Abstract
Objectives: Longer-term electrocardiographic effects of multiple inappropriate ICD shocks were investigated to study their hypothesized pro-arrhythmic potential.
Design: Thirteen male patients with ischemic cardiomyopathy who received ≥2 inappropriate shocks within 24 h and for whom 12-lead ECGs were available both before and within 72h after the inappropriate shocks were analyzed. Exclusion criteria included continuous ventricular pacing, underlying AF, events within 6 weeks after lead implantation and concomitant acute medical problems.
Results: A total of 149 inappropriate shocks (mean 11 ± 19) were received. There were no significant differences in any of the measured intervals or morphological indices, nor was there a correlation between the “before-after” differences and the number of shocks received. Non-significant changes showed Percentage of Loop Area increase and relative T-wave Residuum decrease while the opposite changes have previously been associated with arrhythmic risk.
Conclusions: No potentially pro-arrhythmic electrocardiographic changes were found 19 h after multiple inappropriate shocks.
Introduction
Sudden cardiac death due to ventricular arrhythmia is presently the second most frequent cause of death in developed countries.[Citation1] The annual incidence of sudden cardiac death in Europe and North America ranges from 50 to 100 per 100,000 of general population with 40% of cases occurring before the age of 65. The implantable cardioverter-defibrillator (ICD) is currently the mainstay for arrhythmic death prevention in patients on optimal medical therapy.[Citation1]
Both appropriate and inappropriate ICD shocks have been found associated with increased mortality and pro-arrhythmia.[Citation2,Citation3] The MADIT-RIT clinical trial showed that delaying ICD therapy decreases the number of inappropriate therapies and reduces all-cause mortality during follow-up.[Citation4] This fueled an ongoing debate of whether ICD shocks per se, on top of the underlying cardiac substrate, increase mortality. The potential adverse effect of high voltage shocks remains one of the important questions of ICD therapy.[Citation5] Immediately after shocks, pro-arrhythmic electrocardiographic changes have been described.[Citation6–8] The potential contribution of shock-induced cardiac electrical trauma to increased heart failure and mortality in patients who receive appropriate and inappropriate shocks has not been excluded.[Citation9] We therefore studied the effects of ICD shocks on the ECG within 24 h after multiple inappropriate shocks. For that purpose, we assessed T-wave morphology characteristics previously shown to predict arrhythmic events. Total Cosine R to T (TCRT) and Percentage of Loop Area (PLA) have been shown to be powerful indicators of arrhythmic complications in patients with ischemic cardiomyopathy and improved the post myocardial infarction risk stratification when combined with other risk markers.[Citation10,Citation11] Absolute and relative T-wave Residuum (TWR) were significant predictors of survival in a long-term follow-up cohort with cardiovascular disease.[Citation11]
Materials and methods
Patient population
Patients with ischemic cardiomyopathy who received an ICD for standard implantation criteria and were followed at the University Hospitals of Leuven were screened for this analysis. The patient characteristics, medication and ejection fraction at the time of the shocks were collected from the electronic medical record. Inclusion criteria for the present study were ≥2 inappropriate shocks within a 24-h interval and the availability of a 12-lead ECG within 72h after the inappropriate shocks (“ECG after”). The most recent ECG before the shocks taken during routine follow-up without evidence of any acute medical problem was selected as control (“ECG before”). The interval between “ECG before” and the inappropriate shocks was calculated in days according to the respective dates. For the interval between the inappropriate shocks and “ECG after”, the time of the last inappropriate shock was based on the ICD event analysis when available. When not available, the time was estimated based on the medical report. To rule out T-wave memory or concurrent medical problems as confounding factors, patients were excluded if they had continuous ventricular pacing, including cardiac resynchronization therapy, and all had ICDs programmed to minimize ventricular pacing. Patients were further excluded if they were in atrial fibrillation (AF), if the inappropriate shocks occurred within 6 weeks after lead implantation or revision; or if they were admitted with a concomitant acute medical problem. The etiology of the inappropriate shocks was classified as lead failure, T-wave oversensing, AF, and supraventricular tachycardia (sinus tachycardia, atrial re-entry tachycardia). Out of 483 patients, 14 patients were identified meeting these criteria (). The study was approved by the University Hospitals of Leuven Ethical Committee.
Table 1. Demographic and clinical characteristics.
Electrocardiographic methods
ECGs were standard 10-s 12-lead ECG tracings obtained at 250 Hz sampling rate. They were recorded using the MAC 3500 system (GE Healthcare) and stored on MUSE® Cardiologic Information System (GE Medical Systems, Menomonee Falls, WI, USA). The digitized 12-lead ECGs were exported without any filter settings.
ECG analysis, blinded to the clinical data, was performed at the Imperial College, London. The RR, PR, QRS, and QT-interval were measured in representative complexes superimposed on the same isoelectric line to improve accuracy, since measurements in individual leads can be influenced by isoelectric axis projection.[Citation12] The RR-interval was measured as the time interval between the R-waves of 2 consequent sinus beats and averaged over the 10-s duration of each recording. PR-interval was measured from the start of the P-wave to the onset of the QRS complex. QRS-duration was defined as the time between Q-wave onset and the end of the S-wave. QT interval was measured from the start of the Q-wave till the end of the T-wave. Computer-defined end of the T-wave was defined based on pattern matching algorithm.[Citation13] All computer measurement of ECG intervals was visually validated and edited where necessary by one experienced investigator. The QT interval was corrected for heart rate using Fridericia’s formula (QTcF = QT/(60/HR)1/3).
While we fully recognize the conceptual problems with the so-called QT dispersion,[Citation14] this parameter was added to the analysis to the ECG characteristics solely to compare our results with previous reports.[Citation6,Citation7] For this purpose, the ECGs were analyzed at the University of Leuven using Labchart 7 Pro (AD Instruments) to identify the minimum and maximum QT-interval durations of all 12-leads and calculating QT dispersion, defined as the difference between maximum and minimum of all available complexes across all individual leads.
Three T-wave morphological indices, which were previously reported to predict cardiac risk were calculated.[Citation10,Citation11] The general approach for this ECG analysis has previously been described in detail. In brief, 8 algebraically independent leads are subjected to singular value decomposition and the dominant 3-leads were used to characterize QRS and T-wave loops. From these, 3 parameters were derived which have previously been shown to identify cardiac risk:
TCRT parameter (Total Cosine R to T) expresses the cosine of the three-dimensional angle between the QRS and T loops and therefore a measure estimating the spatial deviation between the overall direction of depolarization and repolarization waveforms.[Citation10] Higher TCRT values indicate a smaller angle between the R- and T-wave loop vectors as is seen in normal ECGs.
PLA parameter (Percentage of Loop Area) calculates the area of the T-wave loop as a fraction of the rectangle that encompasses the loop. It expresses the circularity of the T-wave loop thus distinguishing homogeneous smooth repolarization sequences from those affected by localized abnormalities.[Citation10] An irregular loop indicates heterogeneous temporal evolution of ventricular depolarization, hence PLA decreases in an abnormal ECG.
TWR parameter (Relative T-wave Residuum) reflects true heterogeneity of ventricular repolarization within the ECG.[Citation11] As mentioned earlier, the 3 dominant orthogonal leads derived from the 8 independent leads of the 12-lead recordings represent the standard vector-cardiographic T-wave (the so-called dipolar signal components), whereas the remaining 4th to 8th relate signal components beyond the single dipole movement. They reflect repolarization signals within the ECG that cannot be incorporated into the overall T-wave dipole. Therefore, they are a measure of repolarization heterogeneity. Quantitatively, the relative TWR is calculated as the proportion of the sum of squares of the eigenvalues of the 4th to 8th of the T-wave signal to the sum of squares of all 1st to 8th eigenvalues.
Since all calculations are influenced by recording noise and the study involves advanced analysis of serial ECGs, the product noise was calculated as objective characterization of ECG quality. As previously published,[Citation15] the numerical values of the noise were obtained as the product of the average standard deviation residuum and the RMSSD (root mean square of successive differences) residuum.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. Paired t-tests were used to compare before and after measurements. Pearson’s correlation coefficients were used to investigate the relationship between the difference in the ECG measurements (“ECG before” minus “ECG after”) and the number of shocks in each patient. The statistical analysis was performed on all patients included and repeated after the exclusion of inappropriate shocks caused by supraventricular tachycardia. All statistical analyses were performed using SPSS (IBM Statistics, version 22, Armonk, NY, USA). An alpha-level of <0.05 was used to infer statistical significance.
Results
Demographics
The demographic and clinical characteristics of the patients are shown in . Of 14 eligible subjects there was 1 female which was excluded for further analysis to prevent gender induced differences. The investigated population included only 2 patients with ICD implanted because of primary prevention. The medication profile of all patients did not differ between the ECG before and after the multiple inappropriate shocks. All patients were taking beta-blockers or sotalol. One patients was taking beta-blockers in combination with amiodarone. There was no significant difference between the left ventricular ejection fraction at implantation and at the time of the multiple inappropriate shocks (48 ± 15% versus 47 ± 15%, p = 0.59 respectively). The devices were programmed in the VVI back-up pacing mode with a lower rate of 40 bpm in 11 patients (85%), in the AAI-DDD mode with a lower rate of 50 bpm in 1 patients (8%), and in in the DDI mode with a lower rate of 40 bpm and an AV delay of 250 ms in 1 patient (8%). This resulted in a cumulative percentage of RV-pacing <2%. The total number of inappropriate shocks within the investigative 24-h window was 149 (range 2–75 shocks per patient). The patient receiving 75 shocks within 24 h presented at the Emergency Department with multiple inappropriate shocks due to lead fracture. The patient received multiple series of inappropriate shocks because of false arrhythmia detection mistaken for ventricular fibrillation. The average shock intensity was 32.7 ± 2.9 Joules with a total energy delivered of 4743 Joules. In 7 patients (53%), these were caused by lead failure, in 4 patients (31%) by T-wave oversensing, in 1 patient (8%) by supraventricular tachycardia, and in 1 patient (8%) by AF. The mean time interval between “ECG before” and the first inappropriate shock included in the analysis was 68 ± 38 days. The mean time interval between the first inappropriate shock included in the analysis and the post-shock ECG was 18 ± 22 h, in 11 patients (85%) the post-shock ECG was performed within 24 h.
Electrocardiographic analysis
The results of the electrocardiographic analysis are shown in and . The intra-subject change in T-wave morphology parameters is illustrated in . There was no significant difference in any of the investigated values comparing the ECG before and after. There were no significant correlations found between the intra-patient parameter differences and the number of appropriate shocks received. Carefully comparing these results with previous studies we note that 3 patients (23.1%) had a change in TCRT which previously have been described as associated with a significant worse outcome in a comparable patient population with ischemic cardiomyopathy, whereas 4 patients (30.8%) showed a significant change in the opposite direction (). Similarly, 2 patients (15.4%) had a change in PLA which was previously has been associated with a significant worse outcome and 4 patients (30.8%) in the opposite favorable direction (). For TWR, 4 patients (30.8%) had a change associated with significant worse outcome and only 1 (7.7%) had a change in the favorable direction (). When repeating the statistical analysis after exclusion of inappropriate shocks caused by supraventricular tachycardia, the results remained unchanged with no significant differences found.
Figure 1. Intra-subject change in T-wave morphology parameters. (A) Intrasubject changes in total cosine R to T (TCRT). (B) Intrasubject changes in percentage of loop area (PLA). (C) Intrasubject changes in T-wave residuum (TWR).
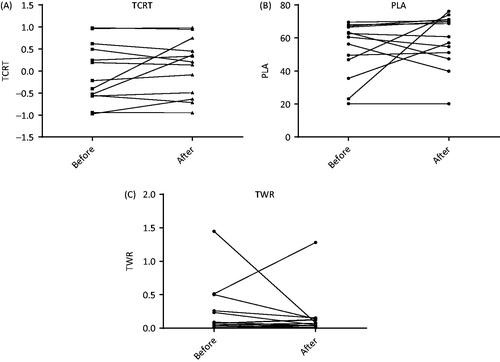
Table 2. Results of the electrocardiographic analysis with paired t-tests.
Table 3. Results of the correlation analysis.
Discussion
In the study, inappropriate ICD shocks were not found to cause any significant electrocardiographic changes in men 19 h after shocks detected by a battery of indices that have previously been shown to characterize arrhythmic and other cardiac risk.
Do shocks lead to ECG changes?
Our findings show that any early change in ECG parameters, as previously described, is not detectable anymore after 72 h. Transient changes in ECG parameters could have been present but the study design did not allow their investigation. Although the validity and clinical relevance of QT and QTcF dispersion is appropriately seriously questioned,[Citation14,Citation16] we separately calculated the dispersion to compare with previous reports and found no significant differences. Gurevitz et al. were the first to report QT dispersion increase in 17 patients undergoing ICD implantation or defibrillator testing. They reported an increase of QT dispersion by 16 ± 44 ms, 10 s after a test shock, by 23 ± 36 ms 1 min after, and 10 ± 14 ms 5 min after a test shock compared to a baseline value of 103 ± 48 ms. Pang et al. studied the dispersion of regional ventricular depolarization after high-energy defibrillation in dogs and found a significant increase of this measure associated with the energy delivered.[Citation17] The changes of QT dispersion after ICD shocks were further investigated by Topaloglu et al.[Citation6] They observed a normalization of QT dispersion, QTc dispersion and maximum QTc 20 min after a single shock. In patients taking amiodarone this normalization occurred significantly earlier, after only 15 min. In both studies, the electrocardiographic data were collected during defibrillator testing. To our knowledge, no other significant electrocardiographic changes have been reported.
The differences in T-wave morphology parameters observed were not significant. Due to the low number of patients, these findings are difficult to compare with literature. Lampert et al. found a significant increase of T-wave alternans up to 15 min after a high-energy shock.[Citation18] However, they did not increase the monitoring interval until T-wave alternans returned to baseline. Zabel et al. reported on 280 patients post-myocardial infarction with 32 ± 10 months’ follow-up and studied a combined endpoint of all-cause mortality, sustained VT and resuscitated VF.[Citation19] They observed a significant lower TCRT (0.16 ± 0.57 versus −0.31 ± 0.57, p < 0.0002) and lower PLA (56 ± 16% versus 48 ± 20%, p = 0.031) for patients were the combined endpoint occurred. Further, in a study on 813 male US veterans (follow-up 10.4 ± 3.8 years) they observed a baseline significant lower TCRT (−0.11 ± 0.65 versus −0.23 ± 0.60, p < 0.02) and higher TWR (0.43 ± 0.62% versus 0.33 ± 0.56%, p < 0.0005) in patients that died during follow-up.[Citation11] The measurements after the inappropriate shocks showed, on average, shorter QRS duration, shorter QTc intervals, increased PLA, and reduced TWR. The intra-subject changes in T-wave morphology parameter was discussed earlier. Although none of these changes were statistically significant in an overall analysis, the small number of investigated patients limits true clinical conclusion and indicates that further investigation is necessary to exclude pro-arrhythmic changes.
It could be questioned whether these previous studies reflect the effect on the ECG of shocks during ambulatory follow-up. Patients were sedated with possible changes in their autonomic tone and not awake as in spontaneous arrhythmias. Lampert et al. observed an increase of T-wave alternans after evoked ICD shocks and have shown a correlation with catecholamine levels in peripheral blood stream.[Citation18] Since the sensation of an unexpected shock is an unpleasant experience, there could be a more drastic increase in catecholamine level with a more pronounced electrocardiographic effect in real life. Furthermore, the defibrillator testing provoked VT or VF by shocks timed in the vulnerable phase of the T-wave. Further shocks were delivered as clinically needed to terminate the ventricular arrhythmias. The post-shock measurements were performed after delivery of the final shock. Since Moubarak et al. showed an increase of spatial dispersion of repolarization measured invasively after provoked ICD shocks, the results of electrocardiographic changes after provoked ICD shocks could be a true effect of a shock or be secondary to the shock provocation and/or to the temporarily occurring ventricular arrhythmia.[Citation8]
Are inappropriate shocks linked to an increased mortality?
At present, the link between mortality and inappropriate ICD therapy remains unclear. Some studies have shown an association between both appropriate and inappropriate shocks and an increased risk of death,[Citation4,Citation20,Citation21] whereas other studies did not show any association.[Citation22,Citation23] In the MADIT-RIT trial, multivariate analyses showed inappropriate therapies to be significantly associated with an increased risk of all-cause mortality, both for inappropriate shocks as inappropriate ATP only. Whereas the number of patients receiving inappropriate shocks doubled in the conventional group compared to the high-rate and delayed therapy group of patients, the number of patients with inappropriate ATP was four to five times larger. The results of the ALTITUDE Survival by Rhythm Study suggested that the increased mortality associated with inappropriate therapies is more likely related to the underlying rhythm rather than to the therapy itself.[Citation21] A first inappropriate shock due to AF of atrial flutter was associated with a significant increased mortality, whereas patients with a first inappropriate shock due to supraventricular tachycardia or noise/artifact/oversensing showed no significant increased mortality.
ICD therapies are not designed for treating underlying atrial arrhythmias, hence they are frequently repeated in a short time span. Therefore, inappropriate shocks or ATP may have an additional deleterious effect on an already vulnerable myocardium and thus potentially affect the tachy-arrhythmia – heart failure – mortality cascade.
Limitations
This study was not powered to perform any subgroup analysis, such as gender, the effect of amiodarone or comparison between ejection fraction ≤35% and >35%. The current findings are limited to patients with ischemic cardiomyopathy. The small sample size was the maximal sample size achievable in our patient population.
Although the exclusion criteria were set up to exclude any case of T-wave memory, it is impossible to eliminate any ventricular pacing. The longer time interval between the last shock and the post-shock ECG limits the comparison with previous literature and since this interval differed between patients the effect of timing between the inappropriate shocks could not be studied. Correlation analysis was performed between the number of inappropriate shocks received and ECG changes. However, because of variable time intervals between shocks, this analysis should be interpreted with caution. Finally, hemodynamic consequences of ICD shocks, which also could be linked with mortality, have not been addressed in this study.
Conclusion
Multiple inappropriate shocks were not found to cause electrocardiographic pro-arrhythmic changes 19 h after shocks detected by ECG analyses that are known to provide arrhythmic and other risk indicators. However, transient ECG changes could have been present and warrant future research.
Funding information
This work was supported by the European Community’s Seventh Framework Program FP7: EU-CERT-ICD (grant agreement no. HEALTH-F2-2013-602299).
R.W./J.E./C.G. received research funding from Biotronik, Boston Scientific Belgium and Medtronic Belgium. R.W./J.E./C.G. have received speakers- and consultancy fees from and participated in clinical trials by different manufactures of cardiac implantable electronic devices (Medtronic, Boston Scientific, Biotronik, St Jude Medical, Sorin). R.W./J.E. are supported as a postdoctoral clinical researcher and C.G. as predoctoral clinical researcher by the Fund for Scientific Research Flanders (FWO).
GG/SVH: Research Council KUL: CoE PFV/10/002 (OPTEC); PhD/Postdoc grants Flemish Government: FWO: projects: G.0427.10N (Integrated EEG-fMRI), G.0108.11 (Compressed Sensing) G.0869.12N (Tumor imaging) G.0A5513N (Deep brain stimulation); PhD/Postdoc grants IWT: projects: TBM 080658-MRI (EEG-fMRI), TBM 110697-NeoGuard; PhD/Postdoc grants iMinds Medical Information Technologies SBO2015, ICON: NXT Sleep Flanders, Belgian Federal Science Policy Office: IUAP P7/19/(DYSCO, “Dynamical systems, control and optimization”, 2012–2017) Belgian Foreign Affairs-Development Cooperation: VLIRUOS programs EU: EU: The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Program (FP7/2007–2013)/ERC Advanced Grant: BIOTENSORS (n 339804).
EU funding: RECAP 209G within INTERREG IVB NWE program, EU MC ITN TRANSACT 2012 (n316679), ERASMUS EQR: Community service engineer (n 539642-LLP-1-2013).
M.M./K.H. receive British Heart Foundation grants PG/12/77/29857 and PG/13/54/30358.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348.
- Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017.
- Pinski SL, Fahy GJ. The proarrhythmic potential of implantable cardioverter-defibrillators. Circulation. 1995;92:1651–1664.
- Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275–2283.
- Sweeney MO. The contradiction of appropriate shocks in primary prevention ICDs: increasing and decreasing the risk of death. Circulation. 2010;122:2638–2641.
- Topaloglu S, Aras D, Sahin O, et al. QT dispersion significantly increases after implantable cardioverter-defibrillator shocks. Ann Noninvasive Electrocardiol. 2007;12:44–49.
- Gurevitz O, Yaacoby E, Segal E, et al. Effect of implantable cardioverter-defibrillator shocks on QT dispersion. Am J Cardiol. 2000;86:1146–1148. A9.
- Moubarak JB, Karasik PE, Fletcher RD, et al. High dispersion of ventricular repolarization after an implantable defibrillator shock predicts induction of ventricular fibrillation as well as unsuccessful defibrillation. J Am Coll Cardiol. 2000;35:422–427.
- Sweeney MO. Point: implantable cardioverter-defibrillator shocks for ventricular tachyarrhythmias increase mortality. Heart Rhythm. 2012;9:985–987.
- Hnatkova K, Ryan SJ, Bathen J, et al. T-wave morphology differences between patients with and without arrhythmic complication of ischemic heart disease. J Electrocardiol. 2001;34 Suppl:113–117.
- Zabel M, Malik M, Hnatkova K, et al. Analysis of T-wave morphology from the 12-lead electrocardiogram for prediction of long-term prognosis in male US veterans. Circulation. 2002;105:1066–1070.
- Reddy BR, Xue Q, Zywietz C. Analysis of interval measurements on CSE multilead reference ECGs. J Electrocardiol. 1996;29 Suppl:62–66.
- Johannesen L, Garnett C, Malik M. Electrocardiographic data quality in thorough QT/QTc studies. Drug Saf. 2014;37:191–197.
- Malik M, Acar B, Gang Y, et al. QT dispersion does not represent electrocardiographic interlead heterogeneity of ventricular repolarization. J Cardiovasc Electrophysiol. 2000;11:835–843.
- Batchvarov V, Hnatkova K, Malik M. Assessment of noise in digital electrocardiograms. Pacing Clin Electrophysiol. 2002;25:499–503.
- Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36:1749–1766.
- Pang Y, Jin Q, Zhang N, et al. High-energy defibrillation increases the dispersion of regional ventricular repolarization. J Interv Card Electrophysiol. 2011;32:81–86.
- Lampert R, Soufer R, McPherson CA, et al. Implantable cardioverter-defibrillator shocks increase T-wave alternans. J Cardiovasc Electrophysiol. 2007;18:512–517.
- Zabel M, Acar B, Klingenheben T, et al. Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation. 2000;102:1252–1257.
- Ruwald AC, Schuger C, Moss AJ, et al. Mortality reduction in relation to implantable cardioverter defibrillator programming in the Multicenter Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT-RIT). Circ Arrhythm Electrophysiol. 2014;7:785–792.
- Powell BD, Saxon LA, Boehmer JP, et al. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J Am Coll Cardiol. 2013;62:1674–1679.
- Gasparini M, Proclemer A, Klersy C, et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309:1903–1911.
- Cay S, Canpolat U, Ucar F, et al. Programming implantable cardioverter-defibrillator therapy zones to high ranges to prevent delivery of inappropriate device therapies in patients with primary prevention: results from the RISSY-ICD (Reduction of Inappropriate ShockS bYInCreaseD zones) trial. Am J Cardiol. 2015;115:1235–1243.
