 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives: The diagnosis of cardiovascular involvement in ankylosing spondylitis (AS) is usually delayed since conventional echocardiography relies mainly on the morphological alterations. The aim of this study was to evaluate the role of echocardiographic methods such as tissue Doppler and strain imaging of left ventricle (LV) and proximal aorta; and concentrations of biomarkers of cardiac fibrosis such as galectin-3 (Gal-3) and soluble suppression-of-tumorogenicity-2 (sST2) in determining early cardiovascular impairment in AS. Design: In this prospective study of 75 AS and 30 healthy subjects (mean age 41.7 ±10.1 years; 37.3% female), we determined layer-specific strain and strain rates in longitudinal, circumferential and radial axes for LV as well as transverse and longitudinal strains of proximal aorta; central pulse wave velocity(cPWV); plasma high sensitivity C-reactive protein(hsCRP), Gal-3 and sST2 levels. Results: Patients with AS had increased levels of hsCRP and sST2 when compared to healthy controls. cPWV, E and e' velocities; longitudinal strain and strain rates at all myocardial layers; and transverse strains of both anterior and posterior aortic walls were reduced in AS patients. Gal-3 levels with strain and strain rates at circumferential and radial axes were similar between the groups. Among all echocardiographic and clinical parameters, AS was independently associated with LV dysfunction (expressed by longitudinal strain of LV) and aortic impairment (expressed by transverse strain of anterior wall). Conclusions: This study demonstrates that functional impairment in AS occurs early in the disease course and strain imaging is an effective tool in discriminating involvement. sST2 may represent the link between inflammation and fibrosis in AS.
Introduction
Ankylosing spondylitis (AS) is an inflammatory rheumatic disease, which affects the axial joints and spine [Citation1]. Among the extra-articular manifestations of AS, cardiovascular involvement is prevalent and cardiovascular diseases constitute the main cause of mortality in AS [Citation2,Citation3].
Sclerosing inflammatory process in AS affects primarily the region around the aortic root; extending to aortic valves, subaortic interventricular septum, anterior mitral leaflet and myocardium [Citation4]. The clinical presentation, therefore, constitutes a wide spectrum of manifestations such as aortitis, aortic dilatation, aortic valvular regurgitation, conduction disturbances, mitral regurgitation and myocardial dysfunction [Citation4]. Two dimensional transthoracic echocardiography (TTE) detects cardiovascular involvement only when the morphological changes become apparent; which accounts for the increased cardiovascular mortality. This calls need for a more detailed approach using different echocardiographic methods to diagnose early cardiovascular involvement.
We hypothesized that functional changes in aorta and left ventricle (LV) in AS precede those in morphology. The aim of this study was, therefore, to evaluate the role of tissue Doppler imaging; strain imaging and biochemical markers in early diagnosis of functional impairment in cardiovascular system. Layer-specific strain imaging of LV and proximal aorta was performed in conjunction to conventional echocardiography. Doppler indices of diastolic dysfunction that relate to myocardial fibrosis were evaluated [Citation5,Citation6]. We also measured central pulse wave velocity (cPWV) and serum concentrations of biomakers of disease activity and cardiac fibrosis. PWV, the rate at which the blood travels in the vasculature, is a measure of aortic elasticity; an early target of AS [Citation7]. Serum concentration of hsCRP is an indicator of AS activity, while galectin-3 (Gal-3) and soluble suppression-of-tumorogenicity-2 (sST2) are novel biomarkers of cardiac fibrosis [Citation8].
Materials and methods
In this prospective observational study, 75 AS patients diagnosed using modified New York criteria who were free of cardiovascular disease or overt cardiovascular involvement were compared with 30 healthy control subjects. The study protocol conformed to 1975 Declaration of Helsinki and was approved by the local Ethics committee. Patients were recruited consecutively among AS patients who attended a routine follow up visit at the rheumatology clinic. For comparisons, 30 age- and sex-matched individuals who were free of any known disease were consecutively recruited. After recording the demographic data of patients; physical examination was conducted to determine limitations in thorax expansion, lumbar lateral flexion, tragus wall distance, cervical rotation and the Schober test. The medications taken by each patient were recorded and then categorized into three groups with respect to mode of action as follows: Nonsteriodal anti-inflammatory drugs (NSAID), disease-modifying antirheumatic drugs (DMARD) or tumor necrosing factor inhibitors (antiTNF). Next, fasting blood samples were collected to determine hsCRP, sST2 and Gal-3 concentrations. All patients completed Bath ankylosing spondylitis disease activity index (BASFI), Bath ankylosing spondylitis disease activity index (BASDAI) and ankylosing spondylitis quality of life (ASQoL) questionnaires with the help of a physiotherapist. cPWV was determined for each patient oscillometrically. Body surface area (BSA) was calculated using Du Bois formula and body mass index (BMI) was calculated as weight (kg), divided by height square (m2) [Citation9].
Cardiovascular diseases that are known to impair cardiac mechanics or those that can alter the circulating levels of cytokines were not included to the study. All patients with known cardiovascular disease such as hypertension, atherosclerotic cardiovascular disease such as coronary-peripheral or carotid disease; moderate severe valvular disease; myocardial diseases and heart failure were excluded from the study. Patients with diabetes mellitus, chronic renal failure (estimated GFR <60 mL/min/1.73 m2), known malignancy, chronic liver disease and thyroid disorders were not included to the study as well.
Of 101 consecutive AS patients; 86 patients who fulfilled the inclusion criteria were involved. Eleven patients who had poor acoustic window that hampered strain imaging, were further excluded from the study. A total of 75 patients (mean age 40.8 ± 10.1, 39% female) were involved in the final analyses.
cPWV measurements
cPWV was measured by an experienced physician using AngioExperience Pro8 (AngETM, Sonotechnik, Austria) device. This is an 8-channel pulse oscillography device with ECG-trigger that allows calculation of pulse propagation. Four cuffs, placed on wrists and ankles on both sides, are used for routine assessment of cPWV [Citation10–13]. cPWV is calculated using the difference of the pulse propagation time between the pulse waves on ankle and wrist. The following formula is applied in which Δdistance is the difference of the distances to ankle and wrist; and Δtime is the difference between onset of pulse waves to the time reference on ECG.
TTE examination
All patients underwent a detailed TTE with Siemens Acuson SC2000 cardiac ultrasound system (Mountain View, CA, USA) and 4V1C (1.75–4.3 MHz) transducer by an experienced physician blinded to the study groups. Conventional 2D echocardiography at parasternal long axis view was used to determine the aortic dimensions at the aortic root, sinus of valsalva, sinotubular junction and ascending aorta as described earlier [Citation14]. Left atrial volume index (LAV) was measured using area length method and indexed to BSA; left ventricular ejection fraction (LVEF) was determined using modified Simpsons Method [Citation14]. Pulsed-wave Doppler was performed at mitral inflow to measure early (E) and late (A) filling velocities; deceleration time (DT) and isovolumetric relaxation time (IVRT). Lateral and septal mitral annular early diastolic (e') tissue velocities were determined by tissue Doppler. Septal tissue velocity measurements were used to acquire deceleration time of e' and IVRTseptal as previously described [5,6]. LV mass was calculated using linear method and was indexed to BSA [Citation14].
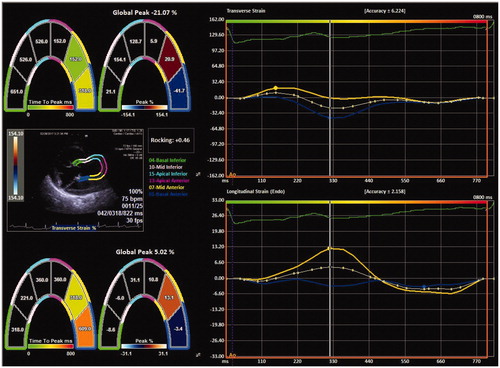
Three consecutive cardiac cycles were recorded at end expiratory apnea for subsequent offline analysis of LV deformation. Syngo Siemens software package which utilizes velocity vector imaging (VVI) was used for the evaluation of layer specific LV strain and strain rates. The methods of image acquisition and post processing for LV strain were carried out as described previously [Citation15]. Layer-specific longitudinal strain (LS), circumferential strain (CS), radial strain (RS) and strain rate (SR) parameters were calculated from appropriate windows as described in the literature [Citation15]. Only absolute values of the strain parameters are reported. Deformation imaging of the aorta was performed from parasternal long axis view. Since the dedicated software does not have aortic wall strain; we used apical two chambers view of LV to evaluate aortic deformation. Biesevicience et al [Citation16] used a similar method to evaluate biomechanics of dilative aortas. The inner contour of the proximal aortic wall was traced during systole, and the region of interest was corrected manually by adjusting the diameter. The apical segments of the LV analysis in standard apical two chambers view were omitted and six segments were reduced to four, dividing aortic wall into two anterior and two posterior segments. Anterior and posterior aortic wall strain parameters were evaluated separately ().
Figure 1. Strain imaging of posterior aortic wall using velocity vector imaging (VVI) in proximal aorta.
For intra-observer variability, 15 randomly selected patients were re-evaluated by the same observer blinded to the first measurements; for inter-observer variability, these patients were re-evaluated by a second observer (DB). The inter- and intra observer correlation coefficients of strain parameters were ≥0.75 (Supplementary Table).
Table 1. Demographic and disease characteristics of the study population.
Biochemical analyses
Fasting serum samples (5 mL) were collected from an antecubital vein without venous occlusion between 9–11 am in the morning. The samples were centrifuged at 3000 rpm for 10 minutes, the serum was collected and stored at −86°C until biochemical analyses. HsCRP, sST2, and Gal-3 concentrations were determined by Enzyme-linked immunosorbent assay (ELISA) method based on the competition principle and microtiter plate separation. Human sST2 kit (Human ST2/IL-1R4 ELISA kit, Aviscera Bioscience, CA, USA) had inter-assay and intra-assay CV% as 8–10% and 4–6%; and the minimum detectable dose of human sST2 was 5 pg/mL. Human Gal-3 kit (Human Galectin-3 ELISA kit, Invitrogen™, CA, USA) had inter-assay and intra-assay CV% as 5.4% and 7.5%; and the minimum detectable dose of human Gal-3 was 0.29 ng/mL. Human hsCRP kit (High Sensitivity C-Reactive Protein ELISA kit, DRG International Inc, NJ, USA) had inter-assay and intra-assay CV% as <4.1% and <7.5%; and the minimum detectable dose of hsCRP was 0.01 mg/L.
Statistical analyses
Statistical analyses were performed using IBM® SPSS® Statistics for Mac, Version 20 software (IBM Corp., Armonk, NY). The continuous variables were tested for normal of distribution using Kolmogorov Smirnov Test. Continuous variables with normal distribution were expressed as mean ± standard deviation (SD); and those without normal distribution were expressed as median (min-max); the categorical variables were presented as number (percentages). The AS patients were compared with controls by independent samples t test or Mann Whitney U test as appropriate. Categorical variables were compared by Chi square. Linear regression analysis was used to describe whether, amongst all clinical and echocardiographic variables, presence of AS was an independent predictor of myocardial involvement (expressed by LV longitudinal strain parameters) or aortic involvement (expressed by transverse strain of anterior and posterior walls). All variables with p≤.10 were included into model. A p value of <.05 was considered statistically significant. The minimum sample size of the study was calculated using G*Power version 3.1 for Mac software, by adjusting the effect size as 0.8, alpha error as 0.05 and power as 0.95.
Results
The demographic and disease characteristics of AS patients with respect to control subjects are expressed in . The mean age of AS patients was 41.7 ± 10.1 years (37.3% female). There was no difference between AS patients and controls in terms of age, sex, current smoking status, BSA and BMI. The median duration of symptoms was 10 and time from diagnosis was 5 years, reflecting a young study population with relatively short duration of AS. The limitations revealed by physical examination and scores of BASFI, BASDAI and ASQoL questionnaires are presented in the . Most of the patients were on NSAID and/or antiTNF drugs.(52%)
cPWV, biochemical markers and echo parameters
The two groups were compared in terms of cPWV, biochemical markers, and echocardiographic parameters, all of which were summarized in . The cPWV was higher in AS compared to control subjects (p = .011). Of biochemical markers, hsCRP and sST2 were elevated in AS patients. Gal-3 concentrations, however, were similar in AS and control groups.
Table 2. Comparison of AS patients and control subjects in terms of cPWV, biochemical markers and conventional echocardiography parameters.
Although still within the normal range, aortic diameters at sinus of Valsalva and ascending aorta in AS patients were higher than those in the control subjects. The aortic diameters at root and sinotubular junction however were similar within groups. Of conventional echocardiographic parameters, there were no difference between two groups with respect to LAVI, LVEF, LVMI, A wave, IVRT, DT and E/e'. Nevertheless, E velocity and E/A ratio were diminished in AS patients. Moreover, tissue Doppler velocities of e' at lateral and septal annuli were reduced and a' velocity and IVRT at septum were increased in patients with AS. There were more patients with aortic regurgitation in AS group but the prevalence of mitral regurgitation was similar.
Strain imaging of ascending aorta and LV using VVI
LV and aortic deformation parameters with respect to study groups are presented in . The layer specific deformation imaging of LV revealed that the LS and LSR at all three layers of the LV myocardium were reduced in AS. The CS and CSR parameters, on the other hand, were similar within the study population. For RS and RSR, there was no difference between the groups.
Table 3. Comparison of deformation parameters in LV and proximal aorta between the study groups.
Of all the aortic deformation parameters, the transverse strain of the anterior and posterior aortic walls were significantly reduced in the AS patients when compared to healthy controls. Linear regression analysis that evaluated associations of different variables with LS revealed that AS was the single independent predictor of myocardial involvement, expressed by impaired LS of different layers of LV. AS was also an independent predictor of impaired strain in anterior aortic wall ().
Table 4. Linear regression analysis to reveal the predictors of strain parameters.
Discussion
The main findings of the current study are (1) Elastic tissue damage of the aortic wall in AS causes functional alterations before morphological changes become obvious; which is evidenced by increased cPWV and decreased aortic transverse strain, despite relatively normal aortic dimensions. (2) Tissue Doppler indices of myocardial fibrosis and strain imaging can be used to reveal myocardial involvement early in the disease course. (3) Increased sST2 may contribute to the pathogenesis of cardiac fibrosis in AS.
The prevalence of cardiovascular involvement in AS varies depending on the diagnostic modality used and the pathology described, ranging between 10–40% [Citation4,Citation17]. Previous studies reported cardiac manifestations to be more frequent in long standing AS, which was defined as AS longer than 15 years [Citation18]. In our study, patients' median AS duration was 5 years and mean age was 41.7 ± 10.1 years, which represents a young study population with 'early AS'. Moreover, the physical examination and disease specific instruments such as BASFI and BASDAI revealed mild limitation with minimal disease activity in the study population. In this context, the pathological changes described by this study have clinical implications for the early diagnosis of cardiovascular involvement.
Aortic involvement in AS
The exact prevalence of aortitis in AS patients is not known, since there is no specific imaging modality that identifies the pathology. Histological studies have shown focal destruction of muscular and elastic structures with thickening of the intima [Citation19]. Echocardiographic studies, therefore, have focused on the morphological changes such as increase in the aortic wall thickness or diameter, both of which occur late in the disease course [Citation18,Citation20]. Despite of relatively young age and short duration of AS in our study population, the aortic diameters were higher than those in healthy individuals at sinus of Valsalva and ascending aorta. Nevertheless, the aortic dimensions were still within the reference range for most patients obscuring the recognition. Additional evaluation of the functional impairment in aortic wall using cPWV and strain imaging provided incremental diagnostic value. Our results revealed increased cPWV, as an evidence for the increased aortic stiffness and deteriorated elastic functions in AS. This is in parallel with published literature [Citation21]. Yet, PWV provides estimate of aortic stiffness and does not describe local vascular characteristics. To reveal the elastic impairment in the proximal ascending aorta locally, we studied strain parameters of aortic wall using VVI. In this context, strain parameters in transverse direction, were decreased. Aorta is an elastic buffering chamber which stores 50% of stroke volume during systole [Citation22]. Our findings indicate that AS impairs aortic deformation in transverse axis and that it is feasible to noninvasively quantify aortic strain using VVI. Similarly, Kim et al [Citation23] used VVI of descending aorta in TEE, to quantify the disruption of local elastic properties with aging and showed that decreased circumferential strain was associated with increased collagen content in aortic wall. Noninvasive methods using VVI for arterial assessment were used in several clinical studies to successfully quantify vascular pathology in Marfan [Citation24] and arteritis [Citation25] but there is no studies on AS. Bell et al, who have analyzed longitudinal aortic strain in magnetic resonance imaging (MRI) have found that it results in underestimation of circumferential strain and should be incorporated into calculation of circumferential strain. Although the longitudinal strain parameters of the ascending aorta were similar in present study population, we did not correct the transverse strain by longitudinal strain [Citation26,Citation27].
Myocardial involvement in AS
Myocardial involvement in AS usually manifests as diastolic dysfunction [Citation28]. Brewerton et al. [Citation29] have conducted an echocardiographic and histological study in which they demonstrated that most of AS patients had deteriorated diastolic function and increased interstitial connective tissue. We have sought to demonstrate myocardial fibrosis both with echocardiographic parameters and biomarkers of cardiac fibrosis, such as Gal-3 and sST2. Taken together, decreased E, e' velocities at septal and lateral annuli and E/A ratio; increased a' and IVRT at septal annuli correspond to impaired LV relaxation. Decreased LS and LSR of LV myocardium further confirmed the presence of subclinical systolic dysfunction in early AS. After adjusting for all clinical, echocardiographic and biochemical parameters, AS remained independently associated with reduced LV strain at all myocardial layers. Layer-specific strain analysis showed that LS and LSR at all myocardial layers were reduced, but CS and RS parameters were preserved in AS group. Although subclinical myocardial systolic dysfunction in AS was previously demonstrated, this is the first study to reveal this distinct pattern of involvement [Citation30]. Autoimmune diseases tend to selectively infiltrate different myocardial layers [Citation31]. MRI of LV in AS, showed focal hyperenhancement of midwall and subepicardial layers [Citation31]. In support of these findings, the association of AS with LS at myo was stronger than other layers in our study. The MRI study also showed correlation of increased myocardial extracellular volume with CRP concentrations [Citation31]. Despite the prevalent use of potent anti-inflammatory drugs, hsCRP levels were closely associated to LS in univariate analyses in the current study as well.sST2 is a decoy receptor belonging to the interleukin-1 receptor family, that stimulates cardiac fibrosis and ventricular remodeling [Citation8,Citation32]. Li et al. have [Citation33] demonstrated increased mRNA levels of sST2 in AS patients and suggested that it may contribute to the inflammatory pathogenesis of AS. Consistent with previous study, we showed increased sST2 in AS, which can be a potential link between inflammation and myocardial fibrosis. sST2 was predictor of subclinical LV dysfunction of all myocardial layers in univariate analyses. After adjustment of other predictors such as presence of AS, however, this association did not persist; which further confirms its effects are through presence of AS. Gal-3 is a soluble beta glucosidase binding glycoprotein which is secreted by macrophages, stimulating proliferation of myofibroblasts and deposition of collagen [Citation34]. It is a well established predictor of heart failure development [Citation34]. Gal-3 levels were not associated with neither presence of AS nor ventricular strain parameters in the current analyses. This suggests that the underlying pathophysiology of fibrosis in AS is different from that of heart failure triggered by other causes.
The main limitations of the study were small sample size; and the lack of a panel of established markers of myocardial fibrosis. Biomarkers of collagen synthesis and breakdown such as collagen type I C terminal telopeptide (CITP) or Procollagen type I C terminal propeptide(PICP) are shown to be related to myocardial fibrosis and could have been integrated into the study panel to give a thorough understanding of the pathophysiological basis of myocardial fibrosis.
In conclusion, our data showed that systemic inflammation in AS is not confined to skeletal system. Involvement of cardiovascular system, manifested as functional impairment in the proximal aorta and as subclinical dysfunction in LV precedes the morphological changes. Strain imaging using VVI is a noninvasive and feasible method to determine decreased transverse strain in the proximal aorta, and decreased longitudinal strain of all myocardial layers in the LV in early diagnosis. We also suggest that increased sST2 may be a possible link between inflammation and fibrosis in AS.
Supplemental Material
Download MS Word (82.2 KB)Acknowledgements
We would like to thank Dr Niyazi Guler for his advice and contribution to the study.
Disclosure statement
The authors report no conflict of interest.
References
- Maghraoui AE. Extra-articular manifestations of ankylosing spondylitis: prevalence, characteristics and therapeutic implications. Eur J Intern Med. 2011;22:554–560.
- Yang DH. Ankylosing spondylitis and cardiac abnormalities. J Cardiovasc Ultrasound. 2012;20:23–24.
- Papagoras C, Voulgari PV, Drosos AA. Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin Exp Rheumatol. 2013;31:612–620.
- Lautermann D, Braun J. Ankylosing spondylitis – Cardiac manifestations. Clin Exp Rheumatol. 2002;20:11–15.
- Mu¨ller-Brunotte R, Kahan T, Malmqvist K, et al. Tissue velocity echocardiography shows early improvement in diastolic function with Irbesartan and atenolol therapy in patients with hypertensive left ventricular hypertrophy. Results form the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA). Am J Hypertens. 2006;19:927–936.
- Mu¨ller-Brunotte R, Kahan T, López B, et al. Myocardial fibrosis and diastolic dysfunction in patients with hypertension: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation versus Atenolol (SILVHIA). J Hypertens. 2007;25:1958–1966.
- Nemes A, Geleijnse ML, Forster T, et al. Echocardiographic evaluation and clinical implications of aortic stiffness and coronary flow reserve and their relationship. Clin Cardiol. 2008;31:304–309.
- Boer RA, Daniels LB, Maisel AS, et al. State of the Art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–569.
- Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. Nutrition. 1989;5:303–311.
- Passon SG, Ku¨llmar V, Blatzheim AK, et al. Carotid strain measurement in patients with pseudoxanthoma elasticum - Hint for a different pathomechanism? Intractable Rare Dis Res. 2018;7:25–31.
- Pingel S, Pausewang KS, Passon SG, et al. Increased vascular occlusion in patients with pseudoxanthoma elasticum. Vasa. 2017;46:47–52.
- Pizarro C, Linnhoff F, van Essen F, et al. Lower extremity and carotid artery disease in COPD. ERJ Open Res. 2016;26;2.
- Schaefer CA, Adam L, Weisser-Thomas J, et al. High prevalence of peripheral arterial disease in patients with obstructive sleep apnoea. Clin Res Cardiol. 2015;104:719–726.
- Lang RM, Badano LP, Mor-Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270.
- Fine NM, Shah AA, Han IY, et al. Left and right ventricular strain and strain rate measurement in normal adults using velocity vector imaging: an assessment of reference values and intersystem agreement. Int J Cardiovasc Imaging. 2013;29:571–580.
- Bieseviciene M, Vaskelyte JJ, Mizariene V, et al. Two-dimensional speckle tracking echocardiography for evaluation of dilative ascending aorta biomechanics. BMC Cardiovasc Dis. 2017;17:27.
- Roldan CA. Valvular and coronary heart disease in systemic inflammatory diseases: systemic disorders in heart disease. Heart. 2008;94:1089–1101.
- Roldan CA, Chavez J, Wiest PW, et al. Aortic root disease and valve disease associated with ankylosing spondylitis. J Am Coll Cardiol. 1998;32:1397–1404.
- Bulkey BH, Roberts WC. Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation. 1973;48:1014–1027.
- Palazzi C, Salvarani C, D'Angelo S, et al. Aortitis and periaortitis in ankylosing spondylitis. Joint Bone Spine. 2011;78:451–455.
- Avram C, Drăgoi RG, Popoviciu H et al. Association between arterial stiffness, disease activity and functional impairment in ankylosing spondylitis patients: a crosssectional study. Clin Rheumatol. 2016;35:2017–2022.
- Belz GG. Elastic properties and Windkessel function of the human aorta. Cardiovasc Drugs Ther. 1995;9:73–83.
- Kim SA, Lee KH, Won HY, et al. Quantitative assessment of aortic elasticity with aging using velocity-vector imaging and its histologic correlation. Arterioscler Thromb Vasc Biol. 2013;33:1306–1312
- Yang WI, Shim CY, Cho IJ, et al. Dyssynchronous systolic expansion of carotid artery in patients with Marfan syndrome. J Am Soc Echocardiogr. 2010;23:1310–1316.
- Cho IJ, Shim CY, Yang WI, et al. Assessment of mechanical properties of common carotid artery in Takayasu’s arteritis using velocity vector imaging. Circ J. 2010;74:1465–1470.
- Bell V, Mitchell WA, Sigurosson S, et al. Longitudinal and circumferential strain of the proximal aorta. J Am Heart Assoc. 2014;3:e001536
- Bell V, Mitchell GF. Influence of vascular function and pulsatile hemodynamics on cardiac function. Curr Hypertens Rep. 2015;17:68.
- Okan T, Sari I, Akar S et al. Ventricular diastolic function of ankylosing spondylitis patients by using conventional pulsed wave Doppler, myocardial performance index and tissue Doppler imaging. Echocardiography. 2008;25:47–56.
- Brewerton DA, Gibson DG, Goddard DH et al. The myocardium in ankylosing spondylitis. A clinical, echocardiographic, and histopathological study. Lancet. 1987;2(1):995–998.
- Ustun N, Kurt M, Nacar AB, et al. Left ventricular systolic dysfunction in patients with ankylosing spondylitis without clinically overt cardiovascular disease by speckle tracking echocardiography. Rheumatol Int. 2015;35:607–611.
- Biesbroek PS, Heslinga S, Konings TC, et al. Insights into cardiac involvement in ankylosing spondylitis from cardiovascular magnetic resonance. Heart. 2017;103:745–752.
- Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac bio-marker: ST2: a review. Molecules. 2013;18:15314–15328.
- Li GX, Wang S, Duan ZH, et al. Serum levels of IL-33 and its receptor ST2 are elevated in patients with ankylosing spondylitis. Scand J Rheumatol. 2013;42:226–231.
- Meijers WC, van der Velde AR, de Boer RA. ST2 and galectin- 3: ready for prime time? EJIFCC. 2016;27:238–252.
