Abstract
Objectives. Catheter ablation is regarded as first-line therapy for symptomatic atrioventricular nodal reentry tachycardia (AVNRT). Ablation induces intended myocardial damage and the extent of myocardial damage may differ between ablation methods. The objective of this MAGMA AVNRT(NCT00875914) substudy was to compare high-sensitive cardiac troponin T (hs-cTnT) levels as a surrogate marker for myocardial damage after manually guided (MAN) AVNRT ablation versus AVNRT ablation using remote magnetic navigation (RMN). Design. In total, 70 patients (mean age 44 ± 14 years, 26% male) undergoing catheter ablation for AVNRT in the MagMa-AVNRT-Trial were randomized to remote magnetic navigation (n = 34, 49%) or manually guided catheter ablation (n = 36, 51%). hs-cTnT was measured the day after the procedure. Results. The median follow-up time was 6.2 ± 1.1 years. Acute success was 100% in both groups. hs-cTnT release was significantly lower in the remote magnetic navigation group (52 ng/L versus 95 ng/L, p < .01), even though the ablation time was longer and number of applications was higher with remote magnetic navigation (4.2 min vs 2.8 min, p = .017; 4.9 vs 3.3 applications, p = .01). hs-cTnT released per minute ablation time was also lower with remote magnetic navigation (12 ng/L versus 34 ng/L, p < .01). Both groups exhibited similar clinical long-term follow up regarding recurrence and complications. Conclusion. Remote magnetic navigation controlled catheter ablation of AVNRT has similar clinical outcome, but leads to less hs-cTnT release than manually guided catheter ablation. This might correspond to less unintended myocardial damage with RMN, which might be advantageous in complex ablation procedures.
Introduction
AVNRT, the most common supraventricular tachycardia (SVT), is typically seen in young adults with no structural or ischemic heart disease [Citation1]. Manually guided RFA is an established first line treatment for AVNRT due to the high success rate and few complications (3%) [Citation1]. In previous studies, the single procedure success was 94–97% and with repeated ablation success was 96–100% [Citation2–4].
The preferred target during AVNRT ablation is the slow pathway [Citation1] and the conventional approach is manually guided ablation (MAN) using fluoroscopic anatomical landmarks and characteristic intracardiac electrocardiograms. More recent methods, such as the use of 3D electro-anatomical Mapping or irrigated catheter ablation, have not led to substantially altered clinical outcomes [Citation5]. Radiofrequency ablation (RFA) induces local damage to the myocardium [Citation6,Citation7], resulting in a rise in cardiac troponin levels. A positive correlation between cardiac troponin and both total ablation time [Citation8,Citation9], and number of radio frequency applications [Citation6,Citation9,Citation10] has been demonstrated.
Mapping and ablation using magnetic navigation allows for more precise spatial placement of catheters and a reduction in fluoroscopy time [Citation1]. Remote magnetic navigation (RMN) is clinically as effective as MAN ablation for AVNRT and there has not been demonstrated difference in long term outcomes (arrhythmia, AV block, death) [Citation11]. The MagMa-AVNRT-Trial found similar acute success comparing manually and magnetically guided ablation, but an approximate 50% reduction in fluoroscopy time and dosage (for both patient and operator) were found using RMN [Citation12]. This MagMma-AVNRT sub-study investigates if there is a difference in applied myocardial damage (with hs-cTnT as a surrogate marker) between conventional manual ablation and magnetic navigated ablation. Recurrence and late complications with a longer follow-up time (>5 years) were also assessed.
Materials and methods
Study population
In the randomized multicenter MagMa-AVNRT-Trial, patients with AVNRT were randomized to manually guided RFA (MAN) or RFA using RMN (Niobe Magnetic navigation system, Stereotaxis Inc., St Louis, MO, USA). The fluoroscopy time, safety and efficiency were compared between the two groups [Citation12]. The study protocol was approved by the local ethics committee (REK 2008/13927) and registered in clinicaltrials.gov (Identifier: NCT00875914). For this Mag-Ma AVNRT- sub-study, all 70 patients from one center (Haukeland) were randomized at a 1:1 ratio between April 2009 and June 2013 for MAN or RFA using RMN were assessed.
All of the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Electrophysiological procedure
The EP procedure was performed by 3 experienced operators (>2.5 years of experience with RMN and MAN). The same operators performed either procedure based on randomization.
A diagnostic catheter was positioned in the coronary sinus, in the right ventricular apex and in the HIS position. In the MAN group, any commercially available 4 mm tip ablation catheter was permitted. For the RMN group, a RMT non-irrigated 4 mm tip ablation catheter was used in combination with the Niobe system. After placement of the diagnostic and therapeutic catheters, the Niobe system allowed the operator to navigate the ablation catheter from outside the operating room. The Niobe Magnetic navigation system is described in detail elsewhere [Citation13].
Patients underwent a conventional electrophysiological (EP) study, and AVNRT was diagnosed using the standard criteria. All except 3 patients in the MAN group and 4 patients in the RMN group (p = ns) needed isoproterenol in addition to pacing to induce AVNRT. Slow pathway ablation or modification at low position in both groups was performed with a combination of anatomical and electrophysiological approaches. Standard RF energy setting (60 °C, 30–35 W) was delivered for 60 seconds in both groups. Crossover between groups was allowed.
Procedure time, number and duration of radiofrequency lesions, power, temperature and fluoroscopy time for both the patient and operator were registered. The procedure was performed mainly in the morning in both groups.
High-sensitive cardiac troponin T measurement
Patients were hospitalized with continuous ECG monitoring until the next day. The 12-lead ECG and hs-cTnT values (high-sensitive cardiac troponin T assay from Roche Diagnostics) were measured the morning after the procedure (16–20 hours after ablation) ().
Follow-up
A 12-lead ECG was collected, and subjects were interviewed by telephone regarding the recurrence of symptoms 6 months after RFA and again at the end of the study. The median follow-up time was 6.2 years ± 1.1 year. ECGs were obtained from 48 patients (69%). Four patients (6%) were lost to follow-up at the last interview.
Statistical methods
All statistical analyses were performed using Stata (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The hs-cTnT values, hs-cTnT value per procedure time, Radiofrequency (RF) time, number of RF applications, X-ray dose, X-ray time, procedure time and duration of symptoms were positively skewed and were log transformed prior to using the Student’s t-test. These results are reported as the geometric means. Other continuous variables are presented as the means plus or minus the standard deviation (±SD). Proportions were analyzed using the Chi Square test or Fisher’s exact test, as appropriate. Multiple linear regression was used to create a model to compare hs-cTnT values between the RMN and MAN groups, adjusting for RF time, RF applications, power and temperature. Log transformation of the hs-cTnT values was performed to meet the assumptions of linear regression. All hypothesis testing was two-tailed, and a p-value less than 0.05 was considered statistically significant.
Results
Patient characteristics and procedural data
Patients’ baseline characteristics are presented in . There were no significant differences in baseline characteristics between groups. Procedural data are presented in .
Table 1. Baseline characteristics of the patients.
Table 2. Procedural data and recurrences/arrhythmia at follow up.
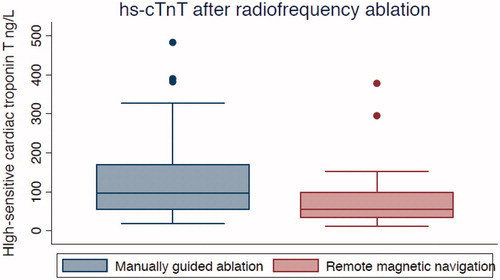
Figure 1. High-sensitive cardiac troponin T (ng/L) measured 16–20 hours after RFA. The box displays the interquartile range (25th–75th percentiles) and the median. The length of the whiskers is 1.5 times the interquartile range. Values outside the whiskers (dots) are defined as outliers.
A total of 70 patients (26% male, mean age 44 (±14) years) were randomized to RMN (n = 34, 49%) or MAN (n = 36, 51%). All patients (except two who had a slow-slow AVNRT) had typical slow-fast AVNRT. One patient crossed over to the manually guided catheter group because of device failure before initiation of ablation. This patient was included in the MAN group. Acute success was 100% in both groups as defined by non-inducibility of AVNRT and either disappearance of the slow pathway (RMN n = 19, MAN n = 13; p = .10) or a maximum of one echo beat. The only periprocedural complication was a temporary total AV block that resolved spontaneously after a few seconds and was considered to be caused by mechanical contact in the RMN group. This patient had a normal PQ time at follow up.
Fluoroscopy time and dosage were significantly lower in the RMN-group for both the patients (173 seconds vs. 348 seconds, p < .01; 91 vs. 192 cGycm2, p < .01) and operator (75 seconds vs. 348 seconds, p < .01; 46 vs. 192 cGycm2, p < .01). The procedure time was not significantly longer with RMN (78 min vs. 74 min, p = .32).
High-sensitive cardiac troponin T release
Total hs-cTnT (ng/L) release was significantly lower in the RMN group (52 vs. 95 ng/L, p < .01). hs-cTnT release per minute of ablation was also lower in the RMN group (12 vs. 34 ng/L/min p < .01). However, RF time and number of applications were higher in the RMN group (4.2 min vs 2.8 min, p = .017; 4.9 vs 3.3 applications, p = .01).
In the multiple linear regression analysis used to predict the hs-cTnT levels for MAN vs. RMN ablation, based on RF time (min), temperature and power, the hs-cTnT value in the MAN group was 72% higher (geometric mean, p = .004) compared to that of the RMN group. RF time (p = .015) and temperature (p = .000) were significant predictors for higher hs-cTnT values, whereas power was not (p = .213). A higher number of RF applications were a predictor for higher hs-cTnT values (p = .01) in a multiple linear regression analysis to predict hs-cTnT using MAN vs RMN ablation, based on temperature, power and RF applications.
Follow up
Success after 6 months was 100% in both groups. (In the MagMa-AVNRT Trial success after 6 months was 97% with RMN versus 98% with MAN (p = .67)Citation12]. At the long term follow up, we found atrial fibrillation in one patient in each treatment arm. In the MAN group, two patients (5.6%) underwent a second ablation (after 4 years and 8 years). 2 patients in the MAN group (5.6%, 49 and 53 years old) developed an intermittent third-degree AV block and required permanent pacemakers after 17 and 24 months and one patient was diagnosed with ventricular tachycardia.
Discussion
Main findings
This study demonstrates that patients with AVNRT treated with remote magnetic navigation have significantly lower hs-cTnT values as a surrogate marker for myocardial damage the day after ablation, compared to patients treated with manually guided RFA. In accordance with previous studies we found a positive correlation between post-procedural cardiac troponin levels and both total ablation time and number of RF applications [Citation6,Citation8–10]. And as formerly demonstrated in atrial fibrillation ablation [Citation8] there were lower troponin values per minute ablation with RMN versus MAN.
RFA induces intentional myocardial injury [Citation6,Citation7]. There is a system dependent difference between the stiff catheters used with MAN and the soft and flexible catheters used with RMN. RMN provides better catheter stability than MAN, but a decrease in force applied [Citation14]. Electrode-tissue contact force is a major determinant of lesion size and temperature during RFA [Citation15,Citation16]. Lower troponin T values per minute ablation probably reflects lower tissue contact force with RMN. However, RMN and MAN are equally effective regarding short and long-term success.
In a former study EGM modification as a surrogate for electrical transmurality was faster obtained with RMN in comparison to optimized contact force guided catheter ablation when treating atrial fibrillation [Citation17]. When studied in a stationary myocardial phantom similar lesion dimensions were observed with RMN and MAN, whereas with simulated wall motion of 6 mm in the myocardial phantom, lesion dimensions were larger with RMN [Citation18,Citation19]. A recent study presented by Grossi and colleagues compared lesions formation in contact force (CF) and RMN guided PVI in patients with paroxysmal AF. They used signal shrinkage and signal fragmentation as markers of lesion quality and energy attenuation and impedance drop as markers of lesion size. Comparing 283 radio frequency (RF) lesions in 44 patients they found that contact force catheters produce lesions that are larger, but less homogeneous than those produced by RMN catheters in terms of surrogate parameters [Citation20]. One can hypothesize that RMN allows smaller, but still effective ablation lesions, which inflicts less collateral myocardial damage. This might be of advantage in complex ablation procedures where precision is an absolute prerequisite (congenital heart disease), but might request higher power output in some cases.
RMN is also demonstrated to be a safe and effective tool in VT ablation. In a meta-analysis comparing RMN with MAN in VT ablation, patients treated with RMN had a significantly lower VT recurrence, higher procedural success, lower procedural complications, and lower fluoroscopy and procedural time compared with MAN [Citation21].
Using RMN for AVNRT ablation has economic aspects with higher per-procedural costs. Centers that hold the remote magnetic technology mainly use it for complex ablations. However, this not funded study from clinical everyday praxis demonstrates that using RMN for AVNRT ablation is a good alternative to the manual approach in centers that already possess RMN technology. The use of RMN when treating AVNRT led to similar or longer procedure times than those reported for manually guided catheter ablation in previous studies [Citation11]. But there was no significant difference in procedure time between RMN and MAN in this subgroup of the MagMa-AVNRT-Trial.
Radiation exposure remains a concern in invasive cardiology, especially for operators who are exposed in every procedure. After placement of the ablation catheters, navigation of the magnetic system is performed from outside the operating room, minimizing radiation exposure for the operator when using RMN. As demonstrated in the MagMa-AVNRT-Trial and in this subgroup, RMN requires less fluoroscopy time for both the patient and operator than manually guided RFA [Citation22]. Further, 3D electro-anatomical mapping makes it possible to perform slow pathway ablation with very little radiation exposure [Citation23,Citation24], but the ablation itself is still performed manually.
Limitations
A weakness of the study is lack of CF data which is likely an independent variable determining lesion formation, independent of RF power and delivery time. hs-cTnT values where measured 16–20 hours after ablation due to hospital routines but not prior to or soon after the RFA procedure. The study population was quite young and healthy and there was no significant difference between the groups regarding age, gender, the low prevalence of known coronary disease, hypertension, smoking or diabetes. There were no patients diagnosed with heart failure and only one patient in each treatment arm with kidney failure, both with GFR > 50 ml/min. We assume the probability of preexisting elevated TnT levels in our rather healthy group to be very low.
Conclusion
Remote magnetic navigation and manually guided RF ablation for AVNRT are equally effective regarding short and long-term success. However, this substudy from RMN controlled AVNRT catheter ablation in the MagMa-AVNRT trial leads to less hs-cTnT release than manually guided catheter ablation which might correspond to successful ablation with less myocardial damage. This supports the idea that RMN is advantageous also in complex ablation procedures. There is need for more research to address this.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm. Heart Rhythm. 2016;13:e92–135.
- Spector P, Reynolds MR, Calkins H, et al. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia††conflicts of interest: Dr. Spector is a consultant for Medtronic, Inc., Minneapolis, Minnesota; Biosense Webster, Inc.; and Boston Scientific Corporation, Natick, Massachusetts. Am J Cardiol. 2009;104:671–677.
- Kimman G. Ten year follow-up after radiofrequency catheter ablation for atrioventricular nodal reentrant T achycardia in the early days forever cured, or a source for new arrhythmias? Pace. 2006;28:1302–1309.
- Calkins H, Yong P, Miller JM, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. The Atakr Multicenter Investigators Group. Circulation. 1999;99:262–270.
- Chrispin J, Misra S, Marine JE, et al. Current management and clinical outcomes for catheter ablation of atrioventricular nodal re-entrant tachycardia. EP Eur. 2017;23:543–550.
- Katritsis D, Hossein-Nia M, Anastasakis A, et al. Use of troponin-T concentration and kinase isoforms for quantitation of myocardial injury induced by radiofrequency catheter ablation. Eur. Heart J. 1997;18:1007–1013.
- Gerstenfeld E, Jacobson J, Bazan V, et al. Comparison of high power, medium power, and irrigated-tip ablation strategies for pulmonary vein isolation in a canine model. J Cardiovasc Electrophysiol. 2007;18:849–853.
- Solheim E, Off MK, Hoff PI, et al. Remote magnetic versus manual catheters: evaluation of ablation effect in atrial fibrillation by myocardial marker levels. J Interv Card Electrophysiol. 2011;32:37–43.
- Hirose H, Kato K, Suzuki O, et al. Diagnostic accuracy of cardiac markers for myocardial damage after radiofrequency catheter ablation. J Interv Card Electrophysiol. 2006;16:169–174.
- Vasatova M, Pudil R, Tichy M, et al. High-sensitivity troponin T as a marker of myocardial injury after radiofrequency catheter ablation. Ann Clin Biochem. 2011;48:38–40.
- Shurrab M, Danon A, Crystal A, et al. Remote magnetic navigation for catheter ablation of atrioventricular nodal reentrant tachycardia: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2013;11:829–836.
- Reents T, Jilek C, Schuster P, et al. Multicenter, randomized comparison between magnetically navigated and manually guided radiofrequency ablation of atrioventricular nodal reentrant tachycardia (the MagMa-AVNRT-trial). Clin Res Cardiol. 2017;106:947–952.
- Ernst S, Ouyang F, Linder C, et al. Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation. 2004;109:1472–1475.
- Aksu Md T, Bozyel Md S, Golcuk Md E, et al. Atrial fibrillation ablation using magnetic navigation comparison with conventional approach during long-term follow-up. J Atr Fibrillation. 2015;8:1276.
- Ikeda A, Nakagawa H, Lambert H, et al. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart clinical perspective. Circ Arrhythmia Electrophysiol. 2014;7:1174–1180.
- Shah DC, Namdar M. Real-time contact force measurement. Circ Arrhythmia Electrophysiol. 2015;8:713–721.
- Bun S-S, Ayari A, Latcu DG, et al. Radiofrequency catheter ablation of atrial fibrillation: electrical modification suggesting transmurality is faster achieved with remote magnetic catheter in comparison with contact force use. J Cardiovasc Electrophysiol. 2017;28:745–753.
- Bhaskaran A, Barry MA, I. Al Raisi S, et al. Magnetic guidance versus manual control: comparison of radiofrequency lesion dimensions and evaluation of the effect of heart wall motion in a myocardial phantom. J Interv Card Electrophysiol. 2015;44:1–8.
- Alaiti MA, Maroo A, Edel TB. Troponin levels after cardiac electrophysiology procedures: review of the literature. PACE - Pacing Clin Electrophysiol. 2009;32:800–810.
- Grossi S, Grassi F, Galleani L, et al. A comparison of contact force and remote magnetic navigation on lesion formation for the ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2018;41:1–9.
- Turagam MK, Atkins D, Tung R, et al. A meta-analysis of manual versus remote magnetic navigation for ventricular tachycardia ablation. J Interv Card Electrophysiol. 2017;49:227–235.
- Reents T, Jilek C, Schuster P, et al. Multicenter, randomized comparison between magnetically navigated and manually guided radiofrequency ablation of atrioventricular nodal reentrant tachycardia (the MagMa-AVNRT-trial). Clin Res Cardiol. 2017;106:947–952.
- Scaglione M, Ebrille E, Caponi D, et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol. 2013;36:1460–1467.
- Swissa M, Birk E, Dagan T, et al. Radiofrequency catheter ablation of atrioventricular node reentrant tachycardia in children with limited fluoroscopy. Int J Cardiol. 2017;236:198–202.
