Abstract
Objective. To evaluate the effects of miR-210 on cardiac stem cells (CSCs) against hypoxia-induced injury. Methods. CSCs were isolated from rat ventricular wall and cultured until passage 4. After exposure to hypoxia for 6 h, the expression of miR-210 was determined. Thereafter, transfection of miR-210 mimic and inhibitor was carried out. 1 week later, in vitro experiments were performed to measure the expression of caspase-8-associated protein 2 (Casp8ap2), Caspase 8, protein tyrosine phosphatase, non-receptor type 2 (PTPN2) and CXC chemokine receptor 4 (CXCR4), as well as migration and apoptosis of CSCs under hypoxic condition. Results. Hypoxia induced a significant up-regulation of miR-210 expression in CSCs. Notably, the expression of Casp8ap2, Caspase8, PTPN2 was dramatically inhibited by overexpression of miR-210 in CSCsmiR-210 Group (P < .05), but no changes in CXCR4 (P > .05), compared with the control. Additionally, a decreased apoptosis of CSCs was detected in CSCsmiR-210 Group (26.22 ± 1.15%, P < .001), compared with Control Group (34.97 ± 0.63%). Moreover, the migration of CSCs was significantly promoted in CSCsmiR-210 Group (45.73 ± 2.4, P < .001), compared with Control Group (19.6 ± 1.11). Meanwhile, down-regulation of miR-210 reversed these results (P < .05). Conclusions. miR-210 was a hypoxia responsive element in CSCs, and its up-regulation inhibited apoptosis of CSCs and promoted their migration under hypoxic condition, through regulating its target genes Casp8ap2/Caspase 8 and PTPN2, which may provide a new strategy for cell therapy of ischemic heart disease.
Keywords:
Introduction
Cardiac stem cells (CSCs) transplantation has been emerged as a promising therapeutic of myocardial regeneration for ischemic heart disease, due to their capacity of differentiation into cardiomyocytes, endothelial cells and smooth muscle cells [Citation1–3]. However, it has still been a burning issue that the survival of CSCs transplanted into ischemic regions is largely restricted by their rather limited potential against hypoxia, although there have been lots of researches in this field [Citation3–7]. Thereby, it is critical to enhance the CSCs ability of anti-hypoxia for such therapy.
MicroRNAs (miRs), a kind of single-stranded noncoding RNAs that are composed of 19–23 nucleotides, have been verified as crucial manipulators of most cellular functions in the level of posttranscriptional regulation of gene expression, through a direct inhibition of their target genes. It has been demonstrated that miR-210 is one of hypoxia-regulated miRs, which may play critical roles in cellular anti-hypoxia procedure. As reported by Fasanaro et al., the expression level of miR-210 was drastically increased in endothelial cells after exposure to hypoxia for 4 h, and abrogation of miR-210 with its specific inhibitor increased the apoptosis of these cells [Citation8]. Moreover, the similar results were found in bone marrow-derived mesenchymal stem cells (BMSCs) by Kim et al., confirming the cytoprotection of miR-210 again [Citation9]. Notably, it has been documented by Luan et al., that overexpression of miR-210 could attenuate the hypoxia-induced injury in PC-12 cells [Citation10]. Therefore, it is speculated that miR-210 may play the similar protective role in CSCs against hypoxia, although there has not presented reports referring to the effects of miR-210 in this kind of cells.
To evaluate the effect of miR-210 on activity of CSCs under hypoxic condition, the in vitro experiments were carried out as following. CSCs of Sprague-Dawley (SD) rat were harvested and transfected by miR-210 mimic or inhibitor, followed by determining the expression of miR-210 and its candidate target gene caspase 8 associated protein 2 (Casp8ap2) and protein tyrosine phosphatase, non-receptor type 2 (PTPN2), as well as cell migration and apoptosis to assess activity of CSCs.
Material and methods
This animal experiment has been approved by Liaoning Administrative Committee for Laboratory Animals and carried strictly out referring to the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Isolation of CSCs
As described previously in our papers [Citation4], the ventricular wall tissues of SD rats (4 days) were harvested digested with collagenase to isolate cells, followed by an enrichment of c-kit+ and CD45− by the method of immunomagnetic bead. The expression of GATA-4 (61.36%), Flk-1 (45.92%), Nkx2.5 (37.84%), c-TNT (0.23%) and CX-43 (0.41%) was determined to identify CSCs by using fluorescence-activated cell sorting. The isolated CSCs were cultured until passage 4 for the following experiments.
The expression of miR-210 in CSCs under hypoxia
A sealed GENbox hypoxic chamber was used to establish a condition of deep hypoxia (0.2% O2, DH), imitating the low oxygen microenvironment (0.2% to 1%) within the core of the myocardial ischemic area. The CSCs were exposed to this DH and serum deprivation (SD) for 6 h, before the expression of miR-210 and U6 (the internal control) was measured by RT-PCR.
The transfection of miR-210
To regulate endogenous miR-210, miR-210 inhibitor or mimics were transfected to CSCs with lentivirus (Shanghai Genechem Co., Ltd., Shanghai, China) at the final multiplicity of infection (MOI) of 10. After a 48 h incubation, CSCs were re-fed with fresh medium, and continuously cultured for 5 days. Thereafter, the expression of miR-210 was observed, the measure of Caspase8, Casp8ap2, PTPN2 and CXCR4 expression was performed by using Western Blotting, and apoptosis and migration under condition of DH/SD were determined to evaluate the effects of miR-210 on CSCs against hypoxia.
Migration and apoptosis of CSCs
As described previously in our papers [Citation4,Citation11], an 8-mum pore-size transwell migration chamber (Millipore, Billerica, MA, USA) was used for determination of CSCs migration. The CSCs were added to upper chambers, and this system was put into the above mentioned sealed GENbox hypoxic chamber. 6 h later, CSCs migrated to the outside of bottom of upper chambers were stained with crystal violet and quantitatively analyzed by the absorbance measure. The assessment of apoptosis was performed by using the method of annexin V/propidium iodide staining as described in our previous published papers [Citation4,Citation12], after a 6 h exposure to DH/SD.
Statistical analysis
Based on the facts that investigators were blinded to the treatment, all off line results were analyzed. Data were exhibited as mean ± standard deviation. With SPSS 19.0 software package (SPSS Inc, Chicago, USA), independent 2-samples Students t test was used for comparison between two groups, and one-way analysis of variance (ANOVA) with Bonferroni post hoc correction was performed to compare measurements among four groups. The difference is considered statistically significant as P < .05.
Results
The assessment of transinfection of miR-210
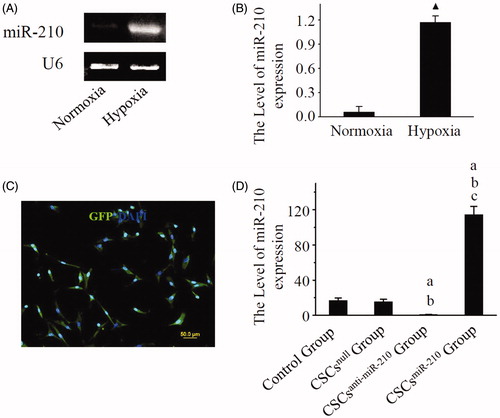
A significant up-regulation of miR-210 expression was detected in CSCs following a 6 h exposure to DH/SD (P < .05), as shown by results of RT-PCR (). 5 days after transinfection, more than 90% CSCs expressed GFP (). And there was a significant up-regulated expression in CSCsmiR-210 Group, but no changes in CSCsnull Group and CSCsanti-miR-210 Group under normoxic condition ().
Figure 1. The identification of miR-210 expression. Representative RT-PCR photographs of miR-210 and U6 expression in CSCs are shown in A, and comparison of the ratio of miR-210 to U6 between normoxia and hypoxia is performed in B. C displays the representative photo of GFP expression in CSCs after transfection. D shows representative photographs for the ratio of miR-210 expression to U6 in CSCs. B and D present mean values and SD from three independent observations. GFP: Green fluorescent protein. CSCs: Cardiac stem cells. ▲ P < .001 vs. Normoxia. a: P < .001 vs. Control Group. b: P < .001 vs. CSCsnull Group. c: P < .001 vs. CSCsanti-miR-210 Group.
The expression of miR-210 target genes
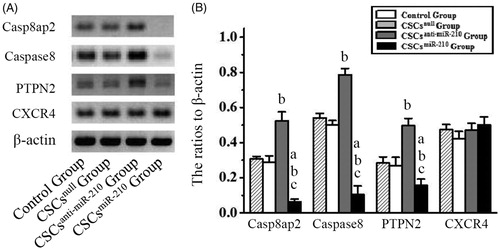
Twenty-four-hours after exposure to HP/SD, a significant decrease in Casp8ap2, Caspase8, PTPN2 expression was detected in CSCsmiR-210 Group, compared with other three groups (P < .05, ). Meanwhile, there was a significant increase in CSCsanti-miR-210 Group (P < .05), but no differences in CSCsnull Group (P > .05), related to the control. However, these changes were not found in CXCR4 expression among four groups (P > .05), indicating that miR-210 could not influence CXCR4 expression.
Figure 2. The expression of Casp8ap2, Caspase8, PTPN2 and CXCR4 in CSCs. The representative western blotting pictures of Casp8ap2, Caspase8, PTPN2 and CXCR4 are shown in A. Subsequently, the quantitative analysis is performed and the results are compared among the four groups in B. B presents mean values and SD from three independent observations. Casp8ap2: Caspase 8 associated protein 2. PTPN2: Protein tyrosine phosphatase, non-receptor type 2. CXCR4: CXC chemokine receptor 4. a: P < .001 vs. Control Group. b: P < .001 vs. CSCsnull Group. c: P < .001 vs. CSCsanti-miR-210 Group.
Up-regulated miR-210 inhibited hypoxia-induced apoptosis of CSCs
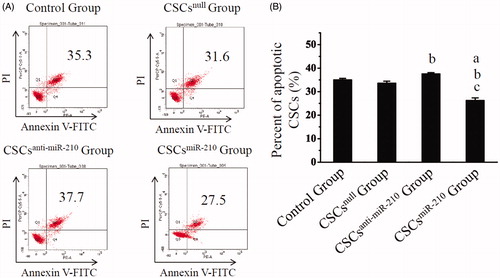
As displayed by annexin V/propidium iodide staining (), the percentage of apoptotic CSCs were significantly decreased in CSCsmiR-210 Group (26.22 ± 1.15%), compared with Control Group (34.97 ± 0.63%, P < .001), CSCsnull Group (33.55 ± 0.91%, P < .001) and CSCsanti-miR-210 Group (37.55 ± 0.53%, P < .001). Obviously, it was higher in CSCsanti-miR-210 Group than Control Group (P = .034), and no differences were detected between CSCsnull Group and the control (P = .429). It was therefore indicated that up-regulated miR-210 could inhibit hypoxia-induced apoptosis of CSCs.
Figure 3. The in vitro assessment of CSCs apoptosis. Annexin V/PI staining are shown in A, and the percentages of apoptotic CSCs for each group are compared in B. B presents mean values and SD from three independent observations. a: P < .001 vs. Control Group. b: P < .001 vs. CSCsnull Group. c: P < .001 vs. CSCsanti-miR-210 Group.
In vitro migration of CSCs was improved by up-regulated miR-210
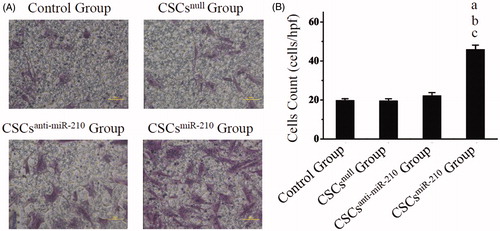
The representative pictures of CSCs migration are shown in , and cell count revealed the most migrated cells in CSCsmiR-210 Group (45.73 ± 2.4, P < .001) among the four groups, with no differences between CSCsanti-miR-210 Group (22 ± 1.8, P = .747) or CSCsnull Group (19.4 ± 1.22, P = 1) and Control Group (19.6 ± 1.11, ). These findings suggested that up-regulated miR-210 could promote migration of CSCs under the condition of hypoxia.
Figure 4. The in vitro evaluation of CSCs migration. The images of CSCs migration are exhibited in A, and comparisons of quantitative analysis are shown in B. B presents mean values and SD from three independent observations. a: P < .001 vs. Control Group. b: P < .001 vs. CSCsnull Group. c: P < .001 vs. CSCsanti-miR-210 Group.
Discussion
It has been demonstrated in the present study that (1) hypoxia induced the expression of miR-210 in CSCs; (2) overexpression of miR-210 attenuated apoptosis of CSCs and promoted their migration under hypoxic condition through regulating miR-210 and its target genes Casp8ap2 and PTPN2.
Although many animal experiments have reported CSCs transplantation could provide protective effects on myocardial regeneration and LV function after myocardial infarction, no consensus was reached as to whether this therapeutic method may provide similar benefits at clinical trials [Citation13,Citation14]. It is widely agreed that limited ability of CSCs against hypoxia will severely restrict the survival of these cells transplanted into the hypoxic microenvironment [Citation3–7,Citation15], however, the following fact must be considered that a complete revascularization fails to be achieved in many patients with severe and complicated coronary artery disease, even after percutaneous coronary intervention (PCI) or coronary artery bypass grafting treatment. Therefore, it is a promising strategy to enhance the CSCs capability against hypoxia for improvement of the efficiency of such therapy. miR-210 has been proved as a strong anti-hypoxic miR for other various cells [Citation8–10,Citation16]. As expected, the present study revealed that up-regulated miR-210 dramatically decreased the expression of its target genes Casp8ap2/Caspase 8 and PTPN2 in CSCs, resulting in attenuation of apoptosis and improvement of migration, which represent a greater potential of CSCs to repair impaired myocardium. Thereby, it is suggested that this new method of posttranscriptional regulation of gene expression may be a promising therapeutic strategy for clinical application, to heighten the protective effects of CSCs transplantation.
Casp8ap2 and PTPN2 have been identified as two functional target genes of miR-210 in other stem cells [Citation9,Citation17], which has been further confirmed in CSCs by our in vitro study. Increasing evidences have revealed that Casp8ap2, as integral member of the apoptosis signaling complex, is an important pro-apoptotic factor, which facilitates Caspase 8 activation and release from the complex, and subsequently triggers the downstream apoptotic pathway [Citation18,Citation19]. In the present study, the expression of Casp8ap2 and Caspase 8 was decreased, and consequently the hypoxia-induced apoptosis of CSCs was inhibited after up-regulation of miR-210, suggesting that these two proteins are involved in the anti-apoptotic mechanisms of miR-210. Additionally, it has been demonstrated that down-regulated PTPN2 could promote the migration of adipose-derived stem cells, therefore speculating that overexpression of miR-210 increased the migration of CSCs also through the way of PTPN2 [Citation17,Citation20]. Although CXCR4 has been proved as a key regulator of CSCs migration [Citation21,Citation22], its expression did not change after miR-210 transfection in the present experiment, indicating that CXCR4 is not target gene of miR-210 and may not be involved in the mechanisms of miR-210-regulating CSCs migration.
In spite of the encouraging results, there are several questions that must be presented. Firstly, based on the difference in cells activity between rat and human, this animal research may not contribute to the following clinical application. Secondly, there may be other protective mechanisms involved in miR-210 promoting the activity of CSCs under hypoxic condition, which need to be explored in the following studies. Thirdly, the present study only delineated the effect of miR-210 on CSCs ex vivo, a further in vivo experiment should be performed to confirm the protective function. Finally, the other effects of miR-210 were not evaluated, for example differentiation of CSCs, thus these should be researched in the future.
In summary, hypoxia can up-regulate miR-210 expression in CSCs. Overexpression of miR-210 can inhibit its target genes Casp8ap2, Caspase8 and PTPN2 in CSCs, resulting in improvement of apoptosis and migration. Thereby, miR-210 may be an important anti-hypoxic target, which may provide a new strategy to protect transplanted CSCs against ischemia and hypoxia, and upregulation of miR-210 may become a promising pretreatment method for the clinical application of CSCs transplantation.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [No. 81400196], Ph.D. Programs Foundation of Ministry of Education of China [20132104110004], and Science and Technology Research Program of the Educational Department of Liaoning Province [No. L2013297].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bolli R, Ghafghazi S. Stem cells: Cell therapy for cardiac repair: what is needed to move forward? Nat Rev Cardiol. 2017;14:257–258.
- Hinkel R. Pim1 overexpressing ckit(+) cardiac stem cells in cardiac regeneration: preconditioning as next-generation stem cell therapy? J Am Coll Cardiol. 2016;68:2465–2466.
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942.
- Zhang GW, Gu TX, Sun XJ, et al. Edaravone promotes activation of resident cardiac stem cells by transplanted mesenchymal stem cells in a rat myocardial infarction model. J Thorac Cardiovasc Surg. 2016;152:570–582.
- Ashur C, Frishman WH. Cardiosphere-derived cells and ischemic heart failure. Cardiol Rev. 2018;26:8–21.
- Hou J, Wang L, Long H, et al. Hypoxia preconditioning promotes cardiac stem cell survival and cardiogenic differentiation in vitro involving activation of the HIF-1alpha/apelin/APJ axis. Stem Cell Res Ther. 2017;8:215.
- Bellio MA, Rodrigues CO, Landin AM, et al. Physiological and hypoxic oxygen concentration differentially regulates human c-Kit + cardiac stem cell proliferation and migration. Am J Physiol Heart Circ Physiol. 2016;311:H1509–H1519.
- Fasanaro P, D'Alessandra Y, Di Stefano V, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883.
- Kim HW, Haider HK, Jiang S, et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168.
- Luan Y, Zhang X, Zhang Y, et al. MicroRNA-210 protects PC-12 cells against hypoxia-induced injury by targeting BNIP3. Front Cell Neurosci. 2017;11:285
- Zhang GW, Gu TX, Guan XY, et al. bFGF binding cardiac extracellular matrix promotes the repair potential of bone marrow mesenchymal stem cells in a rabbit model for acute myocardial infarction. Biomed Mater. 2015;10:065018.
- Zhang GW, Gu TX, Guan XY, et al. Delayed enrichment for c-kit and inducing cardiac differentiation attenuated protective effects of BMSCs' transplantation in pig model of acute myocardial ischemia. Cardiovasc Ther. 2015;33:184–192.
- Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857.
- Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904.
- Wen T, Wang L, Sun XJ, et al. Sevoflurane preconditioning promotes activation of resident CSCs by transplanted BMSCs via miR-210 in a rat model for myocardial infarction. Oncotarget. 2017;8:114637–114647.
- Martinez SR, Ma Q, Dasgupta C, et al. MicroRNA-210 suppresses glucocorticoid receptor expression in response to hypoxia in fetal rat cardiomyocytes. Oncotarget. 2017;8:80249–80264.
- Kim JH, Park SG, Song SY, et al. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013;4:e588
- Imai Y, Kimura T, Murakami A, et al. The CED-4-homologous protein FLASH is involved in Fas-mediated activation of caspase-8 during apoptosis. Nature. 1999;398:777–785.
- Barcaroli D, Bongiorno-Borbone L, Terrinoni A, et al. FLASH is required for histone transcription and S-phase progression. Proc Natl Acad Sci USA. 2006;103:14808–14812.
- Persson C, Savenhed C, Bourdeau A, et al. Site-selective regulation of platelet-derived growth factor beta receptor tyrosine phosphorylation by T-cell protein tyrosine phosphatase. Mol Cell Biol. 2004;24:2190–2201.
- Hatzistergos KE, Saur D, Seidler B, et al. Stimulatory effects of mesenchymal stem cells on cKit + cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circ Res. 2016;119:921–930.
- Zuo K, Kuang D, Wang Y, et al. SCF/c-kit transactivates CXCR4-serine 339 phosphorylation through G protein-coupled receptor kinase 6 and regulates cardiac stem cell migration. Sci Rep. 2016;6:26812.
