Abstract
Background. The efficacy of clopidogrel is often attenuated in the setting of renal impairment. High on-treatment platelet reactivity (HPR) is an independent correlate of adverse event. Here we performed a quantitative evaluation of the prevalence and impact of HPR in patients with chronic kidney disease (CKD). Methods. We systematically searched PubMed, EMBASE and the Cochrane Library from their inception to 1 March 2018 for cohort studies assessing the risk ratio (RR) of prevalence of HPR in CKD versus non-CKD patients and association of cardiovascular outcome with HPR in CKD patients treated with clopidogrel. Outcome measures included major adverse cardiac event, myocardial infarction and stent thrombosis. RRs and 95% confidence intervals (CIs) were used as estimates of effect size in random-effect models. Results. Ten studies comprising a total of 3028 CKD patients and 11138 non-CKD patients were included in the evaluation. Compared to patients with normal renal function, patients with CKD had a significantly higher risk of HPR (OR: 1.34, 95% CI: 1.23–1.46). In CKD patients, HPR was associated with increased risk of MACE (RR 2.99, 95% CI 1.19 to 7.53; p < 0.00001), myocardial infarction (RR1.74, 95% CI 1.29 to 2.33; p = 0.0002), and stent thrombosis (RR 2.98, 95% CI 1.42 to 6.26; p = 0.004). Conclusions. Based on pooled analysis, CKD appeared correlated with HPR and this association had prognostic significance. Further studies with standardised laboratory methods and specifically defined protocols are required to validate the clinical relevance of such response variability to clopidogrel in CKD patients.
Introduction
Chronic kidney disease (CKD) is associated with higher cardiovascular morbidity and mortality with accumulating evidence [Citation1–3]. As the irreversible inhibitors of the ADP P2Y12 receptor [Citation4], clopidogrel is recommended as the standard treatment for preventing thrombotic complications in patients with ischemic heart disease [Citation4]. However, clinical observations demonstrate that the efficacy of clopidogrel in reducing ischemic events is attenuated in the setting of renal impairment [Citation5–7].
Clopidogrel induced antiplatelet effect to be highly variable and some patients persist with enhanced platelet reactivity despite clopidogrel treatment [Citation8]. High platelet reactivity (HPR) and CKD have common risk factors, suggesting a shared mechanistic links between renal dysfunction, platelet aggregation, and increased thrombosis [Citation9]. Recently, some studies evaluating the prevalence of laboratory-defined clopidogrel resistance in CKD and the recurrence of cardiovascular events during follow-up in patients with HPR have been conducted. However, studies about whether decreased kidney function is associated with HPR have yielded conflicting results. In addition, the question whether HPR has a significant clinical implication on the occurrence of atherothrombotic events has not been definitely answered.
Therefore, the aim of this study is to systematically review all the available studies that investigated the prevalence of HPR on clopidogrel therapy in CKD patients and the possible association between HPR and the occurrence of adverse cardiovascular events in patients with CKD.
Methods
Search strategy
A comprehensive search of PubMed, EMBASE and the Cochrane library from their inceptions to January 1, 2018, was conducted to identify studies describing the association between CKD and platelet reactivity and cardiovascular events in clopidogrel-treated patients with coronary heart disease (CAD). The search terms comprised the following items and were combined using the Boolean operator AND: drug name (including “antiplatelet therapy”, “thienopyridine”, “clopidogrel,” “clopilet” or “plavix”); CKD (including “chronic kidney disease,” “renal impairment,” or “renal dysfunction” or “declined renal function”) ; ischemic heart disease (including “coronary heart disease”, “cardiovascular disease”, “percutaneous coronary intervention”) were used in combination with words relating to responsiveness to the therapy (“residual platelet reactivity” , “nonresponse” or “non-response” or “low response” or “low-response” or “resistance” or “resistant”) and the clinical consequences (“prognosis” or “outcome” or “cardiovascular events” or “cardiac death” or “adverse events” or “major adverse cardiac events”) to identify studies describing associations of CKD with responsiveness to clopidogrel therapy in patients with coronary heart disease. We further performed a manual search of references from all identified publications.
Selection criteria
Two authors (Drs Wu and Gong) identified studies eligible for further review by performing an initial screen of identified titles or abstracts. Any discrepancies were reviewed by a third reviewer (Dr Zhou) and resolved by consensus. According to the pre-specified protocol, all studies evaluating the association of CKD (from stage 3 to stage 5) with the presence of high platelet reactivity were included. Case-reports, case-series without a control group, reviews were excluded. The included studies were classified as having a case-control design or a cohort design. To be included in the meta-analysis, a study had to provide original data on the prevalence of high platelet reactivity or clinical outcomes after use of clopidogrel and criteria of HPR.
Data extraction and quality assessment
Data were extracted by two authors (Drs Zhou and Wu) in terms of study design, inclusion criteria, patient characteristics, sample size, treatment dosages, duration of follow-up, methods used to determine the response to the therapy, definition of HPR and number of patients with clinical events according to the quality of response to clopidogrel. The quality of the observational studies was assessed using the Newcastle-Ottawa scale. End points were assessed at the longest follow-up available. All the outcomes were defined in accordance with what was used in the contributing studies.
Statistical analysis
We performed a detailed meta-analysis to evaluate the prevalence of HPR in CKD versus non-CKD patients and association of cardiovascular outcome (including major adverse cardiac event, myocardial infarction and stent thrombosis) with HPR in CKD patients treated with clopidogrel.
All data were analyzed using Cochrane Review Manager software (version 5.0). The fixed-effect model weighted by the Mantel-Haenszel method was used, followed by a test of homogeneity. Between-study and between-subgroup heterogeneities were evaluated by calculating the I2 statistic and the Cochrane Q (χ2) statistic, with a P value of 0.05 set for significance of the test of heterogeneity. If the hypothesis of homogeneity was rejected (p value <0.05), the random effect model using the DerSimonian-Laird method was employed. Potential publication bias of studies with different sample sizes was examined by visual inspection of funnel plots. CKD was defined as estimated glomerulous filtration rate (eGFR) or creatinine clearance (Crcl) <60ml/min/1.73m2. In analyzing the prevalence of HPR, sensitivity analyses were performed in order to determine the source of heterogeneity, according to the main characteristics of the study (methods used to determine platelet reactivity: VerifyNow-LTA-VASP, CKD stages: CKD stage 3- CKD stage 4–5, Clopidogrel loading dose: yes-no). In analyzing the prognostic impact of HPR in patients with CKD, we performed subgroup analyses according to different outcomes
Results
Search results and study characteristics
As shown in , our search initially yielded 180 references in total. After screening for title and abstract, 103 articles were excluded, including reviews, case reports and basic research. 77 studies remained for evaluation via detailed reading and other 67 articles were excluded for not matching inclusion criteria, not corresponding to our definitions and other reasons. Finally, 10 studies were included in the final analysis.
Characteristics of included studies are listed in . Two studies [Citation10,Citation11] used clopidogrel alone, while the remaining studies used dual antiplatlet therapy with clopidogrel and aspirin. Three studies [Citation9,Citation10,Citation15] included a loading dose of clopidogrel of 300 or 600mg, the other studies included a maintenance dose of 75 mg/day of clopidogrel.
Table 1. Characteristics of included studies.
Characteristics of patients in CKD versus non-CKD in studies included were listed in . Patients with CKD tended to be older, and had more often hypertension and diabetes mellitus. The distribution of cardiovascular risk factors is imbalanced among studies.
Table 2. Characteristics of patients in CKD versus non-CKD patients in studies included.
Three assays were used to assess response to clopidogrel therapy. These included light transmission aggregometry (LTA) with the use of adenosine diphosphate as agonist with different concentration in four studies [Citation7,Citation8,Citation11,Citation14], VerifyNow P2Y12 point-of-care testing in six studies [Citation9,Citation11,Citation13–15,Citation17], and vasodilator-stimulated phosphoprotein (VASP) in four paper [Citation10–12,Citation16]. It should be noted that among these papers, one paper [Citation11] used VerifyNow P2Y12 and VASP simultaneously, and another paper [Citation14] used VerifyNow P2Y12 and LTA.
Occurrence of high platelet reactivity
The OR for prevalence of HPR in each study and overall is shown in . In order to avoid the risk of data overlap, studies [Citation11,Citation14] used two laboratory assay methods simultaneously were excluded. Overall, 42.3% patients (1218/2881) were classified as HPR in CKD patients and 38.3% (4170/10883) in non-CKD patients. Compared to patients with normal renal function, patients with CKD had a significantly higher risk of HPR (OR: 1.34, 95% CI: 1.23–1.46, ).
Figure 2. Overall summary estimates of odds ratios and 95% confidence intervals (CI) for prevalence of high platelet reactivity (HPR) in patients with CKD and non-CKD patients. Squares represent the effect size; extended lines, 95% CI; diamond, total effect size.
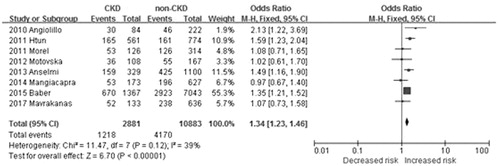
There was no significant heterogeneity among studies (I2 = 45%, p = 0.08). After excluding one paper with the lowest number of patients [Citation8], the heterogeneity of the model decreased to 34% (p = 0.17) and odds ratio remained statistically significant (OR 1.18, 95% CI 1.12 to 1.24; p < 0.001).
As shown in , sensitivity analyses were performed after stratification of the studies into the different variables. We noted a different magnitude of effect according to the methods used to determine clopidogrel response; the OR was 1.42 (95% CI, 1.29–1.56) for VerifyNow compared with 1.72 (95% CI, 1.39–2.13) for LTA, and 1.11(95% CI, 0.87–1.41) for VASP (P for heterogeneity = 0.005).
Table 3. Sensitivity analyses on basis of method of diagnosis, CKD stages, therapy, clopidogrel loading dose.
There was no apparent heterogeneity of effect between the studies in patients with stable CAD or ACS; the OR was 1.20 (1.15–1.47) (95% CI, 1.15–1.47) for stable CAD compared with 1.19 (95% CI, 1.13–1.25) for ACS.
The magnitude of effect was different according to the CKD stages; the OR was 2.32 (95% CI, 1.71–3.14) for CKD stage 4–5 compared with 1.30 (95% CI, 1.16–1.46) for CKD stage3 (P for heterogeneity = 0.001). There was no apparent heterogeneity of effect between the studies with or without clopidogrel loading dose (P for heterogeneity = 0.80).
Cardiovascular events
To further examine the association between HPR and occurrence of cardiovascular recurrences we performed subgroup analyses, by selecting studies according to some specific variables. The OR under a random-effects model for myocardial infarction, all-cause mortality, and MACE associated with a poor response to HPR in CKD patients in each study and overall is shown in .
Figure 3. Risk of major adverse cardiac events (A), Myocardial infarction (B), Stent thrombosis (C) for patients with CKD.
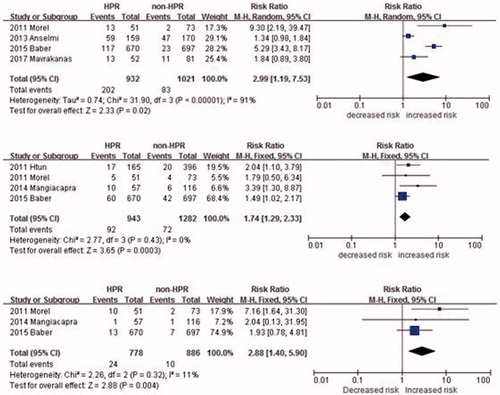
Four studies reported the HPR with major adverse cardiac events (MACE) in CKD patients. Patients with HPR had increased risk of MACE compared with patients with optimal response to clopidogrel (RR 2.99, 95% CI 1.19 to 7.53; p < 0.00001) (). A heterogeneity was found (I2 = 91%).
Four studies included data for the outcome of myocardial infarction. Compared to patients showing an optimal response to antiplatelet treatment, patients with persistent platelet reactivity despite antiplatelet treatment had a significantly higher risk of myocardial infarction (RR 1.74, 95% CI 1.29 to 2.33; p = 0.0002). There was no evidence of statistic heterogeneities among the included studies (P for heterogeneity = 0.43, I2 = 0%) ().
Three studies included data for the outcome of stent thrombosis. Compared to patients showing an optimal response to antiplatelet treatment, patients with persistent platelet reactivity despite antiplatelet treatment had a significantly higher risk of stent thrombosis (RR 2.98, 95% CI 1.42 to 6.26; p = 0.004). There was no evidence of statistic heterogeneities among the included studies (P for heterogeneity = 0.26, I2 =25%) ().
Discussion
Results of the present meta-analysis show that after receiving clopidogrel therapy, CKD patients tend to have higher prevalence of high residual platelet reactivity compared to subjects with preserved renal function. In addition, the presence of high platelet aggregability or low platelet inhibition by clopidogrel is associated with increased hazard of ischemic events and worse outcome compared to normal platelet reactivity in the CKD patients.
The CKD patients tend to have more accelerated atherosclerotic disease and a greater risk of thrombotic complications. Consequently, it might be expected that CKD patients are more likely to derive enhanced benefit from therapeutic approaches shown to improve cardiovascular outcomes in high-risk patients, including more potent antiplatelet treatment regimens as with adjunctive clopidogrel therapy. However, a post hoc analysis [Citation6] of CHANCE (Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events) trial showed that clopidogrel plus aspirin compared with aspirin alone in patients with normal renal function and mild renal insufficiency resulted in a significant reduction in new stroke events and combined vascular events, but this benefit was not apparent in moderate CKD patients. Similarly, a subanalysis of Clopidogrel for the Reduction of Events During Observation trial showed that benefit of long-term clopidogrel use was less apparent in mild CKD and not apparent at all in moderate CKD patients [Citation18]. The present review of pharmacodynamic observations might partly explain the elevated prevalence of ischemic complications among patients with advanced CKD. However, it must be noted that increased HPR does not explain alone the increased rate of clinical events in CKD patients. On the other hand, the persistence of HPR despite clopidogrel therapy underscores the need for more potent platelet inhibiting strategies to reduce recurrent ischemic event risk, including high clopidogrel dosing [Citation19] and novel antiplatelet agents [Citation20,Citation21].
Our meta-analysis suggested that CKD appeared significantly associated with HPR when the measurement was verifyNow P2Y12 or LTA, while the association was not statistically significant when the measurement assay was VASP. This is in accordance with a previous paper [Citation22] which found that the capability of three assays in triage residual platelet reactivity in patients with declined eGFR were different. In fact, standardized definitions to define individual responsiveness to clopidogrel are still lacking [Citation23,Citation24]. This is due not only to the numerous assays currently available to assess clopidogrel induced antiplatelet effects but also to the methodological variability within each technique [Citation25]. Since the assessment of platelet function inhibition by clopidogrel is highly test-specific and results vary on the basis of the assay used, unambiguous guidelines with respect to assay selection is needed.
The studies included in this meta-analysis have provided different results about HPR between CKD and non-CKD groups. One study [Citation9] suggested that renal dysfunction does not independently influence HPR but rather is a marker of comorbid conditions that augment platelet reactivity in the setting of CKD. Diabetes mellitus, older age, current smoking, hypertension and congestive heart failure, for example, are consistent correlates of HPR [Citation26–28] and also increase in prevalence as renal function worsens. Although most studies included in this meta-analysis made multivariable analysis to adjust for underlying confounding risk factors, the distribution of baseline characteristics among studies are imbalanced. Consequently, randomized controlled trials specifically addressing the role of platelet activation and on-treatment residual platelet reactivity for the occurrence of adverse events in CKD patients with cardiovascular disease are anticipated.
Some potential limitations of our study need to be discussed. First, clopidogrel was downgraded by the international guidelines in favour of more potent platelet drugs such as prasugrel and ticagrelor in acute coronary syndromes, thus the clinical application of these results is probably more useful in stable CAD patients. However, the majority of recent studies were performed in patients with high-risk acute coronary syndrome. More dedicated research in stable CAD patients with CKD is warranted. Second, as a potential source of bias, we have to consider that testing for platelet reactivity using different types of measurements was characterized by lack of standardization of the assays. This may limit the comparability of studies. However, by sorting studies according to methodological variables, this may partially offset the above limitation. Third, studies included in our meta-analysis have different inclusion and exclusion criteria and we cannot exclude that the concomitant presence of other demographic and clinical variables may influence our results. Since meta-analysis is performed on aggregate data, the meta-regression analysis would have allowed for the adjustment for these potential confounders. Thus, caution is necessary in overall results interpretation.
In our meta-analysis, CKD appeared correlated with HPR and this association had prognostic significance. With the aim to plan more specific strategies and optimal management in this high-risk population, maybe screening for high platelet reactivity should be suggested. Further studies with standardised laboratory methods and specifically defined protocols are required to validate the clinical relevance of such response variability to clopidogrel in CKD patients.
Ethical approval
For this type of study formal (meta-analysis) consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Yahalom G, Schwartz R, Schwammenthal Y, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. 2009;40:1296–1303.
- Mostofsky E, Wellenius GA, Noheria A, et al. Renal function predicts survival in patients with acute ischemic stroke. Cerebrovasc Dis. 2009;28:88–94.
- Amarenco P, Callahan A, 3rd, Campese VM, et al. Effect of high-dose atorvastatin on renal function in subjects with stroke or transient ischemic attack in the SPARCL trial. Stroke. 2014;45:2974–2982.
- Polzin A, Kelm M, Zeus T. Impaired clopidogrel antiplatelet effects and age: Young patients at risk. Int J Cardiol. 2015;187:216–218.
- Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107:87–92.
- Zhou Y, Pan Y, Wu Y, et al. Effect of estimated glomerular filtration rate decline on the efficacy and safety of clopidogrel with aspirin in minor stroke or transient ischemic attack: CHANCE Substudy (Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events). Stroke. 2016;47:2791–2796.
- Htun P, Fateh-Moghadam S, Bischofs C, et al. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol. 2011;22:627–633.
- Angiolillo DJ, Bernardo E, Capodanno D, et al. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146.
- Baber U, Mehran R, Kirtane AJ, et al. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents registry. Circ Cardiovasc Interv. 2015;8:e001683.
- Morel O, El Ghannudi S, Jesel L, et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011;57:399–408.
- Muller C, Caillard S, Jesel L, et al. Association of estimated GFR with platelet inhibition in patients treated with clopidogrel. Am J Kidney Dis. 2012;59:777–785.
- Motovska Z, Odvodyova D, Fischerova M, et al. Renal function assessed using cystatin C and antiplatelet efficacy of clopidogrel assessed using the vasodilator-stimulated phosphoprotein index in patients having percutaneous coronary intervention. Am J Cardiol. 2012;109:620–623.
- Viviani Anselmi C, Briguori C, Roncarati R, et al. Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical outcome following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc Interv. 2013;6:1166–1175.
- Gremmel T, Muller M, Steiner S, et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28:2116–2122.
- Mangiacapra F, Cavallari I, Barbato E, et al. Impact of chronic kidney disease on platelet reactivity and outcomes of patients receiving clopidogrel and undergoing percutaneous coronary intervention. Am J Cardiol. 2014;113:1124–1129.
- Mavrakanas TA, Alam A, Reny JL, et al. Platelet reactivity in stable cardiovascular patients with chronic kidney disease. Platelets. 2018;29:455–462.
- Maruyama H, Fukuoka T, Deguchi I, et al. Response to clopidogrel and its association with chronic kidney disease in noncardiogenic ischemic stroke patients. Intern Med. 2014;53:215–219.
- Best PJ, Steinhubl SR, Berger PB, et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008;155:687–693.
- Chen YT, Chen HT, Hsu CY, et al. Dual antiplatelet therapy and clinical outcomes after coronary drug-eluting stent implantation in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:262–271.
- Alexopoulos D, Panagiotou A, Xanthopoulou I, et al. Antiplatelet effects of prasugrel vs. double clopidogrel in patients on hemodialysis and with high on-treatment platelet reactivity. J Thromb Haemost. 2011;9:2379–2385.
- Butler K, Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with severe renal impairment. J Clin Pharmacol. 2012;52:1388–1398.
- Guo LZ, Kim MH, Kim TH, et al. Comparison of three tests to distinguish platelet reactivity in patients with renal impairment during dual antiplatelet therapy. Nephron. 2016;132:191–197.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–1516.
- Michelson AD, Frelinger AL, 3rd, Furman MI. Current options in platelet function testing. Am J Cardiol. 2006;98:4N–10N.
- Labarthe B, Theroux P, Angioi M, et al. Matching the evaluation of the clinical efficacy of clopidogrel to platelet function tests relevant to the biological properties of the drug. J Am Coll Cardiol. 2005;46:638–645.
- Redfors B, Ben-Yehuda O, Lin S-H, et al. Quantifying ischemic risk after percutaneous coronary intervention attributable to high platelet reactivity on clopidogrel (From the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents Study). Am J Cardiol. 2017;120:917–923.
- Franchi F, Rollini F, Angiolillo DJ. Defining the link between chronic kidney disease, high platelet reactivity, and clinical outcomes in clopidogrel-treated patients undergoing percutaneous coronary intervention. Circ Cardiovasc Inte. 2015;8:e002760.
- Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–1223.