Abstract
Objectives. The aim of this study was to investigate the association between echocardiographic measures of diastolic left ventricular dysfunction and decreased arterial oxyhaemoglobin saturation measured with pulse oximetry (SpO2). Design. This is a cross-sectional population-based survey of Norwegian adults. Values obtained using echocardiography, pulse oximetry, and spirometry were included. The primary outcome was abnormal mitral Doppler inflow, defined as normal: E/A ratio 0.75–1.5 and EDT ≥ 140 ms; abnormal: E/A ratio <0.75 or >1.5 or EDT <140 ms. The associations between this outcome and possible predictors, including SpO2 ≤ 95%, were analysed using univariable and multivariable logistic regression. Results. A total of 1782 participants aged 50 years or older (54% women, mean age 67.5 years) were included in the analysis. Abnormal mitral Doppler inflow was found in 595 participants. After adjusting for age, gender, previous myocardial infarction, smoking history, dyspnoea, obesity, and decreased lung function, SpO2 ≤ 95% predicted abnormal mitral Doppler flow with an odds ratio (OR) of 1.6 [95% confidence interval (CI) 1.1–2.4]. Hypertension and BMI > =30 were also significant predictors of impaired filling, with OR of 1.7 (95% CI 1.1–2.7) OR and 1.5 (95% CI 1.2–1.9), respectively. Conclusion. Decreased SpO2 was a significant predictor of abnormal mitral Doppler flow. Diastolic dysfunction should be considered when SpO2 ≤ 95% is found.
Introduction
In the general adult population, the prevalence of pre-clinical diastolic dysfunction (PDD) is 20–30% [Citation1]. Although diastolic dysfunction (DD), defined using Doppler techniques, is not often accompanied by symptomatic heart failure (HF), it is associated with increased all-cause mortality [Citation2]. In the community, isolated DD is present in more than 40% of patients with HF. The mortality of patients hospitalized with HF and preserved ejection fraction (HFpEF) is similar to that reported for systolic HF [Citation2,Citation3]. The time course for the development of asymptomatic DD into HFpEF has not been explored thoroughly [Citation4]. Asymptomatic DD does not necessarily progress to HFpEF. If causal factors, like arterial hypertension, are treated, HFpEF may revert to a pre-clinical state [Citation1]. In a cross-sectional survey that included randomly selected adult residents aged 45 years or older in the US in 1997–2000, the prevalence rates of HFpEF and DD were 1% and 28%, respectively [Citation2]. The prevalence of asymptomatic DD increases with age and the presence of cardiovascular comorbidities [Citation2,Citation5].
In a cross-sectional study of patients with obstructive pulmonary disease treated in Norwegian general practice, decreased SpO2 ≤ 95% was associated with a comorbid diagnosis of coronary heart disease [Citation6]. Pulse oximetry is a simple and inexpensive non-invasive method that is widely used for estimating arterial oxyhaemoglobin saturation (SpO2). There is no clear cut-off point for an abnormal SpO2, but a value ≤ 95% is used in most studies of adults [Citation7].
Two important reasons for a decreased SpO2 are HF and ventilation/perfusion mismatch in pulmonary diaseases. HF affects pulmonary function and gas exchange [Citation8], and SpO2 may decrease accordingly [Citation9]. In acute HF secondary to myocardial infarction, the decrease in SpO2 is linked to the severity of the disease [Citation9]. Pulmonary diseases like chronic obstructive pulmonary disease (COPD) and sleep apnea may lead to alveolar hypoxia, and, when severe, to elevated pulmonary artery pressures and also to left ventricular DD [Citation10].
There is limited knowledge of the association between decreased SpO2 values and heart disorders in the general population. The aim of this study was to investigate the association between left ventricular diastolic dysfunction, assessed with transthoracic echocardiography, and decreased SpO2 in an adult population.
Methods
Study population
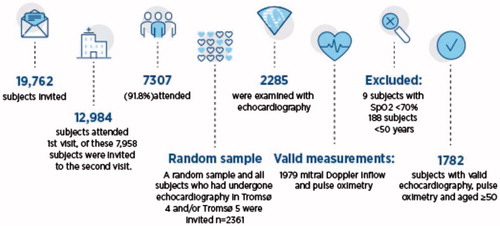
In total, 1782 participants filled the inclusion criteria and had acceptable recordings. The Tromsø Study is a repeated population-based survey, and the first survey was arranged in 1974 [Citation11]. The participants were recruited from the sixth Tromsø Study, which took place in Tromsø, Norway 2007–2008. The study sample () was chosen with the aim of analysing participants with technically acceptable echocardiography results who also had valid results for pulse oximetry [Citation12]. Participants younger than 50 years were excluded to ensure the validity of the Doppler measurements of DD because the reference values for the Doppler measurements change considerably with age.
All of the participants gave written informed consent. The Regional Committee for Medical and Health Research Ethics in North Norway approved the Tromsø six survey, and this study was approved by the Data Inspectorate and the Norwegian Directorate of Health.
Questionnaires and self-reported health
A questionnaire was mailed to participants with the invitation. The following variables from the questionnaire were used in the analysis: smoking (current/previous/never), antihypertensive treatment (current/previous/never), self-reported previous myocardial infarction (yes/no), and self-reported diabetes (yes/no). During the first examination, each participant completed a second questionnaire covering dyspnoea. Perceived breathlessness was classified into five categories consistent with the Medical Research Council dyspnoea grades [Citation13]: Grade 1, no dyspnoea; Grade 2, mild dyspnoea (discomfort in breathing during ordinary physical activities, i.e., discomfort when walking rapidly on level ground or up a moderate slope); Grade 3, moderate dyspnoea (discomfort in breathing after walking 100 yards or after a few minutes on level ground); Grade 4, severe dyspnoea (discomfort in breathing during less than ordinary physical activities such as walking calmly on level ground or washing and dressing); and Grade 5, very severe dyspnoea (discomfort in breathing at rest). In the analyses, Grades 3–5 (moderate, severe, and very severe dyspnoea) were combined into one category.
Measurements
Pulse oximetry
SpO2 values were measured using a digital handheld pulse oximeter (Onyx II, model 9559; Nonin Medical, Inc.; Plymouth, MN, USA). The participant rested for at least 15 min before the examination, and the best of three measurements was recorded. The manufacturer’s testing has shown that only values between 70% and 100% are accurate to within ±2%, and values <70% were regarded as invalid.
Echocardiography
Echocardiography was performed by two expert cardiologists using a VingMED CFM 750 (Vingmed Sound A/S, Horten, Norway) with a combined 3.25 MHz mechanical and 2.5 MHz Doppler probe. The examination was terminated in participants with ongoing atrial fibrillation because of the low validity and reliability in this condition. The examination was performed using the standard apical and parasternal long- and short-axis views. Standard two-dimensional guided M-mode registration of the anteroposterior left atrial (LA) diameter, internal left ventricular (LV) dimensions, and the thickness of the septum and posterior wall were recorded using the leading edge-to-leading edge convention [Citation14]. Measurement of peak flow velocity in early diastole (E-wave) and during atrial contraction (A-wave), peak E/A ratio and the E-wave deceleration time (EDT) were performed online during one heart cycle. The influence of heart rate was minimized by measuring the EDT as the time between the peak E-wave and the upper deceleration slope extrapolated to the zero baseline [Citation15]. The calculation of LV ejection fraction (LVEF) was based on M-mode-derived diameters measured in the short axis [Citation16], and systolic dysfunction was defined as LVEF <50%. LA diameter was indexed by body surface area (BSA). A reproducibility study of the echocardiographic data was performed [Citation17]. Tissue Doppler imaging (TDI) of the septum at the mitral annulus was performed with measurement of early diastolic mitral annular velocity (e´) and subsequent calculation of average septal and lateral E/e′.
Classification of diastolic dysfunction
Mitral Doppler, E/e′, and LA size indices were used to evaluate DD. The mitral Doppler assessments and LA size were classified according to values reported in a previous community-based study [Citation17]. TDI of the mitral annulus was classified according to current guidelines [Citation18].
According to mitral Doppler assessment, diastolic function was defined as follows: normal, E/A ratio 0.75–1.5 and EDT ≥ 140 ms; abnormal, E/A ratio <0.75 or >1.5 or EDT <140 ms. Abnormal mitral Doppler was used as the main outcome in the analyses.
The cut-off value for the ratio of TDI and mitral passive inflow E/e′ were average septal and lateral E/e′ ratio >14 [Citation18].
LA size was indexed by BSA and was categorized into four groups as normal (<2.4 cm/m2), mildly abnormal (2.4–2.6 cm/m2), moderately abnormal (2.7–2.9 cm/m2), and severely abnormal (≥3.0 cm/m2) [Citation19]. The moderately and severely abnormal groups were combined in the analysis.
Spirometry
We included spirometry values because a decrease in SpO2 was strongly associated with severe airflow limitation in another paper from the same survey [Citation12]. Spirometry was performed using a SensorMedics Vmax 20c Encore system (Viasys Healthcare Respiratory Technologies, Yorba Linda, CA, USA). The American Thoracic Society/European Respiratory Society criteria were followed [Citation20]. Norwegian reference values for pre-bronchodilator spirmetry were used because reversibility tests were not performed [Citation21]. Calibration was performed every morning and as required by the spirometer. Three trained technicians conducted the spirometry alternately. The test was performed with the participant sitting and using a nose clip. At least three exhalations were required. The difference between the best and second-best forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) was not to exceed 5% or 150 mL. Current drug therapy was not interrupted before the test. In the analysis, FEV1% predicted was categorized into three groups: FEV1% predicted ≥ 80%, FEV1% predicted 50–80%, and FEV1% predicted <50%.
Other measurements
Body mass index (BMI) was calculated as weight/height2 (kg/m2) and was dichotomized into ≥30 and <30 kg/m2.
Blood pressure was recorded three times at 1-min intervals after 2 min of rest using the automatic Dinamap ProCare 300 Monitor (GE Healthcare, Norway), and the mean of the second and third readings was used. Hypertension was defined as the self-reported use of antihypertensive medication or blood pressure ≥140/90 mmHg.
Outcome measure
The main outcome was abnormal mitral Doppler inflow.
Statistical analysis
Characteristics of the study population were compared between men and women, and the differences were explored using the independent-sample t test for continuous variables or chi-square test for categorical variables. The frequency of abnormal mitral Doppler inflow was analysed according to gender, age, smoking status, self-reported diseases, dyspnoea, hypertension, spirometry, BMI, LVEF <50%, atrial size group, ratio of TDI and mitral passive inflow E/e′, and SpO2 ≤ 95%. The chi-square test was used to identify significant differences.
Univariable analysis of associations between abnormal mitral Doppler inflow and explanatory variables was also performed using binary logistic regression, and the variables associated with abnormal mitral Doppler inflow with a p ≤ .1 were entered into a multivariable analysis. Altogether, 348 subjects did not have M-mode recordings of a quality justifying estimation. We chose to include participants with acceptable Doppler measurements, irrespective of M-mode recordings, in our study population to keep a large sample size. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). The statistical analyses were performed using IBM SPSS Statistics (version 25.0; IBM Corp., Armonk, NY, USA).
Results
Of the 1782 participants 970 were women (54%) and 812 were men (46%). A summary of the participants’ characteristics grouped by gender is shown in . Abnormal mitral Doppler inflow was found in 595 participants (33%), with no significant differences between men and women. SpO2 ≤ 95% was found in 6.4%, significantly more frequently in men than in women (p < .001). Significantly more men than women had hypertension (p = .02), and LVEF <50% (p = .03), whereas women more frequently reported breathlessness (p = .003).
Table 1. Characteristics of 1782 participants grouped by gender.
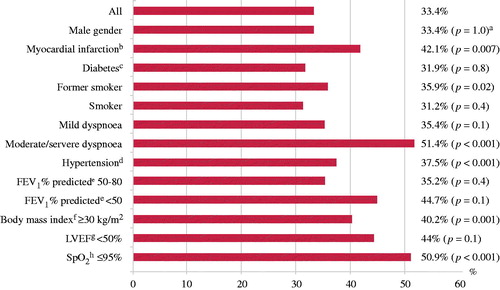
Self-reported myocardial infarction, former smoker, dyspnoea, hypertension, BMI ≥ 30 kg/m2, were all significant predictors of abnormal mitral Doppler inflow (). Among participants with reduced SpO2 (n = 114), 9.7% (n = 58) had abnormal mitral Doppler flow, compared to 4.7% (n = 56) of the 1187 participants with normal SpO2.
Figure 2. Frequency of abnormal mitral Doppler inflow by subject characteristics (in %) in the study sample of 1782 adults. aChi square statistic was used. bMissing n = 30. cMissing n = 37. dMissing n = 18. eMissing n = 19. fMissing n = 2. gMissing n = 323. hSpO2: arterial oxygen saturation as measured by pulse oximetry.
The results of the univariable and multivariable logistic regression analyses are presented in . SpO2 ≤ 95% was an independent predictor of abnormal mitral Doppler inflow both in Model 1 [OR 1.6 (CI 1.1–2.5)] and in Model 2, also adjusted for dyspnoea [OR 1.6 (CI 1.1–2.4)]. Other significant independent predictors in the fully adjusted Model 2 were age [OR 1.07 (CI 1.05–1.08)], self-reported myocardial infarction [OR 1.4 (CI 1.0–2.0)], former smoking [OR 1.3 (CI 1.0–1.7)], hypertension [OR 1.5 (CI 1.2–1.9)], BMI ≥ 30 kg/m2 [OR 1.2 (CI 1.1–1.8)], and moderate–severe dyspnoea [OR 1.7 (CI 1.1–2.4)].
Table 2. Factors associated with abnormal mitral Doppler inflow in the logistic regression model.
The Nagelkerke R-square for Model 1 and Model 2 were 0.13.
Since 348 participants included in this study did not have M-mode recordings of a quality justifying estimation, we made sensitivity analysis in a subgroup only including participants with M-mode recordings (n = 1434). In particular, the association between abnormal mitral Doppler inflow and SpO2 ≤ 95% persisted when the analyses were restricted to participants with EF≥50%, and other associations as well.
Discussion
To our knowledge, this is the first study to assess, in an adult population, the association between abnormal Doppler inflow and low pulse oximetry values. SpO2 ≤ 95% was found to be a significant predictor of abnormal mitral Doppler inflow also when adjusted for dyspnoea (OR 1.6). The study participants with SpO2 ≤ 95% were twice as likely to have abnormal mitral Doppler inflow as participants without SpO2 ≤ 95%. The strongest predictor of abnormal mitral Doppler inflow was moderate to very severe dyspnoea (OR 1.7).
In the current study of people older than 50 years, 33.0% had abnormal mitral Doppler inflow. This prevalence is higher than that reported in a Belgian study (27.3%) Our higher prevalence probably reflects that our population was older (average 67 versus 53 years) [Citation22]. The use of only one measure for DD might have contributed to the high prevalence. In a US study of people aged 45 years or older, the prevalence of pre-DD was 27.4% [Citation2]. In an Australian study of older participants (aged 60–85 years), the prevalence of pre-DD was 29.1% [Citation5].
BMI ≥30 was an independent predictor of abnormal mitral Doppler inflow. Obesity has been found to be both a risk factor for and cause of HF and is associated with haemodynamic changes that predispose to cardiac remodelling and ventricular dysfunction [Citation23].
Strengths and limitations
The participation rates of the Tromsø Study surveys have been high [Citation13]. In this survey, 66% of those invited took part. A lower participation rate among the oldest (80–87 years) might have led to an underestimation of age as a predictor of abnormal mitral Doppler inflow. In this age group, poor health is a likely reason for non-participation. The participants in the survey were mainly native Norwegians, which limits the generalizability to other ethnic groups. The data are cross-sectional and therefore causal relationships between abnormal mitral Doppler inflow and pulse oximetry values cannot be inferred.
Current recommendations for evaluation of DD require assessment of four different variables. If more than half of the variables are in the normal range, DD is considered not to be present [Citation18]. As stated above there is a limitation of our study that we only have abnormal mitral Doppler inflow as a measure of DD. M-mode-derived LVEF has inherent limitations that are known to both over- and underestimate the severity of dysfunction, and M-mode measurements do not necessarily reflect the true minor axis dimension [Citation24]. M-mode derived EF is estimated by Tiecholz formula assuming a spherical left ventricle, both over and underestimating the true EF due to the elliptical form of the ventricle and not accounting for regional hypokinesia due to myocardial scarring after myocarditis, myocardial infarctions or the causes of myocardial disease. However, because most of the participants were healthy, the shortcomings of the method are of less importance.
Echocardiographic measurements are more difficult to obtain in patients with COPD. Air trapping and emphysema may lead to impaired acoustic quality [Citation25,Citation26]. Accordingly, the association between abnormal FEV1% predicted and abnormal mitral Doppler inflow might have been blurred in this study.
There are also limitations related to the pulse oximetry measurements. Carboxyhaemoglobin from smoking can elevate the SpO2 value and may have led to an overestimation of SpO2 in heavy smokers. The accuracy of SpO2 measurements was not verified independently in this population-based survey. This could have been performed using arterial blood gas analyses in a subsample of the survey, but this was not part of the study protocol.
Causal relationships
Hypoxia may lead to DD [Citation10], and in the current study SpO2 ≤ 95% is a predictor of abnormal mitral Doppler inflow. Opposite, diastolic HF may affect pulmonary function [Citation27] and gas exchange [Citation8,Citation28,Citation29], and SpO2 may decrease accordingly [Citation9]. Arterial hypoxaemia in early stage patients with HFpEF could be due to HFpEF itself and/or to comorbidity with respiratory disease [Citation30]. However, due to the cross-sectional design, the study cannot establish causal relationships between abnormal mitral Doppler inflow and pulse oximetry values.
Conclusion
Decreased oxygen saturation measured by pulse oximetry (SpO2 ≤ 95%) was independently associated with abnormal mitral Doppler inflow. Age, hypertension, obesity, self-reported myocardial infarction, dyspnoea and former smoker, were also independent predictors of abnormal mitral Doppler inflow. Diastolic dysfunction should be considered when low SpO2 values are found.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Wan SH, Vogel MW, Chen HH. Pre-clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416.
- Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202.
- Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216.
- Echouffo-Tcheugui JB, Erqou S, Butler J, et al. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta-analysis. JACC Heart Fail. 2016;4:237–248.
- Abhayaratna WP, Marwick TH, Smith WT, et al. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart. 2006;92:1259–1264.
- Dalbak LG, Straand J, Melbye H. Should pulse oximetry be included in GPs' assessment of patients with obstructive lung disease? Scand J Prim Health Care. 2015;33:305–310.
- Vold ML, Aasebo U, Wilsgaard T, et al. Low oxygen saturation and mortality in an adult cohort: the Tromso study. BMC Pulm Med. 2015;15:9.
- Apostolo A, Giusti G, Gargiulo P, et al. Lungs in heart failure. Pulm Med. 2012;2012:952741.
- Masip J, Gaya M, Paez J, et al. Pulse oximetry in the diagnosis of acute heart failure. Rev Esp Cardiol (Engl Ed). 2012;65:879–884.
- Dhar S, Koul D, D'Alonzo GE Jr. Current concepts in diastolic heart failure. J Am Osteopath Assoc. 2008;108:203–209.
- Jacobsen BK, Eggen AE, Mathiesen EB, et al. Cohort profile: the Tromso Study. Int J Epidemiol. 2012;41:961–967.
- Vold ML, Aasebo U, Hjalmarsen A, et al. Predictors of oxygen saturation </=95% in a cross-sectional population based survey. Respir Med. 2012;106:1551–1558.
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586.
- O'Rourke RA, Hanrath P, Henry WN, et al. Report of the joint international society and federation of cardiology/world health organization task force on recommendations for standardization of measurements from M-mode echocardiograms. Circulation. 1984;69:854A–857A.
- Schirmer H, Lunde P, Rasmussen K. Mitral flow derived Doppler indices of left ventricular diastolic function in a general population; the Tromso study. Eur Heart J. 2000;21:1376–1386.
- Kessler KM. Ejection fraction derived by M-mode echocardiography: a table and comments. Cathet Cardiovasc Diagn. 1979;5:295–299.
- Tiwari S, Schirmer H, Jacobsen BK, et al. Association between diastolic dysfunction and future atrial fibrillation in the Tromso Study from 1994 to 2010. Heart. 2015;101:1302–1308.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338.
- Langhammer A, Johnsen R, Gulsvik A, et al. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord-Trondelag study. Eur Respir J. 2001;18:770–779.
- Kuznetsova T, Herbots L, Lopez B, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112.
- Alpert MA, Lavie CJ, Agrawal H, et al. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–356.
- Armstrong WF, Ryan T. Feigenbaum`s Echocardiography. Seventh edn. Philadelphia (USA): Lippincott Williams and Wilkins; 2009.
- Hawkins NM, Petrie MC, Jhund PS, et al. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–139.
- Wheeldon NM, MacDonald TM, Flucker CJ, et al. Echocardiography in chronic heart failure in the community. Q J Med. 1993;86:17–23.
- Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart fail. 2014;7:367–377.
- Guazzi M. Alveolar gas diffusion abnormalities in heart failure. J Card Fail. 2008;14:695–702.
- Higashi M, Yamaura K, Ikeda M, et al. Diastolic dysfunction of the left ventricle is associated with pulmonary edema after renal transplantation. Acta Anaesthesiol Scand. 2013;57:1154–1160.
- Andrea R, Lopez-Giraldo A, Falces C, et al. Lung function abnormalities are highly frequent in patients with heart failure and preserved ejection fraction. Heart Lung Circ. 2014;23:273–279.