Abstract
Objective. Endothelial function, including the nitric oxide (NO)-pathway, has previously been extensively investigated in heart failure (HF). In contrast, studies are lacking on the NO pathway after heart transplantation (HT). We therefore investigated substances in the NO pathway prior to and after HT in relation to hemodynamic parameters. Design. 12 patients (median age 50.0 yrs, 2 females), heart transplanted between June 2012 and February 2014, evaluated at our hemodynamic lab, at rest, prior to HT, as well as four weeks and six months after HT were included. All patients had normal left ventricular function post-operatively and none had post-operative pulmonary hypertension or acute cellular rejection requiring therapy at the evaluations. Plasma concentrations of ADMA, SDMA, L-Arginine, L-Ornithine and L-Citrulline were analyzed at each evaluation. Results. In comparison to controls, the plasma L-Arginine concentration was low and ADMA high in HF patients, resulting in low L-Arginine/ADMA-ratio pre-HT. Already four weeks after HT L-Arginine was normalized whereas ADMA remained high. Consequently the L-Arginine/ADMA-ratio improved, but did not normalize. The biomarkers remained unchanged at the six-month evaluation and the L-Arginine/ADMA-ratio correlated inversely to pulmonary vascular resistance (PVR) six months post-HT. Conclusions. Plasma L-Arginine concentrations normalize after HT. However, as ADMA is unchanged, the L-Arginine/ADMA-ratio remained low and correlated inversely to PVR. Together these findings suggest that (i) the L-Arginine/ADMA-ratio may be an indicator of pulmonary vascular tone after HT, and that (ii) NO-dependent endothelial function is partly restored after HT. Considering the good postoperative outcome, the biomarker levels may be considered “normal” after HT.
Introduction
Heart failure (HF) is common and despite continuous improvement in HF therapy, the condition carries a poor prognosis, where heart transplantation (HT) resides as the ultimate treatment. Pulmonary hypertension (PH) is a complicating factor in HF, associated with worse prognosis [Citation1]. With the present methods and definitions [Citation2,Citation3], it is difficult to differentiate between isolated post-capillary PH (Ipc-PH), caused by passive congestion of the pulmonary vasculature and combined pre-capillary and post-capillary PH (Cpc-PH), complicated by endothelial dysfunction, excessive vasoconstriction and, in some cases, vascular remodelling [Citation2]. Adequately sub-defining post-capillary PH is, however, of major importance in HT where Cpc-PH with vascular remodelling may increase the risk for acute right heart failure and early mortality after HT.
Endothelial dysfunction is central in PH, but is also present in HF. This dysfunction is, at least in part, caused by impaired nitric oxide (NO) production secondary to endothelial damage [Citation4]. The L-Arginine/NO pathway is a complex system with effects on vasomotor tone as well as on cellular adhesion, platelet aggregation, vascular proliferation and angiogenesis [Citation5]. NO is produced from L-Arginine, which can form either NO and citrulline or urea and ornithine, respectively. The former reaction is catalysed by the nitric oxide synthase (NOS) family and the latter by arginase [Citation6,Citation7].
Moreover, the competitive NOS inhibitor asymmetric dimethylarginine (ADMA) has been shown to be elevated in plasma and serum in various cardiovascular conditions [Citation8,Citation9] as well as to be an independent predictor of outcome in acutely decompensated chronic HF [Citation10]. ADMA is primarily metabolized by dimethylarginine dimethylaminohydrolase-1 (DDAH-1) and to a lesser extent excreted by the kidneys [Citation5]. DDAH-1 is primarily expressed in the kidneys and liver [Citation11] and its activity is reduced by oxidative stress and impaired in the above-mentioned conditions. In contrast, Arginase activity has been shown to be elevated in HF [Citation12]. This, together, leads to decreased NO synthesis and increased production of urea, which in turn results in lower L-Arginine available for NO production [Citation12].
In contrast to in HF, there is scarce knowledge on the NO pathway in relation to HT. One study has shown that NO production is impaired after HT, primarily due to increased inflammation [Citation13]. Another study has shown that the immunosuppressive compound sirolimus, but not mycophenolate mofetil, decrease ADMA in HT patients [Citation14]. However, little is known about the levels of various substances of the nitric oxide pathway, their development over time and their correlation to hemodynamics after HT. We therefore evaluated substances related to the NO pathway in severe HF patients prior to and after HT and related the findings to commonly used hemodynamic parameters. The aim was to define the “normal” post-HT plasma levels of substances in the NO pathway, as well as to identify potential biomarkers and targets for future medical therapies.
Materials and methods
Study design and population
Between June 2012 and February 2014, 43 patients were heart transplanted in Lund and included in a prospective cohort study. To identify a “normal” post-HT population, the 12 patients (median age 50.0 yrs, 2 females) who were hemodynamically evaluated at our lab, at rest, prior to HT, as well as four weeks and six months after HT, prior to august 2014, and who, at the four week and six month evaluations, (i) did not have post-operative rejections requiring specific therapy; (ii) did not have postoperative PH and; (iii) had left ventricular ejection fraction ≥50%, were included in the present study. For comparison, we included 12 healthy, age-matched, non-smokers without drug treatment and no symptoms or signs of common cold.
The study was performed with informed consent and approval by the ethics board in Lund and Uppsala (Approval nos. 2010/114, 2010/343, 2010/442, 2011/368, 2011/777, 2015/270) and in accordance with the Declarations of Helsinki and Istanbul.
Right heart catheterization
Right heart catheterization (RHC) were performed at rest prior to HT, as well as four weeks and six months after HT. RHC was performed via the right internal jugular vein, using a Swan Ganz catheter (Baxter Health Care Corp, Santa Ana, CA). Complete pulmonary hemodynamics were recorded. In the present study we focused on mean pulmonary artery pressure (MPAP), pulmonary artery wedge pressure (PAWP), mean right atrial pressure (MRAP), cardiac output (CO), transpulmonary gradient (TPG), diastolic pressure gradient (DPG) and pulmonary vascular resistance (PVR). MPAP, PAWP and MRAP were recorded. CO was measured by thermodilution. TPG, DPG and PVR were calculated using the formulas: TPG = MPAP-PAWP, DPG = diastolic pulmonary artery pressure-PAWP and PVR = TPG/CO.
Biomarker analysis
Plasma samples from patients, collected from the pulmonary arteries during RHC, were stored at −80 °C in the Lund Cardio Pulmonary Register (LCPR) cohort of Region Skåne’s biobank. The samples were analyzed for plasma concentrations of ADMA, symmetric dimethylarginine (SDMA), L-Arginine, L-Ornithine and L-Citrulline with liquid chromatography – tandem mass spectrometry (LC-MS/MS), at the Swedish National Veterinary Institute in Uppsala, Sweden, as described previously [Citation15]. The L-Arginine/ADMA-ratio as well as the L-Arginine/L-Ornithine-ratio were evaluated and the global arginine bioavailability ratio (GABR) calculated using the formula: GABR = (L-Arginine/(L-Ornithine + L-Citrulline)). Control samples were collected at Uppsala University Hospital and analyzed for L-Arginine, ADMA and SDMA as described previously [Citation16]. The patients and controls were analyzed at different time points. The methods used were the same. However, at the time of analysis of the samples from controls, the L-Ornithine and L-Citrulline analyzes were not available so these markers are lacking in controls.
To monitor acute cellular rejections (ACR), endomyocardial biopsies were routinely performed from the right interventricular septum, and graded according to the 2005 International Society for Heart and Lung Transplantation working formulation [Citation17].
Statistics
A SigmaStat/SigmaPlot version 11.0 (Systat Software Inc, San Jose, CA) was used for statistical analysis. Parametric or non-parametric statistics were used depending on the distribution of data: i.e. One Way Repeated Measures ANOVA (Tukey-test) or One Way Repeated Measures ANOVA on Ranks (Tukey-test), when the same group was compared over time; t-test or Mann-Whitney Rank Sum test, respectively, when two groups were compared and Pearson- or Spearman Correlation, respectively, when measuring the association between two variables. A p value <.05 was considered statistically significant. All values are presented as median and interquartile range.
Results
Study population
Baseline demographical data are shown in . Nine of the 12 patients had pre-operative PH (MPAP ≥25 mmHg). Of these patients, three had combined pre-capillary and post-capillary PH (Cpc-PH, DPG ≥7 mmHg and/or PVR >3 WU) and five had isolated post-capillary PH (Ipc-PH, DPG <7 mmHg and/or PVR ≤3 WU). One patient had very severe orthopnea at the RHC when blood samples were collected and adequate PAWP measurements could not be performed, wherefore PH-subgrouping is lacking in this patient. However, the extensive pre-HT evaluation revealed no signs of pulmonary vascular disease and in a second RHC, MPAP and PAWP were 30 mmHg and 24 mmHg, respectively, with a PVR of 1.5 WU and a DPG of 0 mmHg. Prior to the second RHC the patient had been medically optimized with intravenous furosemide and levosimendan and was subsequently accepted for HT.
Table 1. Baseline characteristics of patients and healthy controls.
Follow-up data, with regards to clinical features and medications, are shown in . Four weeks after HT three patients exhibited myocardial biopsies with rejection grade 1R and six months after HT two patients had rejection grade 1R. None needed specific rejection treatment.
Table 2. Characteristics of the patients four weeks and six months after HT.
Substances in the nitric oxide pathway and their characteristics in relation to heart transplantation
Prior to HT, the plasma concentration of L-Arginine was 56 (48–60) µM, L-Ornithine 93 (71–119) µM, L-Citrulline 26 (19–33) µM, ADMA 0.57 (0.47–0.71) µM, SDMA 0.85 (0.68–1.15) µM, L-Arginine/ADMA-ratio 86 (68–128), L-Arginine/L-Ornithine-ratio 0.57 (0.46–0.74) and GABR 0.43 (0.37–0.55) ( and ). L-Arginine was lower (p < .001) in patients than in controls, whereas ADMA and SDMA were higher (p < .003 and p < .001, respectively), resulting in a markedly lower L-Arginine/ADMA-ratio in patients (p < .001).
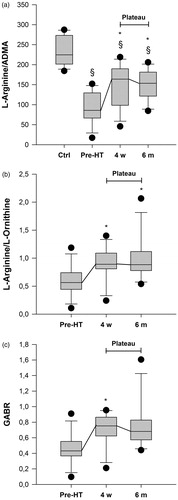
Figure 1. Characteristics of biomarkers related to the nitric oxide pathway prior to and after HT. (a) L-Arginine, (b) ADMA = asymmetric dimethylarginine, (c) SDMA = symmetric dimethylarginine, (d) L-Citrulline, and (e) L-Ornithine. *indicates statistical significance compared to prior to HT. §indicates statistical significance compared to controls.
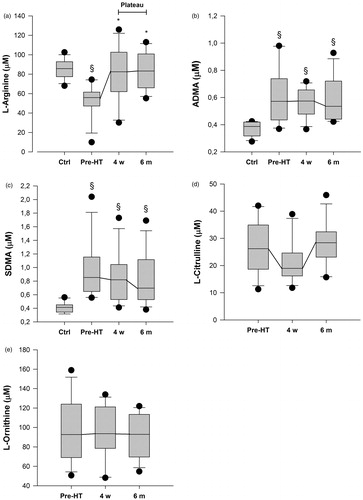
Figure 2. Characteristics of biomarker-ratios related to the nitric oxide pathway prior to and after HT. (a) L-Arginine/ADMA-ratio, (b) L-Arginine/L-Ornithine-ratio, and (c) GABR = global arginine bioavailability-ratio. *indicates statistical significance compared to prior to HT. §indicates statistical significance compared to controls.
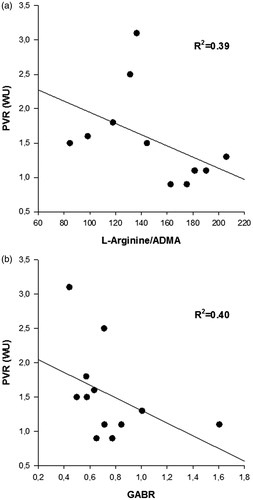
There was a trend (p = ns) towards higher plasma concentration of SDMA, as well as lower L-Arginine/ADMA-ratio, GABR and L-Arginine/Ornithine-ratio in patients with, compared to those without, pre-operative PH ().
Table 3. Biomarker-levels of patients with and without PH at baseline.
Four weeks after HT there was an increase in the plasma concentration of; L-Arginine to 83 (65–101) µM (p < .008), L-Arginine/ADMA-ratio to 164 (105–189) (p < .006), L-Arginine/L-Ornithine-ratio to 0.89 (0.82–1.08) (p < .05) and GABR to 0.75 (0.65–0.85) (p < .05), compared to prior to HT. All concentrations remained stable (p = ns) at the six month evaluation. After HT, L-Arginine was in line with healthy controls (p = ns), whereas L-Arginine/ADMA, although improved, remained decreased (p < .001). There was no change in ADMA, SDMA, L-Ornithine and L-Citrulline levels after HT (p = ns, and ).
Hemodynamic characteristics in relation to heart transplantation
Hemodynamics prior to HT, as well as four weeks and six months thereafter, are shown in . Prior to HT, MPAP was 35 (27–42) mmHg, PAWP 25 (20–30) mmHg, TPG 9 (6–12) mmHg, DPG 1.0 (–0.8–4.8) mmHg, MRAP 15 (12–19) mmHg, CO 3.4 (2.6–4.3) L·min−1 and PVR 2.4 (1.9–3.0) WU.
Table 4. Selected hemodynamic parameters at baseline and during follow-up.
Four weeks after HT there was an increase in CO to 6.0 (5.6–6.6) L·min−1 (p < .001), as well as a decrease in MPAP to 17 (12–21) mmHg (p < .05), PAWP to 8 (5–11) mmHg (p < .001) and PVR to 1.3 (1.1–1.5) WU (p < .05), compared to prior to HT, remaining stable (p = ns) at the six month evaluation. Six months after HT MRAP decreased to 1.5 (0.0–4.0) mmHg (p < .05), as compared to prior to HT. There was no change (p = ns) in DPG and TPG after, compared to prior to, HT. None of the patients had post-operative PH ( and ).
In a univariate analysis prior to HT there was a modest correlation between GABR and creatinine (R2: 0.37, p < .04). Six months after HT, there was an inverse correlation between PVR and the L-Arginine/ADMA-ratio (R2: 0.39, p < .03) as well as GABR (R2: 0.40, p < .03) (). There were no other significant correlations, neither at the pre-operative nor post-operative evaluations, between the L-Arginine/ADMA-ratio or GABR and the hemodynamic parameters, NT-proBNP, creatinine or urea (Supplemental table 1). Moreover, neither the plasma levels of L-Arginine nor ADMA correlated to PVR, or any of the other investigated hemodynamic parameters, after HT (p = ns). In contrast, there was a strong correlation between the L-Arginine/ADMA ratio and aspartate aminotransferase (ASAT) and glutamyl transferase (GT), as well as between GABR and ASAT, GT and alanine aminotransferase (ALAT) (Supplemental table 1). However, these correlations were primarily due to the correlation between L-Arginine and the transferases, whereas no correlation was observed between ADMA and these markers.
Discussion
In the present study, we investigated the plasma concentrations of substances related to the NO pathway in HF-patients prior to and after HT, without post-operative PH and rejections above 1R, as well as with normal left heart function after HT. In support of previous findings, we found impaired L-Arginine, ADMA and L-Arginine/ADMA-ratio prior to HT. Whereas L-Arginine greatly improved, to levels comparable to healthy individuals, already four weeks after HT, ADMA, L-Citrulline and L-Ornithine remained unchanged. Consequently, the L-Arginine/ADMA-ratio was improved, but not fully normalized. The L-Arginine/ADMA-ratio was furthermore inversely correlated to PVR, suggesting a partly restored NO-dependent endothelial function. Based on the good post-operative outcome, without severe complications, our findings indicate the “normal” plasma concentrations of these biomarkers after HT.
The 12 patients included in the present study had severe HF with low CO and high filling pressures as well as severely deranged biomarkers prior to HT. They presented with significantly lower plasma concentrations of L-Arginine but higher ADMA and SDMA compared to healthy controls, as well as compared to patients with mild to moderate HF, recently reported by our group [Citation15]. In fact, the plasma levels of the investigated biomarkers related to the NO pathway were, prior to HT, in line with those of patients with PAH, in our previous report by Sandqvist et al. [Citation15]. These findings may represent the progressive nature of HF, with worsening endothelial function and congestion of the pulmonary circulation. Similar results have been observed in other studies, where ADMA has been shown to correlate to NYHA functional class [Citation8,Citation9,Citation18] and pulmonary pressures [Citation19]. Of the patients included in our study, nine had PH. There was furthermore a non-significant trend towards a worse level of several biomarkers related to the NO pathway in these patients, compared to those without PH. Larger studies are encouraged to define whether the biomarkers in fact differ between HF patients with and without PH.
It is well established that the bioavailabilty of NOS is limited by the availability of L-Arginine, despite L-Arginine in plasma being manifold above Km for NO generation by NOS. This contradiction is referred to as the arginine paradox [Citation20] and has been attributed to ADMA, which competes with L-Arginine for NOS [Citation20]. Therefore, the activity of NOS and NO production increases with higher concentrations of the substrate [Citation21]. The L-Arginine/ADMA-ratio has consequently been suggested to give a more correct insight into NOS activity, NO production and endothelial function, than L-Arginine and ADMA separately, as it reflects the relationship between the substrate and inhibitor [Citation22]. The L-Arginine/ADMA-ratio has indeed been shown to be associated with disease severity [Citation18] as well as mortality [Citation23] in HF. Moreover, low GABR, representing the relationship between substrate and products, was recently found to be associated with impaired outcome in chronic HF [Citation24]. In the present study, we found a marked improvement in the plasma concentration of L-Arginine after HT whereas ADMA remained high. Consequently, the L-Arginine/ADMA-ratio remained low, suggesting that endothelial function, although improved, is still partly impaired during the first six months after HT. The fact that the endothelial function is only partly restored after HT is also supported by that the L-Arginine/ADMA-ratio as well as the GABR shows a moderate inverse correlation with PVR six months after HT. This observation suggests that these ratios may reflect pulmonary vascular tone after HT. In contrast, neither L-Arginine nor ADMA correlated to post-operative hemodynamics and does not seem to reflect vascular tone after HT. Furthermore, as L-Arginine is converted to NO and L-Citrulline, altered NO production may be reflected in changes in L-Citrulline levels. In the present study no alterations in plasma L-Citrulline levels were observed after HT and as NO and cGMP-levels were not measured, we do not provide a clear explanation to these findings. However, circulating L-Citrulline is primarily released form the intestines and not from the endothelium. It is possible that a potential increase in the release of L-Citrulline from the endothelium is not sufficient to significantly alter plasma L-Citrulline levels. Also, with improved cardiac output and possibly reduced inflammation after HT it is a reasonable assumption that that the sensitivity for NO in the vasculature is increased, resulting in partly restored NO mediated vasodilatation irrespective of NO production.
A possible explanation for the improved plasma levels of L-Arginine after HT is decreased arginase activity. Arginase catalyses the formation of urea and ornithine from L-Arginine and has been shown to be elevated in HF [Citation12]. Arginase increases during oxidative stress and infections [Citation25] and as it competes with NOS for L-Arginine it can cause “uncoupling” of NOS, leading, in sequence, to increased superoxide production, diminished NO production and endothelial dysfunction [Citation25]. In our material, the L-Arginine/Ornithine-ratio, which can be used to assess arginase activity, increased after HT. However, the increase was solely due to increase in plasma concentration of L-Arginine and it is unlikely that arginase, to a greater extent, is affected by HT. The present study does moreover not provide a solid explanation to the persistently elevated ADMA levels after HT. Considering the low plasma concentrations of L-Arginine prior to HT, L-Arginine is an appealing therapy in HF. However, despite the strong rationale for such treatment, long-term investigations have been inconclusive [Citation26,Citation27]. It is possible that the lack of effect in these trials is due to that L-Arginine levels do not represent endothelial function. This is supported by the present study, in which correlations between post-operative hemodynamics and L-Arginine were lacking. Based on our findings, with low pre-operative plasma levels of L-Arginine, in combination with the high plasma levels of ADMA and decreased L-Arginine/ADMA-ratio throughout the study, where the L-Arginine/ADMA-ratio indeed reflected post-HT pulmonary vascular tone, it is likely that NO production is reduced both prior to and after HT. Consequently, drugs targeting the NO pathway independent of NO, such as soluble guanylate cyclase (sGC) stimulators, may be more beneficial than L-Arginine as well as NO dependent substances. Indeed, in patients with systolic HF, a phase II trial with the novel sGC-stimulator vericiguat recently demonstrated positive effects on NT-proBNP levels in a dose-dependent manner [Citation28], supporting this hypothesis. Future studies may elucidate the potential of such a therapeutic approach.
Limitations
A major limitation of the present study is the small sample size. However, the size is in line with previous pilot studies in HT which has stimulated the initiation of larger trials. Moreover, in previous studies [Citation29] we have, in larger HT-populations from our hospital, shown similar pre-operative and post-operative hemodynamics as in the present group, suggesting that the current findings can be applied in larger populations. Furthermore, we did not measure NO and cGMP levels so we cannot be certain that the production is decreased. However, the findings of decreased L-Arginine/ADMA-ratio throughout our study, in combination with the fact that the ratio reflect pulmonary vascular tone, points in the direction of NO dependent endothelial dysfunction prior to and after HT. Finally, medical therapies prior to and after HT were individually adjusted. As some of these medications, such as betablockers [Citation24], ACEi and ARB [Citation30] have been shown to decrease circulating ADMA levels, diminished use of these medications after HT could, in theory, increase plasma levels of ADMA and the results in the present study must consequently be interpreted with this as a precaution. However, the use of these drugs represent a cornerstone in heart failure therapy and a true clinical situation so our findings are relevant to generate hypotheses for future, larger, trials.
Conclusions
The present study shows that plasma concentrations of L-Arginine, by an unknown mechanism, normalizes after HT. However, as ADMA plasma levels are unaltered, the L-Arginine/ADMA-ratio remains low and correlates inversely to PVR. This suggests that the L-Arginine/ADMA-ratio reflects pulmonary vascular tone after HT and that NO-dependent endothelial function is post-operatively improved, yet not normalized. Moreover, considering the good post-operative outcome without complications, our results could represent normal plasma magnitudes of the investigated substances after HT and the plasma derived L-Arginine/ADMA-ratio may therefore be used to assess pulmonary vascular tone after HT. Finally, based on the low L-Arginine/ADMA-ratio prior to and after HT, it is reasonable to conclude that, if targeting the NO pathway in HF, treatment with NO independent vasoactive substances may be more beneficial than NO dependent drugs. Larger trials on this subject are therefore encouraged.
Jakob_Lundgren_et_al._Supplemental_table_1.docx
Download MS Word (14 KB)Acknowledgements
We acknowledge the support of the staff at the Hemodynamic Lab at The Section for Heart Failure and Valvular Disease, Skåne University Hospital, and the Department of Cardiology, Clinical Sciences, Lund University, Lund, Sweden. In specific, we acknowledge the work of Ms. Karin Paulsson, Ms. Anneli Ahlqvist and Ms. Marie Wildt for their support in the assembly of the plasma samples within the Lund Cardio Pulmonary Register as well as Mrs. Sofia Berg Blomqvist and Mrs. Karin Kjellström at Uppsala University Hospital, Uppsala, Sweden for sampling of blood plasma from healthy subjects. We furthermore acknowledge the biobank services and retrieval of blood samples performed at Labmedicin Skåne, University and Regional Laboratories, Region Skåne, Sweden. Finally, we are grateful to Mrs. Elisabeth Fredriksson at the National Veterinary Institute (SVA) for carrying out the LC-MC/MS analyses.
Disclosure statement
Dr. Lundgren and PhD Sandqvist report unrestricted research grants from The Swedish Society of Pulmonary Hypertension, and Dr. Rådegran reports unrestricted research grants from Anna-Lisa and Sven-Erik Lundgren’s, Maggie Stephen’s, ALF’s, Skåne University Hospital’s Foundations and Actelion Pharmaceuticals Sweden AB, during the conduct of the study.
Dr. Lundgren reports personal lecture fees from Actelion Pharmaceuticals Sweden AB and GlaxoSmithKline outside the submitted work. Dr. Wikström and Dr. Rådegran report personal lecture fees from Actelion Pharmaceuticals Sweden AB, Bayer Healthcare, GlaxoSmithKline and Sandoz/Novartis outside the submitted work.
Dr. Wikström is, and has been primary-, or co-, investigator in; clinical PAH trials for GlaxoSmithKline, Actelion Pharmaceuticals Sweden AB, Pfizer, Bayer Healthcare and United Therapeutics, and in clinical heart failure trials for Novartis. Dr. Rådegran is, and has been primary-, or co-, investigator in; clinical PAH trials for GlaxoSmithKline, Actelion Pharmaceuticals Sweden AB, Pfizer, Bayer Healthcare and United Therapeutics, and in clinical heart transplantation immuno-suppression trials for Novartis.
Adjunct prof. Hedeland and prof. Bondesson report no conflicts of interest.
Since the completion of the study and initial submission of the manuscript, PhD Sandqvist has started working at Actelion Pharmaceuticals Sweden AB. The company has no role in data collection, analysis, interpretation of data or publishing of the manuscript.
Additional information
Funding
References
- Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231.
- Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertensiondagger. Eur Heart J. 2016;37:942–954.
- Lundgren J, Soderlund C, Radegran G. Impact of postoperative pulmonary hypertension on outcome after heart transplantation. Scand Cardiovasc J. 2017;51:172–181.
- Cooper CJ, Jevnikar FW, Walsh T, et al. The influence of basal nitric oxide activity on pulmonary vascular resistance in patients with congestive heart failure. Am J Cardiol. 1998;82:609–614.
- Visser M, Paulus WJ, Vermeulen MA, et al. The role of asymmetric dimethylarginine and arginine in the failing heart and its vasculature. Eur J Heart Fail. 2010;12:1274–1281.
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666.
- Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34:906–911.
- Usui M, Matsuoka H, Miyazaki H, et al. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–2430.
- Hsu CP, Lin SJ, Chung MY, et al. Asymmetric dimethylarginine predicts clinical outcomes in ischemic chronic heart failure. Atherosclerosis. 2012;225:504–510.
- Zairis MN, Patsourakos NG, Tsiaousis GZ, et al. Plasma asymmetric dimethylarginine and mortality in patients with acute decompensation of chronic heart failure. Heart. 2012;98:860–864.
- Palm F, Onozato ML, Luo Z, et al. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol. 2007;293:H3227–H3245.
- Quitter F, Figulla HR, Ferrari M, et al. Increased arginase levels in heart failure represent a therapeutic target to rescue microvascular perfusion. Clin Hemorheol Microcirc. 2013;54:75–85.
- Holm T, Aukrust P, Aagaard E, et al. Hypertension in relation to nitric oxide, asymmetric dimethylarginine, and inflammation: different patterns in heart transplant recipients and individuals with essential hypertension. Transplantation. 2002;74:1395–1400.
- Potena L, Fearon WF, Sydow K, et al. Asymmetric dimethylarginine and cardiac allograft vasculopathy progression: modulation by sirolimus. Transplantation. 2008;85:827–833.
- Sandqvist A, Schneede J, Kylhammar D, et al. Plasma L-arginine levels distinguish pulmonary arterial hypertension from left ventricular systolic dysfunction. Heart Vessels. 2018;33:255–263.
- Henrohn D, Sandqvist A, Egerod H, et al. Changes in plasma levels of asymmetric dimethylarginine, symmetric dimethylarginine, and arginine after a single dose of vardenafil in patients with pulmonary hypertension. Vascul Pharmacol. 2015;73:71–77.
- Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720.
- Seljeflot I, Nilsson BB, Westheim AS, et al. The L-arginine-asymmetric dimethylarginine ratio is strongly related to the severity of chronic heart failure. No effects of exercise training. J Card Fail. 2011;17:135–142.
- Shao Z, Wang Z, Shrestha K, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–1158.
- Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134(10 Suppl):2842S–2847S.
- Creager MA, Gallagher SJ, Girerd XJ, et al. L-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253.
- Boger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600.
- Anderssohn M, Rosenberg M, Schwedhelm E, et al. The L-Arginine-asymmetric dimethylarginine ratio is an independent predictor of mortality in dilated cardiomyopathy. J Card Fail. 2012;18:904–911.
- Tang WH, Shrestha K, Wang Z, et al. Diminished global arginine bioavailability as a metabolic defect in chronic systolic heart failure. J Card Fail. 2013;19:87–93.
- Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res. 2013;98:334–343.
- Rector TS, Bank AJ, Mullen KA, et al. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation. 1996;93:2135–2141.
- Schulman SP, Becker LC, Kass DA, et al. L-arginine therapy in acute myocardial infarction: the Vascular Interaction With Age in Myocardial Infarction (VINTAGE MI) randomized clinical trial. JAMA. 2006;295:58–64.
- Gheorghiade M, Greene S, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA. 2015;314:2251–2262.
- Lundgren J, Radegran G. Hemodynamic characteristics including pulmonary hypertension at rest and during exercise before and after heart transplantation. J Am Heart Assoc. 2015;4:e001787. doi:10.1161/JAHA.115.001787
- Chen JW, Hsu NW, Wu TC, et al. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am J Cardiol. 2002;90:974–982.