Abstract
Background. Reconstruction of the right ventricular outflow tract with a conduit is an established surgical procedure in congenital heart disease and reinterventions are common. Objective. An increasing number of patients have a conduit, but there are few population-based studies of long-term outcomes after conduit surgery, reoperations, and transcatheter pulmonary valve replacement. Methods. In April 2015, all adult patients with a conduit were identified in the Swedish National Registry for Congenital Heart Disease (SWEDCON). Data on patients who died before age of 16 years are not included in the registry and thus not included in the study. Results. We found 574 patients with a mean age 36.1 years. The largest proportion had tetralogy of Fallot (45%). In total there were 762 operations and 50 transcatheter pulmonary valve replacements. Mean age at first conduit operation was 20.2 years. Long-term survival up to 48 years including perioperative mortality (<1%) was 93% at 20 years. The most common cause of death was cardiac-related. Higher age at first conduit operation was associated with increased mortality risk. Reintervention-free survival was 77% and 54% at 10 and 20 years, respectively. Conduit reinterventions were common. Ten-year reintervention-free survival after first conduit reintervention (n = 176) was significantly lower than after first conduit operation (70% vs 77% p = .04). Higher age at first conduit operation was associated with a reduced risk of reintervention, whereas male sex and complex malformations were associated with increased risk of reintervention. Conclusions. The mortality of repeated conduit reinterventions is low. The need for reintervention of conduits is considerable, and reintervention-free survival after the first conduit reintervention is poorer than after first conduit implantation. The findings in this study only applies for patients reaching 16 years of age.
Introduction
With advancements in cardiac surgery and care, there are now more adults than children with complex congenital heart disease [Citation1,Citation2]. Reconstruction of the right ventricular outflow tract (RVOT) with a conduit is a long established surgical procedure in congenital heart disease (CHD). A conduit is also used in aortic valve disease operated with the Ross procedure where the native pulmonary artery is translocated to the aortic position and a conduit is used to reconstruct the RVOT. The majority of the conduits are homografts and valved dacron conduits. However, all of these conduits have limited durability, and conduit replacement or reintervention is often necessary during the patient’s lifetime [Citation3–7]. Conduit surgery and reintervention is a complex and highly specialized field of surgery, and is most often conducted in tertiary centers. Increasing data on right ventricular volume and function after pulmonary valve regurgitation [Citation8–11] has placed a focus on earlier surgery for pulmonary valve replacement (PVR) and reintervention for dysfunctioning conduits. Introduction of transcatheter PVR (TPVR) with the Melody® Transcatheter Pulmonary Valve or Edwards SAPIEN® Pulmonic Transcatheter Heart Valve has expanded the possibilities for interventions in failing conduits [Citation12,Citation13]. Robust information on mortality and event-free survival after conduit surgery and TPVR are essential for care decision-making and planning. Given that the durability of the conduit inevitably is shorter than the expected life span of the patient, studies on outcomes of repeated conduit replacements and TPVR are needed. In addition, PVR and conduit surgery are now among the fastest growing procedures in CHD surgery [Citation14,Citation15]. Reports on outcome after PVR and conduit replacement are, however, usually from single centers, local registries, and retrospective cohorts. This prompted us to investigate outcomes after conduit surgery in adults using the Swedish National Registry for Congenital Heart Disease (SWEDCON). This population-based approach, including all Swedish tertiary centers, limits selection bias and has full follow-up regarding mortality via the National Death Registry. Furthermore a publicly financed health care system with general and free access to care limits sociodemographic selection bias.
The aim of this study was to examine survival, reintervention-free survival, and predictors of survival in patients after first conduit surgery and after conduit reintervention using data from SWEDCON.
Methods
All seven Swedish tertiary centers participate in a register of adult congenital heart disease since 1998. The SWEDCON registry was created in 2009 with the merging of adult and pediatric cardiology registries into one national database. It comprises three parts: adult congenital heart disease (SWEDCON/GUCH), pediatric cardiology, and congenital heart surgery. SWEDCON/GUCH includes patients aged over 16 years who have attended the GUCH unit. Patients are provided with oral or written information regarding the registry and are included after giving consent. Patients not previously included in SWEDCON/pediatric cardiology have their historical surgical or interventional data added retrospectively, and thereafter all new data is input longitudinally, whereas patients previously included in SWEDCON/pediatric cardiology have their data transferred at the first visit to the GUCH unit. Data include baseline characteristics such as sex, birth date, and diagnosis as well as percutaneous interventions and surgical procedures. Longitudinal input of clinical data such as New York Heart Association (NYHA) functional class and ECG at each visit is performed by the attending physician or nurse. Surgical procedures or percutaneous interventions are either added at the time of the actual procedure or by a nurse or physician at a follow-up visit. SWEDCON/GUCH is also linked to the National Death Registry and updated monthly regarding deaths of registry patients.
The study was initiated after approval by the Regional Ethics Committee and authorization by the board of directors of the SWEDCON registry. We searched SWEDCON/GUCH in April 2015 for all adult patients with a right ventricle to pulmonary artery (RV to PA) conduit identified by their specific group variable. Identification of TPVRs and surgical procedures involving RV to PA conduits was effected using codes for classification of surgical procedures (www.nordclass.se/NCSP_1_16.pdf; Appendix). All procedures from inception to April 2015 were included. Procedures performed in both adulthood as well as childhood and adolescence were collected for further analysis. Reintervention free survival was defined as time from first conduit operation to death, conduit surgery, or TPVR. Conduit type is not systematically collected in the registry, only input as freeform text, and therefore was not used for further analysis. Patients were divided into three diagnostic groups according to the International Classification of Diseases, 10th Revision coding system: tetralogy of Fallot (TOF), Ross-operation for congenital aortic valve disease, and a group with mixed CHD involving the RVOT (pulmonary valve abnormalities or pulmonary atresia, DORV, truncus arteriosus, transposition of the great arteries or other abnormalities).
Validity of data
Validity was assessed by selecting 50 patients in SWEDCON, and their main diagnosis, surgical codes, and date of last surgical procedure were compared with information in their medical charts. Seven missing or false data were found in five patients, one (2%) regarding the main diagnosis and three (6%) regarding the surgical code and date of surgery respectively. The validity of all the data was 95%. SWEDCON is updated monthly regarding deaths, thus follow-up on mortality is close to 100%, and only misses those patients who move abroad.
Statistical analysis
Continuous and categorical data are presented as number, percentage, mean/median or number and percentage, respectively, as appropriate. Cumulative survival and cumulative reintervention-free survival were determined by the Kaplan-Meier method. Point estimates are presented as percentage and standard error (SE). Comparison of survival and event-free survival for different groups of diagnosis was performed by the log-rank test. Multivariable Cox regression was used to identify predictors of survival and reintervention-free survival, and hazard ratios (HRs) and 95% confidence intervals (CIs) were determined. Age at intervention was set as an independent variable, while diagnostic group and sex were covariates.
Two patients were excluded from the survival analysis because of missing data. However, both patients were alive at follow-up. In the reintervention-free survival analysis, eight patients were excluded because of missing or incomplete data.
All statistical tests were two-sided and a p-value <.05 was considered statistically significant. Statistical analysis was performed using SPSS version 20 (IBM Corp., Armonk, NY, USA).
Results
Study population
Approximately 1000 adult patients with previous RVOT surgery were registered in SWEDCON/GUCH in April 2015. A total of 574 patients who had a conduit were identified using a specific group variable for RV to PA conduits, and included 332 men (58%) and 242 women (42%) with a mean age of 36.1 years (median 32.3 years, range 18–78 years). The earliest procedure was in 1967. The largest group were patients with TOF (n = 257, 45%). A total of 812 surgical conduit operations or replacements, including 50 TPVRs, were reported. Median age at first conduit procedure was highest in the Ross group, at 22.7 years (). The majority of patients had one conduit procedure (n = 398); 107 patients had one reintervention, with surgical conduit replacement or TPVR; and 69 patients had two or more reinterventions (maximum four). The mean number of conduits and reinterventions was 1.4 per patient. There were 50 registered TPVRs in 49 patients.
Table 1. Study population.
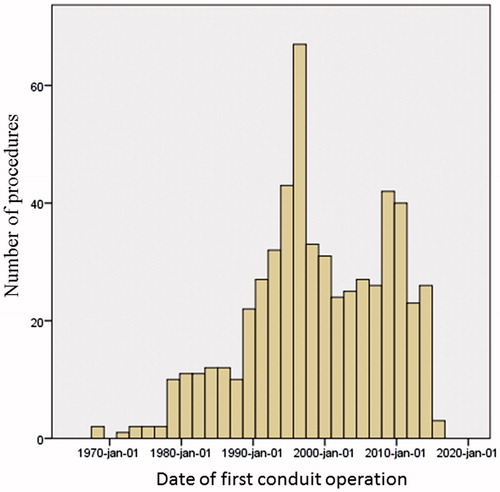
The numbers of procedures by date are presented in . The distribution of TOF and mixed CHD was similar across the timespan. However, the number of Ross procedures showed a distinct peak in the mid to late 1990s, thus explaining a peak in number of conduit operations in that time period. Analysis of reintervention-free survival across time revealed no indication that outcomes were different in any era.
Mortality
Mean follow-up time was 15.9 years (95% CI: 15.1–16.7). In survival analysis (n = 572) from the first conduit operation, the maximum follow-up time was 48 years. Two patients were excluded from analysis because of incomplete data. There were 32 deaths at a mean age of 44 years and the majority of the patients had TOF (n = 18). Mean age at first conduit operation was 32 years (median 34 years, range 3–66) and mean time from latest conduit operation or conduit reintervention to death was 8.5 years (median, 5 years), ranging from 0 to 26.5 years. For 28 of these patients, data on NYHA functional class and QRS width was available. Mean QRS width was 147 ms (range, 80–220) at the latest visit prior to death. Severe functional impairment, i.e., NYHA class 3 or 4, was seen in 14 patients (50%). Only one patient was reported to be NYHA class 1. Cause of death was stated in 28 patients, and the most common cause was cardiac-related (n = 21) of which seven were sudden cardiac death. Three deaths were within 30 days after the first conduit operation. No deaths were reported within 30 days after any conduit reintervention. One patient died during heart transplantation and was not included as a perioperative death.
Cumulative survival, including postoperative 30 day mortality, at 5, 10, 15, and 20 years was 97 ± 1.1%, 96 ± 1.3%, 93 ± 1.9%, and 93 ± 2,0%, respectively, in patients with TOF. In patients with mixed CHD, survival was 98 ± 1.0%, 97 ± 1.2%, 95 ± 1.5%, and 94 ± 1.8%; respectively. In patients with Ross operation for congenital aortic valve disease, survival was 100%, 99 ± 1.5%, 99 ± 1.5%, and 99 ± 1.5%, respectively. There was only one death in the Ross group. Survival after the first conduit operation did not differ among the three diagnostic groups (log-rank p = .192) ().
Figure 2. Kaplan-Meier estimate of patient survival after first conduit operation. Early postoperative deaths are included (n = 3). Mean age at first conduit operation was 20.2 years (95% confidence interval: 18.9–21.6); median age was 16.2 years. In up to 48 years follow-up from first conduit operation, 238 conduit reoperations and TPVRs were conducted in the cohort.
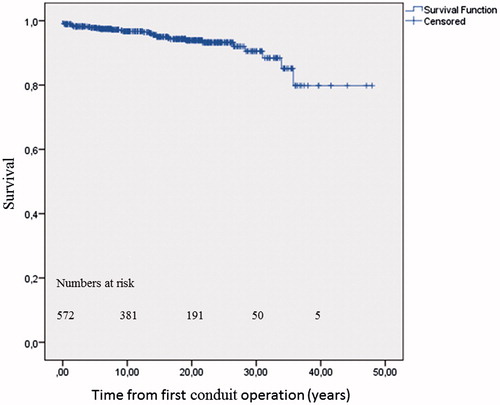
Further analysis using a Cox regression model with the covariates of sex, diagnostic group, and age, identified that higher age at first conduit implantation was associated with increased mortality (HR: 1.07 per year of age (95% CI: 1.05–1.09; p < .0001). Thus, for a 10-year increase in age at first conduit operation the risk of death was doubled. Covariates of sex (p = .24) and diagnostic group (p = .15) showed no statistical significance.
Reintervention-free survival
There were 566 patients included in the reintervention-free survival analysis. Reintervention-free survival after first conduit operation was 89 ± 1%, 77 ± 2%, 63 ± 2%, and 54 ± 3%, at 5, 10, 15, and 20 years, respectively. Median estimated reintervention-free survival was 22.3 years (95% CI: 18.4–26.2). Further analysis according to diagnostic group showed an estimated mean and median reintervention-free survival of 27.5 years (95% CI: 24.2–30.8) and 30.9 (95% CI: 18.6–43.3) in TOF patients (). There was a significant difference in reintervention-free survival between diagnostic groups (log-rank p < .001). The group with mixed CHD had an increased risk compared with patients with TOF and the Ross operation. Cox regression identified that male sex was associated with adverse events, and higher age at first conduit operation had a protective association with event-free survival ().
Figure 3. Kaplan-Meier estimates of reintervention-free survival from first conduit operation according to diagnosis. A: tetralogy of Fallot; B: mixed congenital heart disease; and C: Ross operation for aortic valve disease. Log-rank P < 0.001.
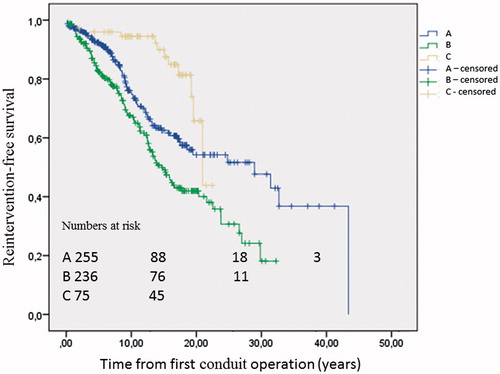
Table 2. Cox regression analysis of factors associated with reintervention-free survival after first conduit operation and after first conduit reintervention.
Conduit reintervention
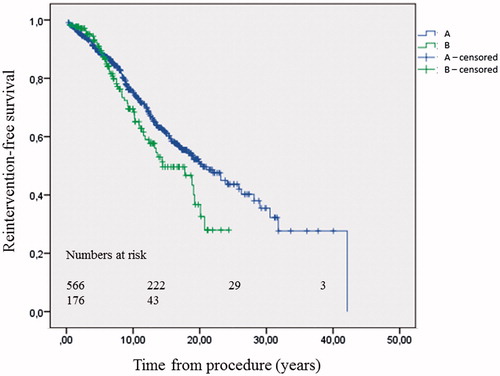
Reintervention-free survival was determined in patients with a second conduit operation or TPVR thus the first conduit reintervention (n = 176). Patients were mainly from the group with mixed CHD (57%) and had a mean age at first conduit operation of 8.6 years (median, 4.6 years). Mean age at first conduit reintervention was 19.1 years and mean follow-up was 9.3 years, (range, 0–26.7 years). Estimated median event-free survival was 15.7 years (95% CI: 10.5–20.8). Point estimates at 10 and 20 years were 70 ± 4% and 47 ± 6%, respectively. In Cox regression analysis, higher age at first conduit operation was now associated with adverse events whereas higher age at conduit reintervention showed a protective association (). Reintervention-free survival time after first conduit reintervention (n = 176) was significantly shorter than after first conduit operation (log-rank p = .04, ).
Figure 4. Kaplan-Meier estimates of reintervention-free survival after first conduit operation, n = 566 (A) and first conduit reintervention, n = 176 (B). Log-rank P = .04.
There were 54 patients with two or more conduit reinterventions. Point estimated reintervention-free survival from the second conduit reintervention was analyzed in 54 patients (37 with mixed CHD; mean age, 21.7 years) and was 72 ± 8% at 10 years. Analysis was however limited because of the small numbers ().
Table 3. Characteristics of patients with conduit reintervention.
Discussion
This unselected national cohort is to our knowledge on of the largest studies of with RV to PA conduits. We used a Swedish national registry to retrospectively study mortality and reintervention-free survival in patients after first conduit operation as well as after conduit reintervention. In Sweden, the standard practice for reconstruction of the RVOT has been implantation of a conduit. Surgical implantation of a valve prosthesis in native RVOT for patients with TOF is uncommon. Furthermore, unpublished data from our group suggests that more than 70% of the conduits were homografts. Patients with conduits are a heterogenic group regarding anatomy and previous surgery for example transannular plasty for TOF. Data on risk factors for reduced event-free survival have previously been identified, namely younger age, non-anatomical position, small conduit size, and conduit type [Citation5,Citation7,Citation16–19]. However, these data were mainly from studies of children, and data from adults were not necessarily presented separately. Outcome data is highly dependent on the indication for PVR. Interpretation of our results and implications for clinical practice should therefore be in the light of past and future indications as well as new methods for reintervention. Optimal timing of PVR and reinterventions is not addressed in this study.
Mortality
In the present study of patients with conduit implantation, we studied late mortality, with up to 48 years follow-up after first conduit operation. Long-term survival was good, with more than 90% survival at 20 years follow-up. Long-term mortality data consisted of both accumulated perioperative mortality at the first conduit operation and reintervention as well as mortality from other causes, predominantly cardiac death. Perioperative mortality was however low, less than 1%. Increasing age at first conduit operation was associated with an increase in long-term mortality in this study. This is supported by a previous report consisting mainly of children which also identified older age at conduit operation as a risk factor for late mortality [Citation7]. One possible explanation is that late correction with a conduit is associated with long-term pulmonary valve stenosis or regurgitation prior to surgery and subsequent remodeling of the RV which could later lead to adverse outcomes [Citation9]. Furthermore, patients with late corrections may have had long time care with a systemic to PA shunt prior to surgical reconstruction, and thereby would have been exposed to systemic ventricle volume loading. Both these explanations could contribute to increased late mortality. Moreover, cardiac death was the most common cause of death and the fact that more than 50% of the patients were in NYHA 3 or 4 at their latest visit prior to death indicated that patients could be suffering from right ventricular failure prior to death. Our finding of increased mortality risk after late surgical correction with a conduit is intriguing and warrants further evaluation. Furthermore, it could contribute to the ongoing discussion of timing of PVR.
Reintervention-free survival
In this study reintervention-free survival after the first conduit operation was estimated to be 77% and 54% at 10 and 20 years, respectively. This is in line with other studies of adults with conduits [Citation3,Citation20] and in line with or better than studies predominantly of children [Citation5,Citation7,Citation18]. Male sex was associated with shorter reintervention-free survival in our study. There are previously reported data on increased risk of conduit failure in male children [Citation5]. There are however, to our knowledge, no data on shorter reintervention-free survival in male than in female patients. This finding is not easily explained and needs further evaluation.
A diagnosis of mixed CHD, including complex lesions and non-anatomical position, was associated with decreased reintervention-free survival in the present study. This was previously described in several studies in both children and adults [Citation5,Citation7,Citation17–19]. Patients who had the Ross procedure (thus normal anatomical position and RV) had the best outcome, with very limited intervention at 10 years, indicating the longevity of the conduit in optimal anatomical conditions.
Our finding of protective hazard ratio with increasing age is consistent with studies that found that younger age was a dominant risk factor for conduit failure in younger patients [Citation5,Citation21,Citation22]. Furthermore, there was a previously described interaction between age, diagnosis, and small conduit size [Citation5,Citation18].
Conduit reintervention
Reintervention-free survival was found to be around 70% at 10 years in patients after conduit reinterventions. Other studies have concluded that patients undergoing reoperation with conduit replacement have equal to (or longer) freedom from conduit dysfunction [Citation5,Citation18,Citation22,Citation23]. However, there are also reports that conduit reoperations are associated with increased risk of hemodynamic conduit dysfunction in adults [Citation20]. Comparison of outcomes for second compared with first operation is however a complex task since there is no independence between groups and there could be a correlation between reintervention-free survival of the first conduit operation and conduit reintervention, for example related to anatomical or immunological factors. Moreover, in this study there were more complex patients in the conduit reintervention group. Reintervention-free survival appeared shorter after the first conduit reintervention compared with the first conduit operation. This raises concern for the future and the growing numbers of patients in need of often repeated conduit reinterventions.
Patients with many reinterventions will likely be even more common in the future with increasing complexity in the planning of care. Previous reports from our group suggested that reintervention per se did not decrease self-reported health status, indicating a possible limited impact of reinterventions on health-related quality of life [Citation24]. Furthermore, risk factors for dysfunction may be different in adults than in children, as reported by Buber and colleagues, who identified lifestyle-related factors such as high body mass index and smoking to be associated with hemodynamic conduit dysfunction [Citation20]. These risk factors are modifiable in contrast to diagnosis or sex, which are not. The increasing number of patients with variable anatomy, previous interventions, and sometimes persistent RV function impairment will be a great challenge to manage in the future
Limitations
In this study, patients operated at a young age have a survival bias compared to patients operated as adults since the registry only includes patients who live to at least 16 years age and could be included in the registry. Patients who underwent conduit surgery and did not live to be transferred to adult cardiology were not included in the registry. This could have had impact on the results in this study, in particular positive effect on conduit operation in young age. Thus, the results in the present study can only be applied on patients who lived long enough to be included in SWEDCON/GUCH (16 years).
Furthermore, this study did not address changes in indications over time or evolution of cardiac surgery, postoperative care and handling of the conduits. Thus, changes in conduit surgery in different eras may have an impact on outcome.
There is no consistent report of conduit type in SWEDCON. However, at least 70% of the conduits are homografts according to unpublished data from our group. Conduit type could affect outcome but should have limited effect on our results. This article studied the concept of conduit implantation and not differences between types of conduits. Furthermore, initial repair (for example transannular plasty) and pulmonary stenosis or regurgitation before first conduit operation likely had an impact on outcome. This is not included in the study. Neither is conduit replacement concomitant with other cardiac surgery studied in detail. Conduit replacements could occur despite no severe dysfunction, for example, concomitant with aortic valve replacement.
Conclusions
Perioperative mortality for conduit surgery was low in relation to total mortality. Higher age at the first conduit operation was associated with increased mortality risk. Reintervention-free survival after the first conduit operation was 50% at 20 years, and reintervention was common practice. Factors associated with decreased reintervention-free survival were complex CHD and male sex. In the lifetime management of patients with RV to PA conduits, the mortality risk of repeated conduit interventions appears to be low in relation to cardiac related death. Shorter reintervention-free survival after conduit reintervention than after first conduit implantation raises concern for the future, with an increasing number of patients in need of conduit reinterventions. Lifelong follow-up, focusing on the hemodynamic conditions of the RV is mandatory in this group of patients. The SWEDCON GUCH registry includes patients who reached at least 16 years of age. Thus the findings in this study only applies for patients reaching 16 years of age.
Appendix_1.docx
Download MS Word (11.9 KB)Acknowledgements
We thank Kjell Pettersson at Akademistatistik for his excellent statistical assistance and all dedicated SWEDCON nurses and physicians for their great effort with the registry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Khairy P, Ionescu-Ittu R, Mackie AS, et al. Changing mortality in congenital heart disease. J Am Coll Cardiol. 2010;56:1149–1157.
- Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation. 2014;130:749–756.
- Skoglund K, Eriksson P, Svensson G, et al. Homograft reconstruction of the right ventricular outflow tract in adults with congenital heart disease: a systematic review. Interact Cardiovasc Thorac Surg. 2015;22:57–62.
- Abbas JR, Hoschtitzky JA. Which is the best tissue valve used in the pulmonary position, late after previous repair of tetralogy of Fallot? Interact CardioVasc Thorac Surg. 2013;17:854–860.
- Caldarone CA, McCrindle BW, Van Arsdell GS, et al. Independent factors associated with longevity of prosthetic pulmonary valves and valved conduits. J Thorac Cardiovasc Surg. 2000;120:1022–1030.
- Christenson JT, Sierra J, Colina Manzano NE, et al. Homografts and xenografts for right ventricular outflow tract reconstruction: long-term results. Ann Thorac Surg. 2010;90:1287–1293.
- Dearani JA, Danielson GK, Puga FJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75:399–410.
- Buechel ER, Dave HH, Kellenberger CJ, et al. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727.
- Frigiola A, Tsang V, Bull C, et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation. 2008;118:S182–S190.
- Oosterhof T, van Straten A, Vliegen HW, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551.
- Therrien J, Provost Y, Merchant N, et al. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782.
- Kenny D, Hijazi ZM, Kar S, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am College Cardiol. 2011;58:2248–2256.
- Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131:1960–1970.
- O'Byrne ML, Glatz AC, Mercer-Rosa L, et al. Trends in pulmonary valve replacement in children and adults with tetralogy of fallot. The American Journal of Cardiology. 2015;115:118–124.
- Srinathan SK, Bonser RS, Sethia B, et al. Changing practice of cardiac surgery in adult patients with congenital heart disease. Heart. 2005;91:207–212.
- Boethig D, Thies WR, Hecker H, et al. Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. Eur J Cardio-Thorac Surg. 2005;27:58–66.
- Brown JW, Ruzmetov M, Rodefeld MD, et al. Right ventricular outflow tract reconstruction with an allograft conduit in non-Ross patients: risk factors for allograft dysfunction and failure. Ann Thorac Surg. 2005;80:655–663.
- Meyns B, Jashari R, Gewillig M, et al. Factors influencing the survival of cryopreserved homografts. the second homograft performs as well as the first. Eur J Cardio-Thorac Surg. 2005;28:211–216.
- Urso S, Rega F, Meuris B, et al. The Contegra conduit in the right ventricular outflow tract is an independent risk factor for graft replacement. Eur J Cardio-Thorac Surg. 2011;40:603–609.
- Buber J, Assenza GE, Huang A, et al. Durability of large diameter right ventricular outflow tract conduits in adults with congenital heart disease. Int J Cardiol. 2014;175:455–463.
- Batlivala SP, Emani S, Mayer JE, et al. Pulmonary valve replacement function in adolescents: a comparison of bioprosthetic valves and homograft conduits. Ann Thorac Surg. 2012;93:2007–2016.
- Ong K, Boone R, Gao M, et al. Right ventricle to pulmonary artery conduit reoperations in patients with tetralogy of fallot or pulmonary atresia associated with ventricular septal defect. Am J Cardiol. 2013;111:1638–1643.
- Oosterhof T, Meijboom FJ, Vliegen HW, et al. Long-term follow-up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J. 2006;27:1478–1484.
- Skoglund K, Berghammer M, Eriksson P, et al. Decline in self-reported health (EQ-5D) over time after surgical reconstruction of the right ventricular outflow tract: a longitudinal cohort study of 103 patients. Congenit Heart Dis. 2015;10:E54–E59.