular insulin (Controls) when needed. (f-0 = fasting P-glucose on day of surgery, etc).
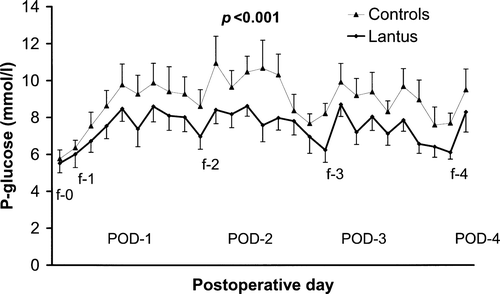
Table II. Average glucose data for postoperative days 1-4 after cardiac surgery for the 43 patients who completed a 4 day comparison of treatment with daily Lantus or Actrapid (Controls). A. Percent of scheduled capillary plasma glucose (P-glu) measurements within Target Values (see Methods). B. Continuous tissue glucose data shown as average glucose calibrated to capillary plasma glucose, maximal daily value, percent of day above 7 mmol/l, and area under the curve above 7 mmol/l.
The average Lantus dose on POD's 2-4 was 0.32±0.6 IU/kg with no tendency to decreased Lantus requirements during that period.
Overall, more than twice as many P-glu values were within Target Interval for Lantus patients as compared with Actrapid patients. Pre-prandial values were more often above target intervals than post-prandial values. Four Lantus patients should have received rescue Actrapid (when pre-prandial P-glu > 10 mmol/l) but did not (2% of pre-prandial measurements). Actrapid patients did not receive rescue Actrapid on 20% of the occasions when they should have.
Hypoglycaemia
Of the 504 timed P-glu values in the study period (pre-lunch POD-1 to after breakfast POD-4), 10 (2%) were between 4.5–5 mmol/l, 1 was between 4 and 4.5 mmol/l. A lower value (fP-glu = 3.6 mmol/l at 6 o'clock on POD-3) occurred once, but it was probably inaccurate as P-glu had been 9.2 and 7.7 mmol/l at 23:00 and 03:00 o'clock, respectively. Asymptomatic hypoglycaemia (3.2 and 3.6 mmol/l) was measured on two occasions after the study period ended (POD 5 & 6: once at 03:00 and once at 06:00 o'clock).
The continuous glucose concentration measurements showed a 17% reduction in both average glucose concentration and peak P-Glu during the first four POD's. The amount of time that P-glu was >7 mmol/l. was reduced 30% in the Lantus group and the area under the curve for glucose >7 mmol/l was likewise reduced by 61%.
Discussion
The goal of this pilot study was to devise a pre-emptive insulin protocol for routinely attenuating hyperglycaemia after CABG surgery in patients with preoperatively diagnosed DM or impaired glucose tolerance. Our principal finding is that once-daily glargine insulin provides a marked improvement in glycaemic control while requiring only a few daily glucose samples. This study also demonstrates the extent and persistence of post-CABG hyperglycaemia in patients, whose glucose levels are normally not even followed in many centres, including our own, due to under-diagnosis of diabetes in patients referred for surgery.
The only randomized intervention studies of aggressive in-hospital hyperglycaemia treatment have been performed on intensive care units. Dramatic improvements in mortality and morbidity due to intensive blood glucose control were found in patients controlled for several days after cardiac surgery Citation7, Citation8. The eventual benefit of treating postoperative hyperglycaemia in non-intensive care patients has not been tested in randomized studies to our knowledge. Furnary's historical data with intravenous insulin treatment on the surgical wards suggests also that glycaemic control is essential for the first three days after CABG surgery Citation6.
Universal insulin treatment of postoperative hyperglycaemia on the intensive care unit was introduced shortly after the van den Berghe's study 5 years ago Citation7. More recently we have begun insulin treatment at the start of surgery. Glucose infusion (100 mg/kg-hr) is not started until patients arrive in the ICU. Although almost 1 unit regular insulin/kg is given during the day of surgery, insulin treatment is routinely ended when patients are discharged from intensive care the morning after surgery. Only patients with previously known diabetes are generally tested and treated for hyperglycaemia on the wards while the majority of patients with postoperative hyperglycaemia remain untreated.
Lantus was given pre-operatively to assure adequate insulin concentrations when intravenous insulin is ended on the morning after surgery. The decrease in regular insulin infused during intensive care agreed surprisingly well with the amount of glargine administered pre-operatively and demonstrates that slowed uptake due to tissue hypoperfusion is not a clinical problem in spite of periods of vasoconstriction.
It is not surprising that the morning fasting glucose values after POD-1 improved markedly with Lantus as the morning's dose was adjusted to that day's fasting value. Unfortunately Lantus was too conservatively dosed for fear of nocturnal hypoglycaemia. As experience was gained during the study, the Lantus dose on POD-1 was increased within the protocol range 0.25–0.3 units/kg. No patient became hypoglycaemic with this dose, but too many didn't come into the fasting target interval until their daily dose was increased to more than 0.3 units/kg. A better Lantus protocol may have been 0.75 units/kg pre-operatively and 0.3 units/kg as morning dose after surgery. These high doses are surprisingly well tolerated without regard to typically poor appetite on the first postoperative day. The observation that the Lantus requirements do not decrease between POD-1 and POD-5 suggest that the stress hyperglycaemia has not diminished. Voluntary food intake probably increases during the first 4-5 POD's but should not affect the Lantus requirements which are based solely on morning fasting values.
Hyperglycaemia is associated with impaired oxygen and nitrogen reactive species, endothelial and leukocyte dysfunction, and myocardial perfusion Citation9–12.
It is not known which hyperglycaemia parameter is most deleterious, e.g. average value, peak values, duration of hyperglycaemia, or time-weighted extent of hyperglycaemia. The continuous measurement of tissue glucose provides valuable insight into the nature of the clinical problem while this limited study cannot identify which glucose parameter is most important clinically: Lantus reduced the average and maximal values by about 20%, while the percent of the day when P-glu exceeds 7 mmol/l was reduced by a third and the area under the glucose curve was more than halved. Some patients had a surprisingly flat hyperglycaemia while others developed peaks to almost 20 mmol/l.
It is important to emphasize that the insulin protocol used immediately after CABG surgery is not generally applicable to medical patients as both the surgical trauma and cardiopulmonary bypass exacerbate the post-operative increase in insulin resistance. This patient population has also a high degree of pre-operative dysglycaemia than a general medical population.
This study has several limitations. First, this study protocol does not purport to provide optimal glycaemic control but rather a major improvement in glycaemic control using a routine requiring a minimal increase in nursing burden and a minimal risk of hypoglycaemia. Furnary correctly insists that only a continuous insulin infusion with blood glucose checks every 1–2 hours will provide optimal glycaemic control Citation6. Further improvement could be expected from a protocol including pre-prandial ultra-fast acting insulin, but with a considerable increase in nursing burden.
The sliding scale protocol for regular insulin can be considered unfairly under-dosed. It is based on the local tradition and is designed to minimize the risk for hypoglycaemia, as it in practice is not only used for pre-prandial glucose but can even be unthinkingly applied for a transient high glucose value when the patient's endogenous insulin is also increasing as after a meal. Standing orders for sliding scale regular insulin is often missed either due to lack of communication, time, or negligence. In this study 20% of pre-prandial P-glu > 10 mmol/l went untreated. Pre-emptive Lantus eliminates that factor. Another purpose for having a control group was to demonstrate the extent of untreated hyperglycaemia in patients referred for surgery as “non-diabetics” and who are normally not even tested on the wards. Only two Actrapid patients did not have glycaemic excursions above 10 mmol/l. A control group without any treatment (as occurs for non-study patients) seemed unethical.
Another major criticism is that there are no randomized interventional studies performed to date which can confirm that the benefits of aggressive hyperglycaemic control seen in the intensive care unit will also be seen in non-critically ill post-CABG patients on the wards. This study can't contribute to answering that question. Furnary's non-controlled studies are however compelling and certainly standard in-hospital medical practice mandates treatment to normoglycaemia Citation6. The adage “do no harm” often dominates with an exaggerated fear of insulin-induced hypoglycaemia impeding adequate treatment. While recent studies have shown no increased mortality with hypoglycaemia, it still behoves a minimal risk of hypoglycaemia before introducing a protocol which does not have a proven positive effect Citation13. Three to five capillary glucose tests per day would safeguard even a more aggressive glargine treatment than used here.
To summarize, this study has demonstrated that even patients without previously known diabetes are hyperglycaemic after CABG surgery, and that a routine protocol with pre-emptive glargine insulin treatment provides a major improvement in glycaemic control with a minimal incidence of hypoglycaemia and without an excessive increase in nursing burden. Fast-acting mealtime insulin could markedly enhance glycaemic control but will increase considerably the nursing burden.
References
- Clement S, Braithwaite SS, Magee MF, Ahmann A, Smith EP, Schafer RG, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004; 27: 553–91
- Anderson, RE, Brismar, K, Ivert, T. Only a minority of patients referred for elective coronary artery bypass surgery have risk factors diagnosed and treated according to established guidelines. J Diab Vasc Dis Research. 2007, (in press).
- Anderson RE, Klerdal K, Ivert T, Hammar N, Barr G, Öwall A. Are even impaired fasting blood glucose levels preoperatively associated with increased mortality after CABG surgery?. Eur Heart J. 2005; 26: 1513–8
- Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. Thorac Cardiovasc Surg. 2005; 130: 1144
- McAlister FA, Man J, Bistritz L, Amad H, Tandon Pl. Diabetes and coronary artery bypass surgery: An examination of perioperative glycemic control and outcomes. Diabetes Care 2003; 26: 1518–24
- Furnary AP, Wu Y. Clinical effects of hyperglycemia in the cardiac surgery population: The portland diabetic project. Endocr Pract. 2006; S3: 22–6
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345: 1359–67
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003; 78: 1471–8
- Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007; 9: 343–53
- Rask-Madsen C, King GL. Mechanisms of disease: Endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab. 2007; 3: 46–56
- Di Filippo C, Cuzzocrea S, Rossi F, Marfella R, D'Amico M. Oxidative stress as the leading cause of acute myocardial infarction in diabetics. Cardiovasc Drug Rev. 2006; 24: 77–87
- Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005; 112: 179–84
- Vriesendorp TM, DeVries JH, van Santen S, Moeniralam HS, de Jonge E, Roos YB, et al. Evaluation of short-term consequences of hypoglycaemia in an intensive care unit. Crit Care Med. 2006; 34: 2714–8