Abstract
Objectives. Hypothermia protects the myocardium from oxidative injury during ischemic stress and reperfusion. We have previously shown that topical negative pressure (TNP) of −50 mmHg significantly increases microvascular blood flow in the underlying myocardium in normal, ischemic, and reperfused porcine myocardium. The present study was designed to elucidate the effect of TNP between −50 mmHg and −150 mmHg on microvascular blood flow in ischemic myocardium during hypothermia. Design. The microvascular blood flow in the myocardium was recorded, in seven pigs, using laser Doppler velocimetry. Analyses were performed in the epicardium and in the myocardium, after 40 minutes of occlusion of the LAD followed by cooling to 31°C. Results. A TNP of −50 mmHg applied to the epicardium, from 23.3±3.8 PU to 104.2±31.3 PU (*p<0.05), and in the myocardium, from 35.0±7.2 PU to 74.2±21.8 PU (*p<0.05). Conclusions. Only a TNP level of −50 mmHg significantly increased the microvascular blood flow in both the epicardium and in the myocardium during hypothermia.
We have previously shown that applying topical negative pressure (TNP) directly to the myocardium significantly increases the microvascular blood flow in the underlying tissue Citation1. TNP is now widely used in wound care, and has been shown to facilitate the healing of chronic and problematic wounds Citation2, Citation3, including diabetic wounds Citation4, and poststernotomy mediastinitis Citation5–8. One of the mechanisms by which TNP promotes wound healing is by stimulating wound edge blood flow, as has been demonstrated in both peripheral Citation9 and in skeletal muscle in sternotomy wounds Citation10. TNP produces a mechanical stress and a pressure gradient across the tissue which may cause a surge of blood to the area. Mechanical forces and increased blood flow are known to stimulate granulation tissue formation, i.e. endothelial proliferation, capillary budding and angiogenesis Citation11–13.
The use of therapeutic hypothermia has increased worldwide over the past few years. Hypothermia is believed to protect the myocardium from oxidative injury during ischemic stress and reperfusion, by preservation of the myocardial function, promoting signaling for cell survival, and by inhibiting apoptotic pathways. The earlier hypothermia is applied to an ischemic myocardium, the more tissue can be salvaged Citation14–16.
TNP is known to stimulate blood flow in various tissues Citation2, for example, the skeletal muscle Citation10, Citation17. We have previously shown that a TNP of −50 mmHg significantly increases microvascular blood flow in the underlying tissue, in normal, ischemic, and reperfused myocardium Citation1. No study has previously been performed to investigate the effects of different negative pressures levels on microvascular blood flow in ischemic myocardium and ischemic epicardium during hypothermia.
In this study, blood flow was measured using laser Doppler velocimetry. The effect of different TNPs between −50 mmHg and −150 mmHg on microvascular blood flow was investigated in the myocardium, at temperature of 31°C, after 40 minutes of occlusion of the left anterior descending artery (LAD) to imitate ischemic coronary disease.
Materials and methods
Experimental animals
A porcine model was use in the present study. Seven domestic landrace pigs of both genders, with a mean body weight of 70 kg, were fasted overnight with free access to water. The study was approved by the Ethics Committee for Animal Research, Lund University, Sweden. The investigation complied with the “Guide for the Care and Use of Laboratory Animals” recommended by the U.S. National Institutes of Health, and published by the National Academies Press 1996.
Anesthesia
All the animals were pre-medicated intramuscularly with ketamine (30 mg/kg) before they were brought into the laboratory. Before commencing surgery sodium thiopental (5 mg/kg), atropine (0.02 mg/kg) and pancuronium (0.5 mg/kg) were given intravenously. Tracheotomy was performed with a Portex endo-tracheal tube (7.5 mm internal diameter, Medcompare™, USA). A servo-ventilator (Siemens Elema 300A, Stockholm, Sweden) was used for mechanical ventilation throughout the experiment. The ventilator settings used were: minute volume = 100 ml/kg, FiO2=0.5, breathing frequency = 16 breaths/minute and positive end expiratory pressure = 5 cmH2O.
Anesthesia and muscular paralysis were maintained by continuous intravenous infusion of Diprivan® (propofol, AstraZeneca, Sweden) at 8–10 mg/kg/hour, Leptanal® at 0.15 mg/kg/hour (fentanyl, Lilly, France) and Pavulon® at 0.6 mg/kg/hour (pancuronium, Organon Teknika, Boxtel, Netherlands).
Data acquisition
Mean arterial pressure, central venous pressure, body temperature, and ventilatory parameters were recorded throughout the experiments.
Surgical procedure
Surgery was performed through median sternotomy. After heparinization (400 IU/kg) a cardiopulmonary bypass (CPB) was installed with an arterial cannula (22 French, DLP® Elongated One-Piece Arterial Cannula (EOPA™), Medtronic Inc., Minneapolis, MO, USA) in the distal ascending aorta, and a venous cannula (32 French, MC2® Two-Stage Venous Cannula, Medtronic Inc.) inserted through the right atrium. Before cannulation of the heart the cannulae were inserted through the thoracic wall to prevent air leakage during TNP application. CPB was conducted in normothermia. Ventricular fibrillation was subsequently induced in the heart. No aortic cross-clamping was performed and no cardioplegia was employed. The mean arterial pressure was maintained between 60 and 80 mmHg. A left ventricular vent (DLP® Vent, Medtronic Inc.) was used to protect the left chamber from overloading. Pulmonary ventilation was applied at 4 liters/minute during the experiments.
CPB was used to facilitate the measurement of microvascular blood flow using laser Doppler velocimetry. Fibrillation of the heart minimizes movement artifacts, while the physiological conditions are, to a large extent, conserved. Moreover, CPB prevents the risk of circulatory failure during LAD occlusion, thereby facilitating experimental analysis in the ischemic myocardium.
Microvascular blood flow was measured by laser Doppler velocimetry (PeriFlux System 5000, Perimed, Stockholm, Sweden), using a technique that quantifies the sum of the motion of the red blood cells in a specific volume, extensively applied in plastic surgery procedures Citation18. In this method a fiberoptic probe carries a beam of light. Light impinging on cells in motion undergoes a change in wavelength (Doppler shift) while light impinging on static objects remains unchanged. The magnitude and frequency distribution of the changes are directly related to the number and velocity of red blood cells. The information is collected by a returning fiber, converted into an electronic signal and analyzed.
Laser Doppler probes were inserted horizontally into the heart muscle 6–8 mm lateral of the LAD at depths of approximately, 1–2 mm (epicardium), and 6–8 mm (myocardium). The mean thickness of the chamber wall was approximately 20–25 mm. All probes were carefully attached to the surface of the heart with a suture (Prolene 7–0; Ethicon Inc., Somerville, NJ, USA), thereby preventing the probe from sliding. A round hole with a diameter of 5 cm was made in the middle of a Phrenic Nerve Pad® (Medtronic Inc.) and placed on top of the heart. The pad was stabilized to the surrounding myocardium with 8–10 sutures (Prolene 5–0; Ethicon Inc.) and to the posterior sternal edges with sutures (Dermalon 2–0; Davis and Geck, St Louis, MO, USA). A retractor was used throughout the experiments to keep the sternal edges apart. A polyurethane foam dressing with an open pore structure of 400 to 600 µm (KCI, Copenhagen, Denmark) was placed between the sternal edges. The foam was continuously sutured to the surrounding skin (Dermalon 2–0; Davis and Geck). The wound was sealed with a transparent adhesive drape. A track pad (KCI) was inserted through the drape and was connected to a continuous vacuum source, (V.A.C. pump unit, KCI). When the negative pressure is applied, the heart will be drawn up towards the phrenic nerve pad and the foam without interfering with the sternal edges. This procedure causes the application of negative pressure to affect only the myocardium exposed by the 5 cm diameter hole. After the experiment the heart was dissected to confirm probe location.
Experimental protocol
The microvascular blood flow was measured continuously by the laser Doppler filament probes. The LAD was occluded for 40 minutes with an elastic vessel loop. Microvascular blood flow was measured before, and after 40 minutes of occlusion.
Hypothermia was subsequently induced by CPB. The microvascular blood flow was measured at 37, 35, 33, and 31°C. Recordings were then made in ischemic myocardium before negative pressure was applied, and at pressures of −50, −75, −100, −125, and −150 mmHg.
Calculations and statistics
Laser Doppler velocimetry measurements were performed on the seven pigs. The output was continuously recorded using PeriSoft software (Perimed, Stockholm, Sweden). Microvascular blood flow was expressed in terms of perfusion units (PU). A repeated-measurement general linear model was used to test the main effect of hypothermia, and the main effect of treatment and subject on ischemic hypothermic myocardium. Post hoc testing of the treatment was performed using Dunnet's test with a control (Baseline/No treatment/0 mmHg). A level of *p < 0.05 was considered statistically significant. A level of p > 0.05 was considered not statistically significant (n.s.).
Results
Hypothermia
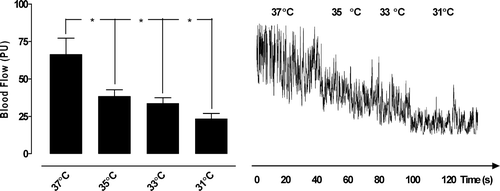
The microvascular blood flow in the ischemic epicardium decreased significantly during the induction of hypothermia: from 66.3±10.9 PU at 37°C to 38.3±4.4 PU at 35°C (*p < 0.05), to 33.7±3.8 PU at 33°C (*p < 0.05), and to 23.3±3.6 PU at 31°C (*p < 0.05) (). The microvascular blood flow in the myocardium also decreased significantly during the induction of hypothermia: from 74.3±11.7 PU at 37°C to 49.6±11.0 PU at 35°C (*p < 0.05), to 37.6±6.5 PU at 33°C (*p < 0.05), and to 33.3±6.0 PU at 31°C (*p < 0.05).
Figure 1. Microvascular blood flow measured in ischemic myocardium exposed to temperatures between 37°C and 31°C, using laser Doppler velocimetry. The measurements were performed at a depth of 1–2 mm in the myocardium (epicardium) in seven pigs. The results are shown as mean values±SEM, in the left panel. A level of *p < 0.05 was considered statistically significant. The right panel shows a representative example of microvascular blood flow changes during induction of hypothermia, from 37–31°C.
Epicardium
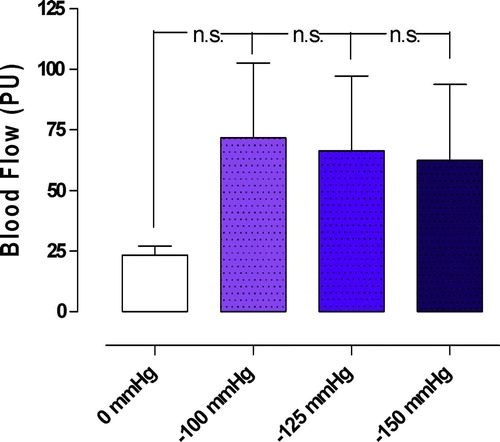
Applying a TNP of −50 mmHg and a TNP of −75 mmHg significantly increased the microvascular blood flow in the epicardium, from 23.3±3.8 PU to 104.2±31.3 PU (−50 mmHg) (*p < 0.05), and from 23.3±3.8 PU to 83.3±34.6 PU (−75 mmHg) (*p < 0.05) (). A TNP of −100, −125, −150 mmHg increased the microvascular blood flow from 23.3±3.8 PU to 71.7±30.9 PU (−100 mmHg) (p > 0.05 n.s.), from 23.3±3.8 PU to 66.3±30.8 PU (−125 mmHg) (p > 0.05), and from 23.3±3.8 PU to 62.5±31.3 PU (−150 mmHg) (p > 0.05) ().
Figure 2. Microvascular blood flow measured at a depth at 1–2 mm in the myocardium (epicardium) in seven pigs, after 40 minutes of occlusion of the left anterior descending artery and hypothermia at 31°C. Recordings were made before and after the application of topical negative pressures of −50 mmHg and −75 mmHg. The results are shown as mean values±SEM, in the left panel. A level of *p < 0.05 was considered statistically significant. The right panel shows a representative example of microvascular blood flow changes before and after application of −50 mmHg. Note the immediate increase in microvascular blood flow response when the negative pressure is applied.
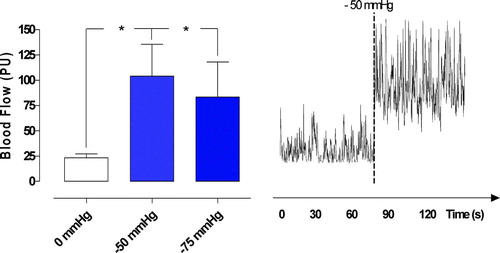
Figure 3. Microvascular blood flow measured before and after the application of topical negative pressures of −100, −125, and −150 mmHg. Measurements were made at a depth of 1–2 mm in the myocardium (epicardium) in seven pigs, after 40 minutes of occlusion of the left anterior descending artery and hypothermia at 31°C. The results are shown as mean values±SEM. None of the measurements was statistically significant.
Myocardium
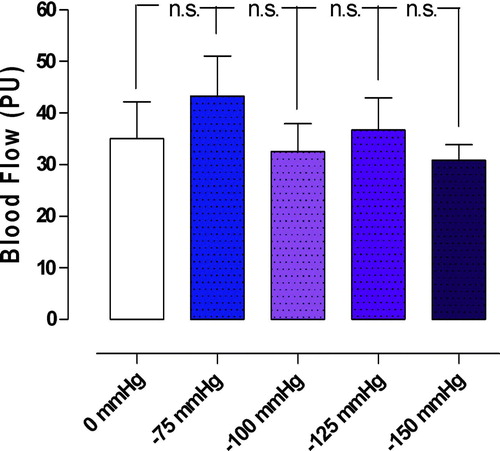
A TNP of −50 mmHg significantly increased microvascular blood flow in the myocardium, from 35.0±7.2 PU to 74.2±21.8 PU (*p < 0.05) (). TNP‘s of −75, −100, −125, and −150 mmHg did not cause statistically significant changes in the microvascular blood flow. Microvascular blood flow changes from 35.0±7.2 PU to 43.3±7.7 PU (−75 mmHg) (p > 0.05), from 35.0±7.2 PU, to 32.5±5.4 PU (−100 mmHg) (p > 0.05), from 35.0±7.2 PU to 36.7±6.3 PU (−125 mmHg) (p > 0.05), and from 35.0±7.2 PU to 30.8±3.0 PU (−150 mmHg) (p > 0.05) ().
Figure 4. Microvascular blood flow measured at a depth at 6–8 mm in the myocardium (myocardium) in seven pigs, after 40 minutes of occlusion of the left anterior descending artery and hypothermia at 31°C. Recordings were made before and after the application of a topical negative pressure of −50 mmHg. The results are shown as mean values±SEM. A level of *p < 0.05 was considered statistically significant. The right panel shows a representative example of microvascular blood flow changes before and after application of −50 mmHg. Note the immediate increase in microvascular blood flow response when the negative pressure is applied.
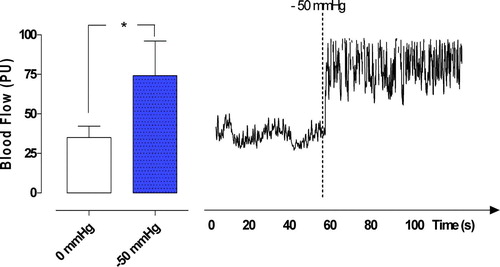
Figure 5. Microvascular blood flow measured before and after the application of topical negative pressures of −100, −125, and −150 mmHg. Measurements were made at a depth of 6–8 mm in the myocardium (myocardium) in seven pigs, after 40 minutes of occlusion of the left anterior descending artery and hypothermia at 31°C. The results are shown as mean values±SEM. None of the measurements was statistically significant.
Discussion
In the present study we found that hypothermia of 31°C significantly decreased the microvascular blood flow in both ischemic epicardium and ischemic myocardium (). The decrease in microvascular blood flow was most prominent between normothermia and 35°C. Microvascular blood flow was decreased at temperatures between 35°C and 31°C, but the decrease was not as great. We also found that TNPs of −50 mmHg and −75 mmHg significantly increased the microvascular blood flow in the epicardium during ischemia and hypothermia (). The only TNP that significantly increased the microvascular blood flow in the myocardium was −50 mmHg (). The reason for the difference in microvascular response in the epi- and myocardium is not known. It may, in part, be caused by the release of a different vasodilator close to the negative pressure source or a redistribution of blood flow towards the epicardium.
Applying different negative pressures to skeletal muscle and subcutaneous tissue causes different changes in blood flow Citation10. When the negative pressure exceeds a certain level it seems to constringe the vessels in the skeletal muscle and a decrease in local blood flow is seen together with a zone of hypoperfusion close to the negative pressure source Citation10, Citation19. The extent of the zone of hypoperfusion is related to tissue density and the amount of negative pressure applied Citation20. In the present study, no zone of hypoperfusion could be observed in ischemic myocardium during hypothermia, which totally agrees with a recent published study that was performed during normothermia Citation21. In a previous study we showed that a TNP of −50 mmHg significantly increased the microvascular blood flow in normal, ischemic, and reperfused myocardium Citation1. We have also previously shown that TNP levels between −75, and −150 mmHg do not alter the microvascular blood flow in normal and ischemic myocardium during normothermia Citation22. TNP is known to increase blood flow velocity and is also known to open up the capillary beds, thus increasing the volume of blood. The mechanical force exerted by TNP and increase in blood flow affect the cytoskeleton in the vascular cells, stimulating endothelial proliferation, capillary budding and angiogenesis, i.e. granulation tissue formation Citation11, Citation12. TNP has recently been shown to increase angiogenesis and decrease matrix metalloproteinases, both of which promote new vessel formation Citation13. Vascular endothelial growth factors (VEGFs) have been shown to play a key role in the promotion of angiogenesis and vascular growth Citation23, Citation24. Moreover, TNP produces a mechanical shear stress that is known to activate VEGFs thus removing the need for exogenous VEGF administration Citation25, Citation26.
Sinus rhythm does differ from ventricular fibrillation. Sinus rhythm of the heart contains both phases of systole and diastole. When the left ventricle contracts it compresses vessels in its walls and reduces coronary flow. The comprehensive force is greater in subendocardial muscle, i.e. equal or higher pressure than in the left ventricular cavity Citation27. The pressure falls toward the epicardium where it reaches levels near atmospheric pressure. In diastole all parts of the ventricular wall is perfused, whereas is systole only the epicardial part is perfused Citation27. During ventricular fibrillation there is no synchronized contraction in the ventricles. However, if perfusion pressure is kept around 100 mmHg, the myocardium will still be adequately perfused Citation28. If the perfusion pressure is reduced to approximately 50 mmHg, the left ventricular flow may be redistributed away from the subendocardium, and ischemia may occur in those parts Citation28. Hottenrot et al. showed in 1972 that perfusion of a fibrillating dog heart for one to two hours did not damage the subendocardial muscle unless a strong maintained electrical stimulus was used Citation29. Buckberg et al. showed in 1977 that the myocardial wall tension is always higher in fibrillating heart than in a beating heart during both normothermia and hypothermia Citation30. They also showed that left ventricular coronary flow remained distributed equally to the ventricular wall in both beating and fibrillating heart at temperatures of 28 and 22°C, but became redistributed toward the subendocardium in fibrillating heart at 37 and 32°C. Moreover, cold fibrillating heart consumed slightly less oxygen per minute than beating hearts at comparable temperatures as fibrillation became less forceful with hypothermia Citation30. To avoid subendocardial ischemia in the fibrillating heart during CPB no sustained electrical stimuli should be used, the ventricle should be protected from distension with, for example by, a ventricular vent, and the prefusion pressure should be kept above 50 mmHg Citation31.
A laser Doppler measurement of the myocardium is complex. In 1988, Ahn et al. assessed the myocardial perfusion in six empty beating porcine hearts with laser Doppler flowmetry. Cardiopulmonary bypass was instituted and the preparation allowed continuous and simultaneous measurement of coronary sinus blood flow and local tissue perfusion. An epicardial and an intramuscular probe were used. They obtained significant linear correlation coefficients between changes of laser Doppler signal and coronary sinus blood flow changes, and between laser Doppler signals and changes of extracorporeal bypass. The correlation between coronary sinus flow and bypass flow was significant in four pigs. Muscular activity of the heart contributed to the laser Doppler signal, and that the magnitude of this “noise level” varied between different experiments even in the same animal, which made it difficult to discriminate between blood flow and muscular contribution Citation32. Ahn et al. also applied Laser Doppler flowmetry to the arrested heart of four pigs and to the fibrillating heart of three pigs during cardiopulmonary bypass. A significant correlation between laser Doppler signal and coronary blood flow was found during cardiac arrest. During ventricular fibrillation after release of the aortic cross-clamp there was significant correlation between laser Doppler signal and extracorporeal blood flow. A residual laser Doppler signal of about 60% of the maximal value was recorded after the bypass flow was discontinued. Ahn et al. concluded that laser Doppler flowmetry permit measurement of myocardial perfusion in the arrested porcine heart, and that muscular activity of the heart contributes to the output signal during ventricular fibrillation Citation33. However, in the studies by Ahn et al., it is unclear if returning venous blood to the heart from venous cava superior, inferior, and also the associating cava (specific for pigs), was occluded in the study when the bypass flow was discontinued, or if the measurements were adjusted to the systemic pressure, which both might had influenced on the measurements Citation34. It has been shown, in a porcine model, that ventricular fibrillation causes venous congestion, an empty left heart, and a greatly distended right heart within 3 min, and that the blood pressure is 50 mmHg after 20 seconds and 20–30 mmHg after 1.5–2.0 minutes, which also might had influenced on the measurements Citation34. In our opinion it is crucial that the CPB flow is kept at a constant level, as well as the blood pressure. Changes of those parameters might change the model. The present study was conducted during low intense ventricular fibrillation to minimize movement artefacts/muscular activity, and minimize tissue trauma, and sliding of the laser Doppler probes, while measuring blood flow in the myocardium. However, a part of the laser Doppler signal is probably caused by muscular activity, as suggested by Ahn et al. Citation32, Citation33. We believe that the majority of the laser Doppler signal represents microvascular blood flow, and that the majority of the changes in the laser Doppler signal before and after the application of TNP represent microvascular blood flow changes.
Recently, we applied a TNP of −50 mmHg over the LAD region in six porcine hearts. The measurements were made before and after application of TNP. The study was conducted during low intense ventricular fibrillation with support by cardiopulmonary bypass. Coronary blood flow was measured with ultrasonic flow meter probes at the proximal part of the LAD, CCX, and LCA. Microvascular blood flow was measured by laser Doppler probes located 5–6 mm lateral of the middle part of the LAD, 5–6 mm down into the myocardial wall. TNP of −50 mmHg significant increased the coronary blood flow in both normal (*p < 0.05) and ischemic (*p < 0.05) myocardium. A significant correlation was found between coronary blood flow and laser Doppler signals in normal myocardium (r2 = 0.81, *p < 0.05), and in ischemic myocardium (r2 = 0.96, *p < 0.05) Citation35.
Poststernotomy mediastinitis is a strong predictor of poor long-term survival after coronary artery bypass grafting (CABG), and it has been suggested that mediastinitis may cause negative, long-term effects on several organs, such as the heart and kidneys Citation36. Over the past years, TNP has been used in the treatment of poststernotomy mediastinitis with excellent clinical results Citation8, Citation37, Citation38. Interestingly, Sjogren et al. found no difference in long-term survival between CABG patients with TNP-treated mediastinitis and CABG patients without mediastinitis Citation38. During TNP treatment of poststernotomy mediastinitis, the topical negative pressure is more or less in direct contact with the myocardium, and these patients may therefore have developed increased coronary collateral blood vessels during TNP treatment, and thus be better prepared when bypass grafts fail to work. Indeed, we have observed that these patients develop a thick layer of well-vascularized granulation tissue on the exposed surface of the heart after 6–8 days of treatment. It may well be that the stimulation of blood flow and development of collateral blood vessels by TNP in part account for the reduced long-term mortality in patients treated with TNP for poststernotomy mediastinitis after CABG.
It is thought that hypothermia reduces the metabolic needs of cells, specifically by reducing the oxygen demand in the hypothermic tissues. The actual mechanism by which hypothermia reduces cell death in ischemic tissues is not completely understood. One of the known effects of hypothermia is that it limits the extent of an infarct if instituted during ischemia and reperfusion. The earlier hypothermia is applied to an ischemic myocardium, the more tissue can be salvaged Citation14–16. In the present study we have shown that, even during hypothermia of 31°C, a TNP of −50 mmHg is able to significantly increase the microvascular blood flow, both in the epicardium and in the myocardium.
In patients with acute coronary syndrome and coronary vessel occlusion, it is of great importance to improve or, if possible, restore the blood flow to the ischemic myocardium to protect it from ischemic stress and, in some cases, acute coronary infarction. Most patients are successfully treated with conventional methods such as percutaneous coronary intervention (PCI) or CABG. However, these procedures do not result in satisfactory results in all patients due to extensive coronary disease or small vessel caliber Citation39. Furthermore, the procedure is not suitable for some patients due to high age, renal failure, or other complicating factors. In some cases of acute ST elevation myocardial infarction there is no reflow during PCI. No reflow situations may also arise during saphenous vein graft intervention, and rotational atherectomy. During no-reflow, epicardial flow is reduced due to an obstruction at the microvasculature level Citation40. This no-reflow condition is usually transient, but patients with refractory no-reflow are associated with a markedly increased risk of 30-day mortality, compared with patients in whom no-reflow is transient Citation41. In the present study we found that a TNP of −50 mmHg significantly increased the microvascular blood flow in both the epicardium and the myocardium. Interestingly, TNP increases blood flow velocity and the volume by opening up the capillary beds Citation2. Furthermore, the method is not dependent on vessel caliber.
Many kinds of treatment have been tried in patients with refractory angina pectoris without satisfactory clinical results Citation39, Citation42. A method oofxxxxof stimulating myocardial neovascularization that is not dependent on vessel caliber would provide an important alternative. Numerous studies have evaluated the efficacy of gene therapy in the treatment of ischemic heart disease aimed at the restoration of myocardial function by stimulating angiogenesis and collateral vessel formation Citation43, Citation44. Interestingly, TNP is known to increase blood flow and stimulate angiogenesis in subcutaneous tissue and skeletal muscle Citation12, Citation13, Citation19. In the present study we have shown that myocardial TNP increases microvascular blood flow in the underlying myocardium. Topical negative pressure may also, theoretically, be able to stimulate angiogenesis in the myocardium.
In conclusion, TNP during hypothermia significantly increases the microvascular blood flow, not only in ischemic epicardium, but also in ischemic myocardium.
Limitations of the study
CPB and low intense ventricular fibrillation was used to minimize movement artefacts/muscular activity while measuring blood flow in the myocardium using laser Doppler technology. Recently, we showed that TNP of −50 mmHg significantly increased the coronary blood flow. A significant correlation was found between coronary blood flow and laser Doppler signals, which support that the two methods both reflects blood flow changes in the myocardium Citation35. A part of the laser Doppler signal is probably caused by muscular activity, as suggested by Ahn et al. Citation32, Citation33. However, we believe that the majority of the laser Doppler signal represents microvascular blood flow, and that the majority of the changes in the laser Doppler signal before and after the application of TNP represent microvascular blood flow changes. The effect of TNP on the beating heart can not be deduced from the present results, although we believe that the effect would be similar to that observed here.
Acknowledgements
We would like to thank Johan Ingemansson (Statistical Solutions IP) for his expert contribution to the statistical analyses. This study was supported by the Anders Otto Swärd Foundation/Ulrika Eklund Foundation, Anna Lisa and Sven Eric Lundgren's Foundation for Medical Research, the Åke Wiberg Foundation, the M. Bergvall Foundation, the Swedish Medical Association, and the Royal Physiographic Society in Lund, the Swedish Medical Research Council, and the Crafoord foundation.
References
- Lindstedt S, Malmsjo M, Ingemansson R. Blood flow changes in normal and ischemic myocardium during topically applied negative pressure. Ann Thorac Surg. 2007; 84: 568–73
- Morykwas MJ, Argenta LC, Shelton-Brown EI, McGuirt W. Vacuum-assisted closure: A new method for wound control and treatment: Animal studies and basic foundation. Ann Plast Surg. 1997; 38: 553–62
- Argenta LC, Morykwas MJ. Vacuum-assisted closure: A new method for wound control and treatment: Clinical experience. Ann Plast Surg. 1997;38:563–76; Discussion 77.
- Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: A multicentre, randomised controlled trial. Lancet. 2005; 366: 1704–10
- Domkowski PW, Smith ML, Gonyon DL, Jr, Drye C, Wooten MK, Levin LS, et al. Evaluation of vacuum-assisted closure in the treatment of poststernotomy mediastinitis. J Thorac Cardiovasc Surg. 2003; 126: 386–90
- Gustafsson RI, Sjogren J, Ingemansson R. Deep sternal wound infection: A sternal-sparing technique with vacuum-assisted closure therapy. Ann Thorac Surg. 2003;76:2048–53; Discussion 53.
- Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: Vacuum-assisted closure versus conventional treatment. Ann Thorac Surg. 2005; 79: 2049–55
- Gustafsson R, Johnsson P, Algotsson L, Blomquist S, Ingemansson R. Vacuum-assisted closure therapy guided by C-reactive protein level in patients with deep sternal wound infection. J Thorac Cardiovasc Surg. 2002; 123: 895–900
- Chen SZ, Li J, Li XY, Xu LS. Effects of vacuum-assisted closure on wound microcirculation: An experimental study. Asian J Surg. 2005; 28: 211–7
- Wackenfors A, Sjogren J, Gustafsson R, Algotsson L, Ingemansson R, Malmsjo M. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen. 2004; 12: 600–6
- Vandenburgh HH. Mechanical forces and their second messengers in stimulating cell growth in vitro. Am J Physiol. 1992; 262: R350–R355
- Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: Microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–96; Discussion 97–8.
- Greene AK, Puder M, Roy R, Arsenault D, Kwei S, Moses MA, et al. Microdeformational wound therapy: Effects on angiogenesis and matrix metalloproteinases in chronic wounds of 3 debilitated patients. Ann Plast Surg. 2006; 56: 418–22
- Alzaga AG, Cerdan M, Varon J. Therapeutic hypothermia. Resuscitation. 2006; 70: 369–80
- Bernard S. New indications for the use of therapeutic hypothermia. Crit Care. 2004; 8: E1
- Bernard S. Hypothermia after cardiac arrest: How to cool and for how long?. Crit Care Med. 2004; 32: 897–9
- Petzina R, Gustafsson L, Mokhtari A, Ingemansson R, Malmsjo M. Effect of vacuum-assisted closure on blood flow in the peristernal thoracic wall after internal mammary artery harvesting. Eur J Cardiothorac Surg. 2006; 30: 85–9
- Zografos GC, Martis K, Morris DL. Laser Doppler flowmetry in evaluation of cutaneous wound blood flow using various suturing techniques. Ann Surg. 1992; 215: 266–8
- Wackenfors A, Gustafsson R, Sjogren J, Algotsson L, Ingemansson R, Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg. 2005;79:1724–30; Discussion 30–1.
- Wackenfors A, Sjogren J, Algotsson L, Gustafsson R, Ingemansson R, Malmsjo M. The effect of vacuum-assisted closure therapy on the pig femoral artery vasomotor responses. Wound Repair Regen. 2004; 12: 244–51
- Lindstedt S, Malmsjo M, Ingemansson R. No hypoperfusion is produced in the epicardium during application of myocardial topical negative pressure in a porcine model. J Cardiothorac Surg. 2007; 2: 53
- Lindstedt S, Malmsjo M, Sjogren J, Gustafsson R, Ingemansson R. Impact of different topical negative pressure levels on myocardial microvascular blood flow. Cardiovasc Revasc Med. in press.
- Biswas SS, Hughes GC, Scarborough JE, Domkowski PW, Diodato L, Smith ML, et al. Intramyocardial and intracoronary basic fibroblast growth factor in porcine hibernating myocardium: A comparative study. J Thorac Cardiovasc Surg. 2004; 127: 34–43
- Horvath KA, Lu CY, Robert E, Pierce GF, Greene R, Sosnowski BA, et al. Improvement of myocardial contractility in a porcine model of chronic ischemia using a combined transmyocardial revascularization and gene therapy approach. J Thorac Cardiovasc Surg. 2005; 129: 1071–7
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci 1999; 112(Pt 19)3249–58
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog. 1998; 14: 356–63
- Baird RJ, Manktelow RT, Shah PA, Ameli FM. Intramyocardial pressure. A study of its regional variations and its relationship to intraventricular pressure. J Thorac Cardiovasc Surg. 1970; 59: 810–23
- Brazier JR, Cooper N, McConnell DH, Buckberg GD. Studies of the effects of hypothermia on regional myocardial blood flow and metabolism during cardiopulmonary bypass. III. Effects of temperature, time, and perfusion pressure in fibrillating hearts. J Thorac Cardiovasc Surg. 1977; 73: 102–9
- Hottenrott C, Buckberg GD, Maloney JV, Jr. Effects of ventricular fibrillation on distribution and adequacy of coronary blood flow. Surg Forum. 1972; 23: 200–2
- Buckberg GD, Brazier JR, Nelson RL, Goldstein SM, McConnell DH, Cooper N. Studies of the effects of hypothermia on regional myocardial blood flow and metabolism during cardiopulmonary bypass. I. The adequately perfused beating, fibrillating, and arrested heart. J Thorac Cardiovasc Surg. 1977; 73: 87–94
- Buckberg GD, Hottenrott CE. Ventricular fibrillation. Its effect on myocardial flow, distribution, and performance. Ann Thorac Surg. 1975; 20: 76–85
- von Ahn HC, Ekroth R, Nilsson GE, Svedjeholm R. Assessment of myocardial perfusion with laser Doppler flowmetry. An experimental study on porcine heart. Scand J Thorac Cardiovasc Surg. 1988; 22: 145–8
- Ahn HC, Ekroth R, Hedenmark J, Nilsson GE, Svedjeholm R. Assessment of myocardial perfusion in the empty beating porcine heart with laser Doppler flowmetry. Cardiovasc Res. 1988; 22: 719–25
- Steen S, Liao Q, Pierre L, Paskevicius A, Sjoberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: A haemodynamic explanation. Resuscitation. 2003; 58: 249–58
- Lindstedt S, Malmsjö M, Gesslein B, Ingemnsson R. Coronary blood flow changes before and during application of myocardial topical negative pressure in a sternal wound model. Int Wound J. 2007:( in press).
- Toumpoulis IK, Anagnostopoulos CE, Derose JJ, Jr, Swistel DG. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005; 127: 464–71
- Sjogren J, Gustafsson R, Dobre M, Koul B, Ingemansson R, Algotsson L. Vacuum-assisted closure therapy in mediastinitis after heart transplantation. J Heart Lung Transplant. 2004; 23: 506–7
- Sjogren J, Nilsson J, Gustafsson R, Malmsjo M, Ingemansson R. The impact of vacuum-assisted closure on long-term survival after post-sternotomy mediastinitis. Ann Thorac Surg. 2005; 80: 1270–5
- Gowda RM, Khan IA, Punukollu G, Vasavada BC, Nair CK. Treatment of refractory angina pectoris. Int J Cardiol. 2005; 101: 1–7
- Kang S, Yang Y. Coronary microvascular reperfusion injury and no-reflow in acute myocardial infarction. Clin Invest Med. 2007; 30: E133–E145
- van Gaal WJ, Banning AP. Percutaneous coronary intervention and the no-reflow phenomenon. Expert Rev Cardiovasc Ther. 2007; 5: 715–31
- Cohn PF. Enhanced external counterpulsation for the treatment of angina pectoris. Prog Cardiovasc Dis. 2006; 49: 88–97
- Takaba K, Jiang C, Nemoto S, Saji Y, Ikeda T, Urayama S, et al. A combination of omental flap and growth factor therapy induces arteriogenesis and increases myocardial perfusion in chronic myocardial ischemia: Evolving concept of biologic coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2006; 132: 891–9
- Sarateanu CS, Retuerto MA, Beckmann JT, McGregor L, Carbray J, Patejunas G, et al. An Egr-1 master switch for arteriogenesis: studies in Egr-1 homozygous negative and wild-type animals. J Thorac Cardiovasc Surg. 2006; 131: 138–45