Abstract
Purpose. Transcatheter aortic valve implantation (TA-TAVI) is a well-established treatment for aortic valve stenosis in high-risk patients and indications have been continuously expanding to also include intermediate-risk patients. However, in low-risk patients, experiences are still sparse and although clinical outcomes have been shown favorable results, HRQoL has remained unexplored. The aim of this report was to describe the long-term health-related quality-of-life (HRQoL) in low-risk patients randomized to TA-TAVI or surgical aortic valve replacement (SAVR). Methods. In a prospective, randomized trial, patients with aortic valve stenosis were randomized to either TA-TAVI or SAVR. TA-TAVI was performed through a mini thoracotomy with the introduction of prosthesis via the apex of the heart and antegradely advancement over the pre-dilated native valve. SAVR was performed during cardiopulmonary bypass with resection of the native valve and replacement with a prosthesis valve through a median sternotomy. Afterwards, patients were followed yearly with echocardiography and HRQoL assessment. Results. A total of 58 patients were included; 29 patients for TA-TAVI and 29 patients for SAVR. The only difference in HRQoL was found in the physical component summary after 1 year; 44 ± 9 in the TA-TAVI group compared with 36 ± 9 in the SAVR group, p = .03. There were no differences in any of the remaining timepoints in neither physical nor mental component summary, p = .19 and p = .98, respectively, and there were no differences in survival during the 5 years. Conclusions. In low-risk patients with aortic valve stenosis undergoing TA-TAVI, no differences appeared in HRQoL compared with SAVR during a 5-year follow-up period.
Introduction
The transcatheter stent valve concept was introduced in 1989 by Andersen et al. [Citation1] as an alternative to conventional aortic valve replacement, and in 2002, Cribier et al. [Citation2] performed the first transcatheter aortic valve implantation in a human. Their success was rapidly followed by numerous studies showing favorable short-term outcomes in inoperable, high-risk patients,[Citation3–5] and the promising outcomes as well as improving technology, which led to the development of a transapical access (TA-TAVI).[Citation6]
Since then, an increasing number of procedures has been performed worldwide [Citation7] and with the recently published 5-year outcomes from the PARTNER trial, it appears that TA-TAVI has long-term durability.[Citation8] Moreover, indications have rapidly expanded to also include intermediate-risk patients.[Citation9] Although TA-TAVI, in some countries, has now become the treatment of choice for degenerative aortic valve stenosis, experiences in low-risk patients remain sparse. Having the good results in high-risk patients in mind, it is of little surprise that life expectancy is also favorable in low-risk patients.[Citation10,Citation11] However, when it comes to health-related quality-of-life (HRQoL), the positive results achieved in high-risk cohorts are not necessarily transferrable onto patients who are younger, more physically active, and not least having other motivations for undergoing treatment.[Citation3,Citation12–18] This is essential because low-risk patients have a higher post-interventional life expectancy than high-risk patients, and long-term HRQoL is therefore of even greater importance. Moreover, it is well-established that the definition of a good procedural outcome should include not only mortality but also a HRQoL component.[Citation19]
In a prospective, randomized study, the aim of this report was to describe the long-term HRQoL in low-risk patients undergoing TA-TAVI compared with patients undergoing surgical aortic valve replacement (SAVR).
Materials and methods
The Danish Data Protection Agency and The Regional Committee on Biomedical Research Ethics of the Central Denmark Region approved the study, which conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study is listed on clinicaltrials.gov (NCT00986193) and according to Danish law, written informed consent was obtained for each study participant. A Data Safety Monitoring Board (DSMB) was appointed before the inclusion of patients, and after inclusion of 59 patients at the Department of Cardiothoracic Surgery and Department of Cardiology, Aarhus University Hospital, Denmark, the study was terminated prematurely after advice from the DSMB as previously described.[Citation10]
Study design
The study was planned as a prospective, multicenter clinical trial with a 1:1 computer-generated randomization of patients to either TA-TAVI or SAVR. Randomization was performed using the web-based, clinical trials support system “TrialPartner”, whereby security demands and guidelines provided by the National Data Protection Agency were met. The data presented in this report were collected only on the subset of patients, who were included at our institution: Aarhus University Hospital, Denmark.
Study participants
All patients were assessed for eligibility at weekly, interdisciplinary Heart Valve Team meetings with participation of cardiologists, cardiac surgeons, and cardiac anesthesiologists. Inclusion criteria were: (1) aortic valve area less than 1 cm2, (2) age above 70 years (increased to 75 years during the study after advice from DSMB), (3) accessible for both SAVR and TA-TAVI, (4) more than 1 year expected survival following intervention. Exclusion criteria were: (1) coronary artery disease to be treated with percutaneous coronary intervention or coronary artery bypass grafting, (2) previous myocardial infarction, (3) PCI within 12 months of intervention, (4) need of other heart surgery, (5) emergency surgery (within 24 h of indication for intervention), (6) unstable cardiac condition (assist device, inotropes, intravenous nitrates in operating room), (7) continuous infection requiring antibiotics, (8) stroke within 1 month of intervention, (9) FEV1 below 40% of expected, (10) renal failure requiring hemodialysis, (11) allergy to acetylic acid, clopidogrel, prasugrel, or X-ray contrast. Eligible patients received detailed information on the study by a study coordinator and a cardiothoracic surgeon before randomization.
Procedures
The interventions are previously described in detail elsewhere.[Citation10] Briefly, TA-TAVI was performed through a mini thoracotomy with a 23 or 26 mm Edwards SAPIEN balloon-expandable Heart Valve system (Edwards Lifesciences). The prosthesis was advanced antegradely over the pre-dilated native valve via the apex of the heart and correct positioning was ensured by transesophageal echocardiography (TEE) and fluoroscopy. A heart-lung machine was present, in case of urgent need for hemodynamic support or conversion to open surgery. SAVR was performed during cardiopulmonary bypass through a median sternotomy. After resection of the native valve, the aortic annulus was measured to ensure the correct size of the PERIMOUNT aortic heart valve bioprosthesis (Edwards Lifesciences). Sub-annular, felt-armed sutures tied the prosthesis valve into place and peroperative performance of the bioprosthesis was checked using TEE.
Post-interventional follow-up
Patients were followed up yearly with HRQoL assessment and echocardiography. HRQoL assessment was performed using the SF-36 Health Survey Questionnaire, which has a well-established validity and reliability.[Citation20] The questionnaire is a self-administered instrument that evaluates the HRQoL of the preceding 4 weeks, and does not target any specific disease or treatment group. It consists of 36 questions covering eight general health dimensions that can be summarized into a physical component summary and a mental component summary. The components are scaled from 1 to 100, with increasing score indicating higher subjective perception of HRQoL.
Endpoints
The primary endpoint was HRQoL evaluated by the SF-36 health survey at 1 year, 2 years, 3 years, 4 years, and 5 years after intervention. A secondary endpoint was the overall survival of 5 years after the intervention.
Statistical analysis
Data are analyzed and presented as intention-to-treat analyses. Continuous, normally distributed variables are reported as means ± standard deviations and compared using unpaired Student’s t-tests or two-way analyses of variance (ANOVA). Continuous, non-normally distributed data are reported as median with total range and compared using the Mann–Whitney–Wilcoxon rank-sum tests. Binary data are presented as absolute numbers and percentages of patients are compared using chi-squared test or the Cochran–Mantel–Haenszel test. All tests were two-sided and a p-value below 0.05 was considered statistically significant. Inclusion of 200 patients was planned; details of the sample size calculation are previously described.[Citation10] All analyses were performed with STATA/IC 12.1 for Mac (StataCorp, College Station, TX).
Results
In the period from November 2008 to May 2011, a total of 59 patients were included at our institution, Aarhus University Hospital, Denmark. One patient randomized to SAVR withdrew consent prior to intervention resulting in 29 patients in the TA-TAVI group and 29 patients in the SAVR group. In total, five patients crossed over from TA-TAVI to SAVR, whereas one patient crossed over oppositely. Baseline characteristics of the two groups are shown in , and a detailed patient flowchart is presented in . As displayed, the two groups were basically similar. As surgical risk predictors logistic EuroSCORE was 8.8 ± 0.8% and 9.9 ± 0.7% and The Society of Thoracic Surgeons Predicted Risk of Mortality (STS) score was 3.2 ± 0.3% and 3.5 ± 0.2% in the TA-TAVI group and the SAVR group, respectively.
Figure 1. Patient flowchart. Flowchart displaying the total number of patients screened for eligibility, the number of eligible patients, and the patients included in the study. One patient withdrew consent prior to intervention and was not included in data analyses. TA-TAVI: transapical transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement.
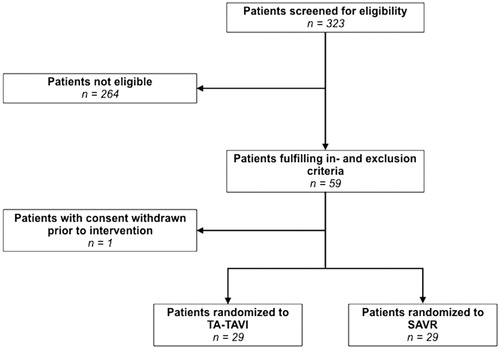
Table 1. Baseline characteristics of patients undergoing transapical transcatheter aortic valve implantation or surgical aortic valve replacement.
Health-related quality-of-life
Quality-of-life during follow-up in the two groups is shown in and graphically illustrated in . There was no overall difference in either physical or mental component summary, p = .19 and p = .98, respectively. At one timepoint, after 1 year, the physical component summary was higher in the TA-TAVI group (44 ± 9) compared with the SAVR group (36 ± 9), p = .03. There were no significant differences at any of the remaining timepoints. Each of the eight HRQoL dimensions after 5 years of follow-up are displayed in , and as shown there were no differences between the groups in any of the dimensions.
Figure 2. Quality-of life (SF-36). Graphical description of quality-of life (SF-36) divided in physical composite summary (A) and mental composite summary (B) during 5 years of follow-up in patients undergoing TA-TAVI (n = 29) or SAVR (n = 29). Horizontal lines indicate standard deviations. TA-TAVI: transapical transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement.
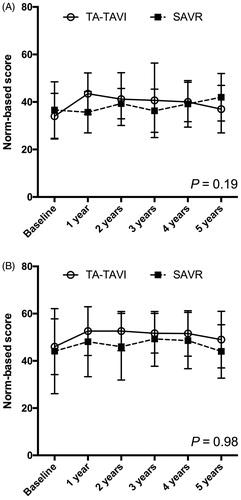
Table 2. Quality-of life (SF-36) in patients undergoing transapical transcatheter aortic valve implantation or surgical aortic valve replacement during 5 years of follow-up.
Table 3. Quality-of life (SF-36) in patients undergoing transapical transcatheter aortic valve implantation or surgical aortic valve replacement after 5 years of follow-up.
Survival outcomes
All patient deaths in the first year occurred within the first month; outcomes that have already been described in detail elsewhere.[Citation10] In short, the 1-year survival rates were 26/29 (90%) and 27/29 (93%) in the TA-TAVI and SAVR groups, respectively. In the TA-TAVI group, one patient died on the waiting list, one died after the intervention due to left coronary artery obstruction, and one died from respiratory problems 38 days after the procedure. In the SAVR group, one patient died from a brain hemorrhage, and another patient died from undefined reasons. Two-year survival rates were 25/29 (86%) in both groups after two years. In the TA-TAVI group, one patient died from a cerebral infarction, and in the SAVR group, one patient died from acute coronary syndrome, whereas another died from undefined reasons. Three-year survival rates were 25/29 (86%) and 24/29 (83%) in the TA-TAVI and SAVR groups, respectively. In the SAVR group, one patient died due to pelvic cancer, whereas no additional deaths had occurred in the TA-TAVI group. Four-year survival rates were 25/29 (86%) and 24/29 (83%) in the TA-TAVI and SAVR groups, respectively, as there were no additional deaths in any of the groups. Lastly, five-year survival rates were 25/29 (86%) and 22/29 (76%) in the TA-TAVI and SAVR groups, respectively. In the SAVR group one patient died from cardiac arrest and another patient died from general frailty, whereas there were no additional deaths in the TA-TAVI group.
After 5 years of follow-up, there were no differences between the groups in any of the echocardiographic measures. Left ventricular ejection fraction was 55 ± 7% and 55 ± 5% (p = .92) and aortic valve area was 1.1 ± 0.6 cm2 and 1.0 ± 0.4 cm2 (p = .32) in the TA-TAVI group and SAVR group, respectively. In the TA-TAVI group, one patient had moderate paravalvular leakage, whereas there was no paravalvular leakage in the SAVR group (p = .15).
Discussion
In low-risk patients with aortic valve stenosis, HRQoL was favorable after TA-TAVI and comparable to patients after SAVR. Likewise, as expected, 5-year survival rate was good and adverse outcomes were generally few. The positive result on HRQoL is an important contribution to the existing knowledge on this field as it favors a further expansion of the clinical indications of TA-TAVI. However, since long-term durability is of even greater importance in low-risk patients with higher life expectancy than high-risk patients, and this report on low-risk patients is based on a small number of patients, there is a need for additional clinical outcome studies
Several studies have assessed HRQoL after transcatheter aortic valve implantation, but importantly, they are all performed in high-risk patients.[Citation3,Citation12–18] Overall, these studies have shown that TA-TAVI generally improves HRQoL compared to their baselines, and the same has been demonstrated in a recently performed meta-analysis.[Citation21] Nevertheless, none of these studies address the more important question: can these patients expect HRQoL comparable with patients who have instead undergone SAVR? Yet, only two studies have been conducted to address this question.[Citation12,Citation14] Similar to us, Gada et al. [Citation14] demonstrated no difference in HRQoL in TA-TAVI patients compared with SAVR patients up to one year after the procedure, and the lack of early HRQoL benefit may seem surprising given that TA-TAVI is a less invasive procedure than SAVR. Nevertheless, it is previously shown that thoracotomy results in greater postoperative pain than median sternotomy due to rib spreading and respiratory motion,[Citation22] and therefore, procedural modifications and localized analgesia may be beneficial in patients undergoing TA-TAVI.[Citation23] Similar to our findings, Amonn et al. [Citation12] found no difference in HRQoL between TA-TAVI patients and SAVR patients five years after treatment. The demonstration of a favorable, long-term HRQoL is clearly of great significance in the low-risk patients as they are expected to live for many years after the intervention.
As mentioned, the reports published so far on HRQoL after TA-TAVI only included high-risk patients. The lack of studies in low-risk patients is important as results achieved in high- or intermediate-risk patients do not necessarily apply to low-risk-patients. This is illustrated by the fact that Amonn et al. [Citation12] reported substantially higher values of physical and mental health (50.0 ± 21 and 75.7 ± 15, respectively) than us after a similar follow-up period even though they included patients of significantly higher risk (logistic EuroSCORE of 26.5 ± 16.1% and STS score of 6.7 ± 3.8%). A potential explanation for this is that highly symptomatic patients often have a strong motivation for having the procedure as they hope to obtain symptom relief, i.e. gaining a better HRQoL. In contrast, patients without significant functional impairment often have high HRQoL before the procedure, and it is reasonable to assume that this discrepancy in motivation affects self-estimated health after the intervention. In essence, such differences in motivational factors between low- and high-risk patients are important to consider as they indicate that the various aspects of HRQoL weighs differently in the two cohorts. Therefore, even though there is no difference in HRQoL between TA-TAVI and SAVR in high-risk patients, the same is not necessarily the case in low-risk patients. In other words, this report’s demonstration of comparable HRQoL in low-risk patients undergoing either TA-TAVI or SAVR is an important contribution to the knowledge on this field.
Secondary to the HRQoL data, this report also presents the survival data during the 5-year follow-up, and as expected, the survival rate was generally high after TA-TAVI. This is despite the fact that the TA-TAVI procedures in this study were performed with 1st generation device systems only. Since then major advances have emerged and the newer generations have smaller delivering systems, a larger 29mm valve size, and an outer skirt to prevent the paravalvular regurgitation that is associated with lower survival rates.[Citation7,Citation8,Citation24–26] Moreover, in the current study pre-procedural measurements of the aortic annulus was performed with transesophageal echocardiography as opposed to today’s use of multi-slice computed tomography. The pre-procedural visualization method is very likely to be responsible for some of the adverse events leading to premature determination of this study.
Originally, the TA-TAVI technique was developed as a less invasive alternative to SAVR in high-risk patients with aortic valve stenosis. Since then, indications have expanded to include intermediate-risk patients also, and in some countries TA-TAVI is now the treatment of choice for all patients with degenerative aortic valve stenosis. Nevertheless, the evidence base for using TA-TAVI in patients with only low surgical risk should include also HRQoL as low-risk patients have different standards when it comes to physical and mental well-being than high-risk patients. Therefore, this report is an important contribution to the existing knowledge on this field facilitating a further widening of the clinical indications for TA-TAVI to also include low-risk patients with aortic valve stenosis.
Limitations
The original survival study [Citation10] was designed to include 200 patients based on a sample size calculation with survival as the main endpoint, but unfortunately, the study was terminated prematurely after inclusion of 59 patients. Indeed, this may limit the power of the current report. However, no sample size calculation was performed on the secondary endpoints such as HRQoL, and the included numbers in the two groups should be enough to demonstrate a clinically meaningful difference on this endpoint. In addition, our results show no tendency of poorer HRQoL in the TA-TAVI group, which supports the conclusion drawn in this report.
Conclusion
In this prospective, randomized trial no differences appeared in HRQoL after TA-TAVI compared with SAVR in low-risk patients with degenerative aortic valve stenosis. Likewise, as expected 5-year survival rates and clinical outcomes are favorable, and perspectively, our results may contribute to further expansion of clinical indications including also low-risk patients. Nevertheless, clinical outcome studies remain warranted in order to ensure the long-term durability.
Notes on contributors
Christian E. Rex is an over graduate medical student, who is currently serving as a research fellow under supervision of Vibeke E. Hjortdal at Aarhus University Hospital. His current research is mainly centered around adults born with ventricular septal defect.
Johan Heiberg is a postdoctoral research fellow at Aarhus University Hospital, who obtained his Ph.D. degree in 2014 in research centered around adults with congenital heart disease. Besides congenital heart disease his current research interests are implementation of point-of-care ultrasound into clinical practice and postoperative quality of recovery after various kinds of surgery. He has currently published more than 20 publications in international, peer-reviewed journals.
Kaj-Erik Klaaborg is a consultant and cardiothoracic surgeon at Aarhus University Hospital, and his primary clinical and research interest is transapical transcatheter aortic valve implantation.
Vibeke E. Hjortdal is a professor in cardiothoracic surgery at Aarhus University Hospital. Her main research interest is congenital heart disease and she has co-authored more than 200 publications in international, peer-reviewed journals.
Acknowledgements
The authors are deeply thankful to V. Laursen for her valuable contribution to data collection.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation, all authors meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors, and all authors are in agreement with the manuscript.
References
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008.
- Lefévre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation. 2006;114:591.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850.
- Huber CH, Nasratulla M, Augstburger M, et al. Ultrasound navigation through the heart for off-pump aortic valved stent implantation: new tools for new goals. J Endovasc Ther. 2004;11:503–510.
- Abdel-Wahab M, El-Mawardy M, Richardt G. Update on transcatheter aortic valve replacement. Trends Cardiovasc Med. 2015;25:154–161.
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. New Engl J Med. 2016;374:1609–1620.
- Nielsen HHM, Klaaborg K-E, Nissen H, et al. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. Eurointervention. 2012;8:383–389.
- Thyregod HGH, Steinbrüchel DA, Ihlemann N, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194.
- Amonn K, Stortecky S, Brinks H, et al. Quality of life in high-risk patients: comparison of transcatheter aortic valve implantation with surgical aortic valve replacement. Eur J Cardiothorac Surg. 2013;43:34–41.
- Georgiadou P, Kontodima P, Sbarouni E, et al. Long-term quality of life improvement after transcatheter aortic valve implantation. Am Heart J. 2011;162:232–237.
- Gada H, Kirtane AJ, Wang K, et al. Temporal trends in quality of life outcomes after transapical transcatheter aortic valve replacement: a placement of AoRTic TraNscathetER valve (PARTNER) trial substudy. Circ Cardiovasc Qual Outcomes. 2015;8:338–346.
- Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (placement of aortic transcatheter valve) trial. Circulation. 2014;129:2682–2690.
- Bona V, Khawaja MZ, Bapat V, et al. Early and late changes in quality of life following transcatheter aortic valve implantation using the transfemoral and transapical approaches. EuroIntervention. 2015;11:221–229.
- Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972.
- Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (placement of AoRTic TraNscathetER valve) trial (cohort A). J Am Coll Cardiol. 2012;60:548–558.
- Arnold SV, Spertus JA, Lei Y, et al. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–597.
- Garratt A, Schmidt L, Mackintosh A, et al. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ. 2002;324:1417.
- Kim CA, Rasania SP, Afilalo J, et al. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160:243–254.
- Grossi EA, Zakow PK, Ribakove G, et al. Comparison of post-operative pain, stress response, and quality of life in port access vs. standard sternotomy coronary bypass patients. Eur J Cardiothorac Surg. 1999;16:39–42.
- Amat-Santos IJ, Dumont E, Villeneuve J, et al. Effect of thoracic epidural analgesia on clinical outcomes following transapical transcatheter aortic valve implantation. Heart. 2012;98:1583–1590.
- Agarwal S, Tuzcu EM, Krishnaswamy A, et al. Transcatheter aortic valve replacement: current perspectives and future implications. Heart. 2015;101:169–177.
- Amat-Santos IJ, Dahou A, Webb J, et al. Comparison of hemodynamic performance of the balloon-expandable SAPIEN 3 versus SAPIEN XT transcatheter valve. Am J Cardiol. 2014;114:1075–1082.
- Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695.
