Abstract
Objective. LncRNA TINCR has been reported to be involved in cardiac hypertrophy, while its involvement in diabetic cardiomyopathy is unknown. Materials and methods. In this study, myocardial biopsy and serum collected from patients with diabetic cardiomyopathy, diabetic patients without cardiopathy and healthy controls, and the expression of TINCR in those tissues was detected by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). ROC curve analysis was performed to evaluate the diagnostic value of TINCR expression for diabetic cardiomyopathy. Human cardiomyocyte cells (Cat# 12440053, Thermo Fisher Scientific) were treated with high glucose, and the expression of TINCR was detected by qRT-PCR. TINCR expression was transfected into cardiomyocyte cells and cell apoptosis under high glucose treated was detected by cell apoptosis assay. Results. We found that TINCR expression level in myocardial biopsy and serum was significantly lower in patients with diabetic cardiomyopathy than in diabetic patients without cardiopathy and healthy controls, while no significant differences were found between diabetic patients without cardiopathy and healthy controls. TINCR expression level can be used to effective diagnose diabetic cardiomyopathy. High glucose treatment showed no significant effects on the expression of TINCR in human cardiomyocyte cells, and TINCR overexpression inhibited apoptosis of cardiomyocytes under high glucose treatment. Conclusion. Therefore, we conclude that lncRNA TINCR is downregulated in diabetic cardiomyopathy and it can inhibit cardiomyocyte apoptosis.
Introduction
Diabetes mellitus is one of the most prevalent disorders, which causes a series of complications in the human body [Citation1]. As one of the most common types of diabetic complications, diabetic cardiomyopathy is featured by the early impairments in diastolic function, being accompanied by the occurrence of myocardial fibrosism cardiomyocyte hypertrophy and cardiomyocyte apoptosis [Citation2]. In the body of diabetic patients, Hyperglycemia-induced oxidative stress has been proved to be one of the major risk factors for occurrence of lesions in the diabetic myocardium, and this process is accompanied with altered signal transduction, abnormal gene expression and activation of apoptotic pathways leading to death of myocardial cells [Citation3]. Therefore, how to improve the survival of myocardial cells under high glucose conditions has become a major task of the treatment and it is a prevention of diabetic cardiomyopathy.
Studies during the last several decades have identified a considerable number of genetic factors that involved in the pathogenesis of diabetic cardiomyopathy [Citation4,Citation5]. However, the involvement of long non-coding RNAs (lncRNAs), which is a subgroup of non-coding RNAs composed of more than 200 nucleotides and plays pivotal roles in human disease [Citation6], is still unknown. A recent study reported that TINCR expression attenuates cardiac hypertrophy [Citation7]. It is known that cardiac hypertrophy is a frequently observed feature of diabetic cardiomyopathy [Citation8]. Therefore it will be reasonable to hypothesize that TINCR may also participate in diabetic cardiomyopathy. In our study, we observed that TINCR expression is downregulated in diabetic cardiomyopathy and expression of this lncRNA protects myocardial cells under high glucose conditions.
Materials and methods
Subjects and specimens
Specimens including myocardial biopsies and serum were collected from 33 diabetic cardiomyopathy patients, 27 diabetic patients without obvious complications and 17 healthy controls. Those participants were diagnosed and treated or only received pathological examinations on potential heart lesions in the First Affiliated Hospital of Changsha Medical University from January 2014 to January 2018. Healthy controls and diabetic patients without obvious complications received myocardial biopsy to suspected heart disease, but the diseases were finally excluded. Diagnosis of diabetic cardiomyopathy was performed according to the standards established by Chinese Medical Association (2014): (1) diagnosed diabetes; (2) diastolic dysfunction without enlarged heart or cardiac enlargement combined with impaired cardiac systolic function; (3) clinical manifestations of heart failure; (4) Excluded heart failure caused by coronary heart disease, hypertension heart disease and rheumatic valvular heart disease; (5) heart biopsy confirms microangiopathy and positive PAS staining. Diagnostic criteria of diabetes: (1) fasting blood glucose ≥7.0 mM; (2) two-hour postprandial blood glucose ≥11.1 mM; (3) HbA1c ≥ 6.5%. Inclusion criteria: (1) patients diagnosed and treated for the first time; (2) patients and/or their families signed informed consent. Exclusion criteria: (1) patients suffer from other types of cardiomyopathy; (2) patients suffer from other diabetic complications; (3) patients received any treatment before admission; (4) patients with other severe diseases. All participants were Han Chinese. The 33 patients with diabetic cardiomyopathy included 15 males and 18 females, and age ranged from 27 years to 65 years, with a mean age of 43.2 ± 6.1 years. The 27 diabetic patients without obvious complications included 14 males and 13 females, and age ranged from 25 to 66 years, with a mean age of 44.4 ± 5.7 years. The 17 healthy controls included 9 males and 8 females, and age ranged from 24 years to 59 years, with a mean age of 41.9 ± 6.2 years. There were no significant differences in age and gender among these three groups. BMI was significantly higher in patients with diabetic cardiomyopathy and diabetic patients without cardiomyopathy than that in healthy controls, while no significant differences in BMI were found between diabetic cardiomyopathy patients and diabetic patients without cardiomyopathy. Ethics committee of the First Affiliated Hospital of Changsha Medical University approved this study and all participants signed informed consent.
Real-time quantitative PCR
Trizol reagent (Invitrogen, USA) was used for all RNA extraction in strict accordance with manufacturer's instructions. RNA concentration was measured using NanoDrop™ 2000 Spectrophotometers (Thermo Fisher Scientific, USA), followed by cDNA synthesis throgh reverse transcription. Primers used in PCR reactions were: 5′-TGTGGCCCAAACTCAGGGATACAT-3′ (forward) and 5′-AGATGACAGTGGCTGGAGTTGTCA-3′ (reverse) for lncRNA TINCR; 5′-GACCTCTATGCCAACACAGT-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse) for β-actin. Conditions of PCR reactions were: 95 °C for 48 s, followed by 40 cycles of 95 °C for 18 s and 57 °C for 40 s. Ct values were processed using 2−ΔΔCT method to mormalized TINCR expression to β-actin endougenous control.
Cell line, cell culture and cell transfection
Full length lncRNA TINCR cDNA was amplified through PCR reaction using primers with ECOR I cutting site at 5’ end. TINCR cDNA were then digested with ECOR I and inserted into ECOR I linearized pIRSE2-EGFP vector (Clontech, Palo Alto, CA, USA) to establish TINCR expression vector. Human cardiomyocyte cell line AC16 was provided by EMD Millipore. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 12% fetal bovine serum (FBS) in an incubator and 1% antibiotics (penicillin and streptomycin) at 37 °C with 5% CO2/95% air). Transfection was performed using lipofectamine 2000 reagent (11668-019, Invitrogen, Carlsbad, USA) to transfect 10 nM vectors into 6 × 105 cells. Empty pIRSE2-EGFP vector transfection was also performed to serve as a negative control. Overexpression of TINCR was confirmed by qRT-PCR before subsequent experiments.
Cell apoptosis assay
Cell apoptosis assay was performed to measure cell apoptotic rate at 24 h after transfection. Cell suspensions (4 × 104 cells/ml) were prepared and cultivated in a 6-well plate with 10 ml in each well. D-glucose was added to a final concertation of 10, 20 40 and 60 mM. Cells were cultivated for 24 h, followed by digestion with 0.25% trypsin. Following propidium iodide and Annexin V-FITC (Dojindo, Japan) staining, apoptotic cells were detected by flow cytometry.
Statistical analysis
All data analyses were performed using SPSS19.0 (SPSS Inc., USA). Expression and cell apoptosis data were expressed as mean ± standard deviation and compared by one-way analysis of variance, followed by LSD test. p < .05 was considered to be statistically significant.
Results
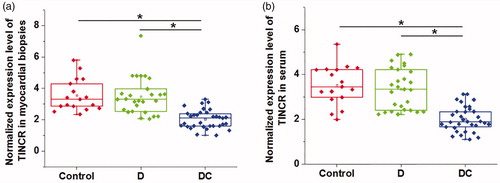
Comparison of TINCR expression among diabetic cardiomyopathy patients, diabetic patients without obvious complications and healthy controls
Differential expression of a gene in lesion tissues and corresponding healthy tissues indicates its involvement in diseases. Therefore, expression of TINCR in myocardial biopsies and serum derived from diabetic cardiomyopathy patients, diabetic patients without obvious complications and healthy controls was detected by qRT-PCR. As shown in , expression of TINCR was significantly downregulated in diabetic cardiomyopathy patients than that in diabetic patients without obvious complications and healthy controls in both myocardial biopsies () and serum () (p < .05). However, no significant differences in the expression of TINCR were found between diabetic patients without obvious complications and healthy controls (p < .05). Therefore, downregulation is likely to be specifically involved in diabetic cardiomyopathy. We further analyzed the correlations between expression levels of TINCR in serum and myocardial biopsies by Pearson’s correlation coefficient. Results showed that expression levels of TINCR in serum and myocardial biopsies were significantly and positive correlation in diabetic cardiomyopathy patients (r = 0.89, R square = 0.79, p < .001), diabetic patients without obvious complications (r = 0.88, R square = 0.78, p < .001) and healthy controls (r = 0.81, R square = 0.66, p < .001).
Figure 1. Comparison of TINCR expression among diabetic cardiomyopathy patients, diabetic patients without obvious complications and healthy controls. This figure shows the comparison of TINCR expression in myocardial biopsies (a) and serum (b) among diabetic cardiomyopathy patients, diabetic patients without obvious complications and healthy controls. The experiments were performed for three times. Data here were represented by five lines, from low to upper: the lowest value, lower 1/4 value, median value, upper 1/4 value and the highest value. Notes: *: p < .05; DC: diabetic cardiomyopathy; D: diabetes only.
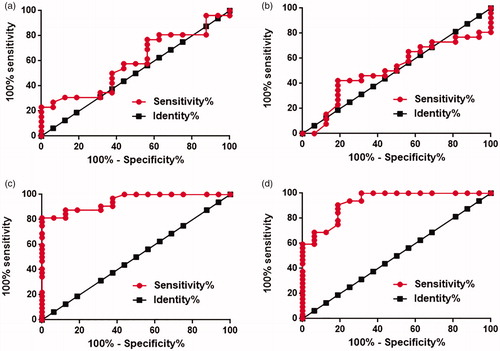
Potential of TINCR expression for the diagnosis of diabetes and diabetic cardiomyopathy
ROC curve analysis was performed to evaluate the diagnostic values of TINCR expression in myocardial biopsies and serum for diabetes and diabetic cardiomyopathy. For the diagnosis of diabetes, area under the curve (AUC) of TINCR expression in myocardial biopsies was 0.59 with standard error of 0.090 and 95% confidence interval of 0.41–0.76 (, p>.05). AUC of TINCR expression in serum was 0.51 with standard error of 0.092 and 95% confidence interval of 0.33–0.69 (, p>.05). For the diagnosis of diabetic cardiomyopathy, area under the curve (AUC) of TINCR expression in myocardial biopsies was 0.95 with standard error of 0.029 and 95% confidence interval of 0.89–1.0 (, p < .001). AUC of TINCR expression in serum was 0.93 with standard error of 0.038 and 95% confidence interval of 0.85 to 1.0 (, p < .01). Therefore, downregulated TINCR expression may serve as a potential diagnostic biomarker for diabetic cardiomyopathy but not diabetes.
Figure 2. Potential of TINCR expression for the diagnosis of diabetes and diabetic cardiomyopathy. This figure showed the ROC curve of the diagnosis of diabetes using TINCR expression in myocardial biopsies (a) and serum (b), and the ROC curve of the diagnosis of diabetic cardiomyopathy using TINCR expression in myocardial biopsies (c) and serum (d). The experiments were performed for three times.
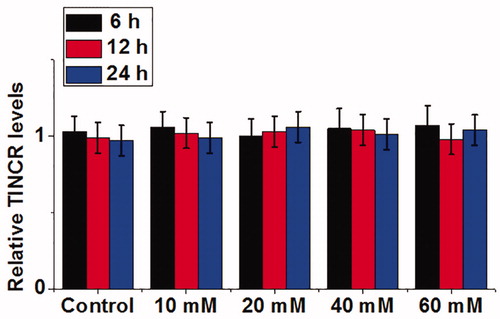
Effects of high glucose treatment on TINCR expression in cells of human cardiomyocyte cell line AC16
Cells of human cardiomyocyte cell line AC16 were treated with d-glucose at concentrations of 10, 20, 40 and 60 mM for 6, 12 and 18 h, and TINCR expression was detected by qRT-PCR. As shown in , compared with cells cultured in normal culture medium (5 mM glucose), no significant differences were found in TINCR expression in cells treated with 10, 20, 40 and 60 mM d-glucose at different time points (p > .05). Therefore, downregulation of TINCR in diabetic cardiomyopathy may not be induced by high glucose.
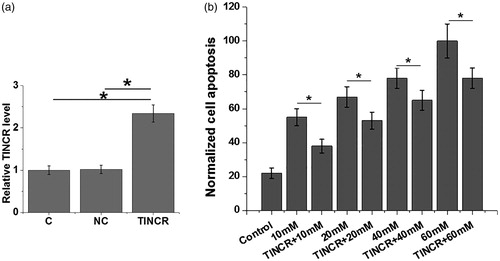
Effects of TINCR overexpression on apoptosis of cells of human cardiomyocyte cell line AC16 under high glucose treatment
As shown in , overexpression rate of TINCR above 200% was reached at 24 h after transfection (p < .05). Cell apoptosis was detected at 24 h after transfection. As shown in , cell apoptosis of cells of human cardiomyocyte cell line AC16 increased with increasing d-glucose concentrations. At each d-glucose concentration, TINCR overexpression significantly reduced the cell apoptosis rate (p < .05). Therefore, TINCR may protect cardiomyocyte cell in patients with diabetes, therefore inhibiting the development of diabetic cardiomyopathy.
Figure 4. Effects of TINCR overexpression on apoptosis of cells of human cardiomyocyte cell line AC16 under high glucose treatment. Overexpression rate of TINCR above 200% was reached at 24 h after transfection (a). The experiments were performed for three times. At each d-glucose concentration, TINCR overexpression significantly reduced the cell apoptosis rate (b). Data here were represented by mean ± standard deviation. Notes: *: p < .05.
Discussion
Previous studies have shown that TINCR is a key player in cardiac hypertrophy and different types of human malignancies [Citation7,Citation9,Citation10]. The key finding of our study is that TINCR may also participate in the development of diabetic cardiomyopathy but not in diabetes. TINCR expression inhibits the apoptosis of cardiomyocyte cells, thus attenuate diabetic cardiomyopathy. However, the specific molecular mechanism remains unclear.
Progression of diabetes is accompanied by the development of a series of complications in major organs as well as changes in the expression pattern of a large set of lncRNAs [Citation11]. In a recently study, altered expression of a considerable number of lncRNAs has also been identified in mice model of diabetic cardiomyopathy [Citation12]. Specifically, lncRNA H19 was significantly downregulated in rat model of diabetic cardiomyopathy, and overexpression of H19 inhibits cardiomyocyte apoptosis by targeting VDAC1 [Citation13]. However, altered expression of H19 seems to be involved in the whole procedure of the development of diabetes but not only in heart tissues [Citation14]. Altered expression of lncRNA MALAT1 has also been observed in diabetic cardiomyopathy [Citation15], but this abnormal expression of MALAT1 has also been observed in tissues other than heart [Citation16]. Therefore, H19 and MALAT1 may not be promising therapeutic targets for diabetic cardiomyopathy. Up to now, the functionality and expression pattern of most lncRNAs in diabetic cardiomyopathy is still unknown.
Although cardiac hypertrophy is not a prerequisite of diabetic cardiomyopathy, it is frequently observed during the development of this disease [Citation8]. It has been reported that TINCR expression improves cardiac hypertrophy [Citation7]. In our study, downregulated expression of TINCR was observed in diabetic cardiomyopathy patients but not in diabetic patients without obvious complications. ROC curve analysis also showed that downregulation of TINCR effectively distinguished patients with diabetic cardiomyopathy form heathy controls but showed no potentials in the diagnosis of diabetes. In addition, TINCR overexpression significantly reduced the cell apoptosis rate of human cardiomyocytes. Therefore, TINCR may serve as a potential therapeutic target and diagnostic biomarker for diabetic cardiomyopathy.
High glucose conditions in diabetic patients induce altered expression of a large set of lncRNAs in the human body [Citation17]. To our surprise, high glucose treatment at different concentrations showed no significant effects on TINCR expression in human cardiomyocytes. Hyperglycemia-induced oxidative stress is the major cause of myocardial cell apoptosis, which is the main pathological change of diabetic cardiomyopathy [Citation3]. Therefore, TINCR may not participate in the hyperglycemia-induced initiation of diabetic cardiomyopathy but play a role in the following occurrence of heart pathological changes.
TINCR achieves its biological roles trough the interactions with multiple signaling molecule such as different mRNA [Citation18] and calmodulin-like protein 5 [Citation19]. However, the interactions between TINCR and those molecule and several anti-proliferative and pro-apototic pathways were not observed in diabetic cardiomyopathy after TINCR overexpression (data not shown). Therefore, the molecular mechanism of the action of TINCR in diabetic cardiomyopathy remains to be further investigated.
In conclusion, the specific downregulation of TINCR is likely involved in the pathogenesis of diabetic cardiomyopathy possibly through the inhibition of cardiomyocytes. Our study provided a novel therapeutic target and diagnostic marker for diabetic cardiomyopathy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Nathan DM. DCCT/Edic Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Dia Care. 2014;37:9–16.
- Huynh K, Bernardo BC, McMullen JR, et al. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375–415.
- Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig. 2014;5:623–634.
- Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122:624–638.
- Frati G, Schirone L, Chimenti I, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113:378–388.
- Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett. 2013;339:159–166.
- Shao M, Chen G, Lv F, et al. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget 2017;8:47565–47573.
- Miki T, Yuda S, Kouzu H, et al. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–166.
- Xu TP, Liu XX, Xia R, et al. SP1-induced upregulation of the long noncoding RNA TINCR regulates cell proliferation and apoptosis by affecting KLF2 mRNA stability in gastric cancer. Oncogene. 2015;34:5648–5661.
- Zhang Z, Lu Y, Zhang Z, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget. 2016;7:22639–22649.
- Ruan Y, Lin N, Ma Q, et al. Circulating LncRNAs Analysis in Patients with Type 2 Diabetes Reveals Novel Genes Influencing Glucose Metabolism and Islet β-Cell Function. Cell Physiol Biochem. 2018;46:335–350.
- Pant T. Microarray analysis reveals deregulated LNCRNAS and MRNAS in DB/DB mice plasma and heart: diagnostic biomarkers of diabetic cardiomyopathy. Biophys J. 2018;114:248a.
- Li X, Wang H, Yao B, et al. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci Rep. 2016;6:36340
- Gao Y, Wu F, Zhou J, et al. The H19/let-7 double-negative feedback loop contributes to glucose metabolism in muscle cells. Nucleic Acids Res. 2014; 42:13799–13811.
- Zhang M, Gu H, Chen J, et al. Involvement of long noncoding RNA MALAT1 in the pathogenesis of diabetic cardiomyopathy. Int J Cardiol. 2016;202:753–755.
- Liu JY, Yao J, Li XM, et al. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506.
- Tang W, Zhang D, Ma X. RNA-sequencing reveals genome-wide long non-coding RNAs profiling associated with early development of diabetic nephropathy. Oncotarget. 2017;8:105832–105847.
- Kretz M, Siprashvili Z, Chu C, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–235.
- Sun BK, Boxer LD, Ransohoff JD, et al. CALML5 is a ZNF750- and TINCR-induced protein that binds stratifin to regulate epidermal differentiation. Genes Dev. 2015;29:2225–2230.