Abstract
Objectives. Severe LV dysfunction and advanced age are associated with VT recurrence after catheter ablation in patients with post-infarction drug-refractory VT. We present retrospective analysis of long-term outcome after single and repeat VT ablation procedures in patients with ischemic heart disease. Design. Patients with recurrent VT post infarction who underwent catheter ablation between 2006 and 2017 in Isala Heart Centre were retrospectively analyzed. Univariate and multivariate analysis were used to identify predictors of arrhythmia recurrence post ablation. Patients were allocated to subgroups based on LVEF: severe (<30%), moderate (30–40%) and mild LV dysfunction (41–51%) and analyzed with log rank test. Results. A total of 144 patients were included. Two years VT free survival after a single procedure was 56.6% with median follow-up 46 [17–78] months. Recurrence of VT postablation wash high among patients with an old anteroseptal MI and LVEF < 30% with multiple morphologies of inducible VTs, indicating an extensive and complex substrate. Patients who underwent repeat ablations (27.1%) had significant more often LV aneurysms (20.5% vs. 7.6%, p = .03) and electrical storms (38.5% vs. 21.9%, p = .04). VT free survival was higher in patients with LVEF 41–51% compared to LVEF < 30% (71.4% vs. 47.8%, p = .01). In multivariate analysis, LVEF < 30% (vs 41–51%) was an independent predictor of arrhythmia recurrence (HR = 2.16, CI 1.15–4.06, p = .02). Conclusions. In patients with ischemic VT, success rate of ablation was highest among patients with preserved LV function and recurrent VT and ES was highest among patients with severe LV dysfunction after single and multiple ablation procedures.
Introduction
Long-term success rates of scar related ventricular tachycardia (VT) ablation are presented in a great variety in previous studies (43–88%) [Citation1,Citation2]. This is caused by inclusion of different ablation strategies, study populations and procedural endpoints. Targeting the scar substrate instead of focusing on ablation of the clinical VT best prevents recurrence of VTs in ischemic cardiomyopathy patients [Citation3–5]. Disadvantage of substrate modification is the risk of damaging healthy tissue and creating more scar, especially in patients with a large scar which requires extensive ablation. Several factors, including procedural success, severe left ventricular (LV) dysfunction and advanced age have been previously associated with both VT recurrence and increased mortality rates after catheter ablation [Citation6–10]. It remains unknown whether VT recurrence reflects adverse prognosis or failure of the procedure, especially in patients with severe LV dysfunction. Clinical relevance of VT free survival and how to achieve this, perhaps with repeat procedures, is a topic of discussion. Significant reduction of VT burden may be of great value despite incidental VT recurrence regarding quality of life improvement. In this single center retrospective study, we present long-term VT free survival after single and multiple ablation procedures in different left ventricular ejection fraction (LVEF) subgroups.
Materials and methods
Patient population and study design
We retrospectively analyzed data of 144 consecutive patients with ischemic cardiomyopathy suffering from recurrent VT, including electrical storm (ES), who underwent their first catheter ablation in Isala Heart Center between 2006 and February 2017. ES was defined as three or more VT episodes within 24 h [Citation11]. VT burden pre-ablation was calculated by number of ‘VT events’ divided by the time from first event until ablation in months. If two or more VT events occurred within 24 h, this accounted for 1 event. VT burden post-ablation was calculated by number of VT events divided by time from ablation date until next intervention (re-ablation or last follow-up date) in months. VT burden reduction was calculated by VT burden pre-ablation minus VT burden post-ablation. Implantable cardioverter defibrillator (ICD) monitor zone was programmed in all patients between 170 and 200 beats per minute, VT therapy zone between 200 and 230 beats per minute and VF zone from 230 beats per minute. In patients with known slow VT (<150 bpm), the monitor window was adjusted. Revascularization was considered incomplete if one of the following was observed in one or more coronary arteries: chronic total occlusion, percutaneous coronary intervention was not successful or when a bypass graft was occluded. Patients underwent substrate-based ablation only or substrate-based ablation with additional mapping during VT, based on operator preference and hemodynamic stability of the patient. Choice for redo ablation was a shared decision between operator and patient, based on; burden of VT recurrence, antiarrhythmic drug (AAD) tolerance, age, complication risk, estimated success rates and patient’s preference.
Ablation procedure
Before the ablation procedure, a transthoracic echocardiogram was performed to rule out thrombus in the LV. Procedures were performed under general anesthesia supervised by a cardiovascular anesthesiologist. In all patients a CARTO (Biosense Webster, J&J) 3D electro anatomical map was created to visualize scar tissue (0.1–0.5 mV), border zones (0.5–1.5 mV) and viable myocardium (>1.5 mV). VT was induced with a standard programmed electrical stimulation (PES) protocol with basic cycle length of 600, 500 or 400 ms with 1–3 extra stimuli with pacing from right ventricular (RV) apex, RV outflow tract and in selected cases from the LV. After induction of a hemodynamically tolerated VT, activation mapping, pace-mapping and entrainment mapping was performed to confirm exit and protected isthmus sites. In selected cases with severe LV dysfunction PES without extrastimuli caused severe hypotension. PES was not performed in these cases due to high risk of VT induced hemodynamic collapse. When VT was not inducible, pace-mapping was performed in the scar zone to identify potential exit sites of clinical VTs. In all patients, local abnormal ventricular activities (fractionated signals, late potentials, diastolic potentials) in low-voltage areas were searched and ablated until all abnormal potentials disappeared. Complete elimination of abnormal potentials was the aimed endpoint of the procedure. Noninducibility was not routinely tested. In patients where noninducibility was testes, we defined complete success as, noninducibility of any sustained monomorphic VT. We defined partial success when only clinical VT was noninducible (excluding polymorphic VT, VF or VT with cycle length <200 ms). If needed, patients were hemodynamically supported with vasopressor drugs (noradrenaline generally), no support devices were used.
Outcomes
Patients visited the outpatient clinic 4–6 weeks after VT ablation and every 6 months including ICD interrogation. Primary endpoint was defined as recurrence of VT at 2-year follow-up after index ablation. VT recurrence was defined as VT (>30 s) documented on a 12 lead ECG, rhythm strip or based on ICD recordings. Secondary endpoints included VT burden reduction after single ablation, procedural related complications, VT recurrence after repeat procedures and all-cause mortality.
Statistical analysis
Data are expressed as mean with standard deviation, interquartile range or percentage in case of non-normal distribution. When continuous variables were normally distributed, we used an independent samples Student’s t-test to calculate the significance of the difference between two variables. In case of non-normally distributed variables, we used the Mann Whitney U test. For categorical variables a Chi-square test or Fisher’s exact test was performed. We considered a p value <0.05 significant. Patients were categorized into subgroups according to their LV dysfunction severity: (LVEF < 30%, LVEF 30–40%, LVEF 41–51%). VT-free survival was estimated for different LVEF subgroups by the Kaplan-Meier curve product-limit method. Univariate and multivariate logistic regression analyses were performed to identify independent predictors of arrhythmia recurrence after VT ablation. Variables with a p < .10 in the univariate analysis were included in the multivariate analysis. For comparison of variables between LVEF subgroups one-way ANOVA was used when continuous variables were normally distributed. When continuous variables were non-normally distributed or in case of small sample sizes Kruskal-Wallis K test was used. For categorical variables a Chi-square test or Fisher’s exact test was performed. Statistical analysis was performed using IBM Statistics version 22.0.
Results
A total of 144 patients with ischemic cardiomyopathy who underwent their first (index) catheter ablation were analyzed. All patients had an ICD, in 54/144 (37.8%) of patients the ICD was initially implanted for primary prevention. In 137/144 (95.1%) patients, at least one spontaneous VA event was documented before ablation. Three patients had spontaneous non-sustained VT pre-ablation and four patients had inducible VT during EPS followed by mapping and catheter ablation. Severe LV dysfunction with LVEF <30% was present in 68/144 (47.2%) patients. shows clinical characteristics of patients divided in three different subgroups based on LVEF. The infarct location was significant different between LVEF subgroups, with higher frequency of anteroseptal locations in LVEF <30% group (). Anti-arrhythmic drug (AAD) therapy prior to ablation was started in 71 (49.3%) patients; including 62 (87.3%) amiodarone, 3 (4.2%) sotalol and 6 (8.5%) quinidine, before index ablation. In 64/71 (90.1%) patients, VT episodes recurred despite AAD therapy. Significant more patients with lower LVEF (LVEF <30% and 30–40%) had failure of AADs before index ablation, compared to patients with LVEF 41–51%. Patients experienced a median of 2 [1–4] VT episodes before ablation and 14/144 patients (9.7%) suffered from ES before index ablation. Median time from first event until ablation was 9 [1–33] months, with no significant difference between LVEF groups (LVEF <30%: 11 [1–51]; LVEF 30–40%: 10 [1–24]; LVEF 41–51%: 6 [0–24], p = .403).
Table 1. Patient and procedural characteristics of index ablation per LVEF group.
Procedural characteristics
Procedural characteristics are shown in . Number of ablations performed was equally divided over 10 years with 74 (51.4%) of the procedures in the first 5 years (2006–2011) and 70 (48.6%) in the last 5 years (2011–2017) and there was no difference between LVEF subgroups. In the LVEF 41–51% subgroup, there was a significant lower number of patients with ≥1 inducible VT during the procedure. (LVEF < 30%: 26.5%, LVEF 30–40%: 39.5%, LVEF 41–51%: 12.1%, p = .02). In addition to substrate-based ablation, detailed mapping and targeted ablation during stable VT was performed in 32 (22.4%) cases. This latter was not significant different between LVEF subgroups. Proportion of additional mapping and targeted ablation during VT was not significant different between first and last 5 years (24.3% vs. 20.3%, p = .59). In two patients, epicardial ablation was performed in addition to endocardial ablation. Operators reported suspicion of epicardial substrate in 13.2% of the patients (n = 19); this was not significant different between LVEF subgroups. In 79 of all 144 patients (54.9%), VT noninducibility was tested postablation. In 61/79 (77.2%) of these patients, complete success was achieved with noninducibility of all VTs. In 18/79 (22.8%) there was partial success leaving only non-clinical VTs inducible. In the remaining 65/144 (45.1) patients, records of inducibility was unavailable or not tested. Procedural success status was not significant different between LVEF groups. In significant more patients, noninducibility was tested to confirm procedural success in the last 5 years compared to the first (68.6% vs 41.9%, p = <.01). When noninducibility was tested, ratio complete/partial success was significant different between last (complete: 85.4%, partial: 14.6%) and first 5 years (complete: 64.5%, partial: 35.5%). with p-value .03.
Complication rate
In 144 procedures, there were a total of 11 (7.6%) procedure related complications. There were four patients with tamponade with need for puncture of the pericardial space. Three patients suffered from bleeding in need for transfusion. Two patients developed AV block after catheter ablation and both needed continuous ventricular pacing. One patient had a stroke. There was one death during catheter ablation due to progression of heart failure with electromechanical dissociation in the absence of pericardial effusion.
VT recurrence and predictors of outcome
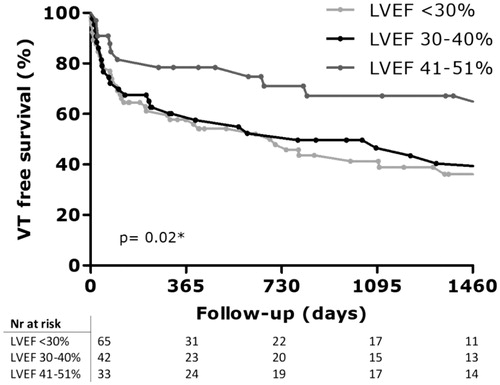
With a median follow-up duration of 46 [17–78] months, 57 patients (39.9%) were free of ventricular tachycardia after index ablation, with 16 of these patients (28%) continuing AAD (n = 1 quinidine, n = 15 amiodaron). One patient was lost to follow-up due to emigration and one patient died during index ablation. One-year VT free survival was 63.2% (n = 91) and 2 years VT free survival was 56.6% (n = 81). Short-term (6 months) VT free survival did not differ between patients ablated in the first 5 years and last 5 years (72.2% vs. 64.3%, log-rank p = .25). Long-term (2-year) VT free survival also did not differ between patients ablated in the first 5 years and last 5 years (58.3% vs. 52.9%, log-rank p = .20). VT free survival curves for different LVEF subgroups are shown in . With a median follow-up duration 46 [17–78] months, VT free survival was significant different between LVEF groups (LVEF < 30%: 32.6%, LVEF 30–40%: 30.2%, LVEF 41–51%: 60.6%), p = .02). Details of VT recurrence after index ablations per LVEF subgroup are displayed in . Median time to VT recurrence was 497 [80–1560] days. Time to recurrence in days was not significant different among LVEF subgroups (LVEF < 30%: 402 [74–1125], LVEF 30–40%: 565 [69–2205], LVEF 41–51%: 1021 [248–1914], p = .06). The total number of patients experiencing ES during follow-up was 38 (26.4%) in our study. The incidence of ES prior to ablation was highest in the LVEF < 30% subgroup and ES during follow-up was also significant different between LVEF groups as summarized in = .02). Of note, a total of 16 (11.2%) patients had ES after the ablation procedure, while they did not present with ES before. A total of 116 patients (80.5%) had reduction of >50% of VT burden compared to pre-ablation, 8 patients (5.5%) had similar VT burden and 9 patients (6.3%) had an increase of VT burden. VT burden reduction was significant different between LVEF groups (). In univariable analysis, the following characteristics were associated with recurrence of ventricular tachyarrhythmia (): failed AAD therapy, total revascularization and LVEF 30–40% vs 41–51%. In multivariable analysis, LVEF <30% vs. 41–51%, remained independently associated with arrhythmia recurrence after index procedure (HR 2.160; 95% CI: 1.149–4.063; p = .02).
Table 2. VT Free survival after single (index) ablation.
Table 3. Potential risk factors for VT recurrence after single ablation.
Repeat ablations
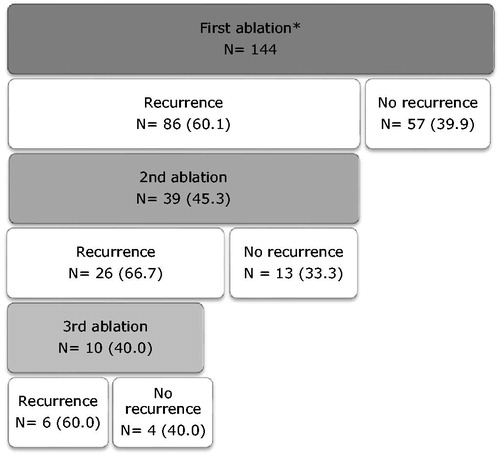
In 39/144 (27.1%) patients at least one redo ablation was performed, with 4/39 (10.3%) patients within 30 days after index ablation. Eleven patients had 2 redo ablations, 1 patient had 3 redo ablations and 1 patient had 5 redo ablations. Clinical characteristics of patients who underwent a single ablation (n = 105) or repeat ablations (n = 39) are displayed in . Median time to redo ablation following VT recurrence was 24 [9–250] days. Patients with single ablation vs. repeat ablations did not differ with regard to age (67 ± 11.1 vs. 64.0 ± 8.5, p = .09) or LVEF (32.7 ± 10.7 vs. 31.5 ± 8.2, p = .49), however they had more often left ventricle aneurysm (6.7% vs. 20.5%, p = .02) and electrical storm (21.9% vs. 38.5%, p = .04). Two patients had epicardial ablations during redo-procedures, with one patient failure of attempt due to adhesions from prior CABG. Overall success rate of multiple redo ablation procedures is displayed in . VT freedom after repeat ablations per LVEF group are shown in . There is a trend of higher percentage VT freedom after repeat ablations in LVEF 41–51% group compared to LVEF <40% (80% vs. 30–40%, p = .09). When patients did have recurrence, it appears that number of VT recurrences was also higher in LVEF <40%. Cumulative VT freedom (VT freedom after all redo procedures and start of AAD) is shown in . Cumulative VT freedom was 63.2% (n = 91) at last follow-up, this was significant different between LVEF groups (LVEF < 30%: 58.8, LVEF 30–40%: 53.3, LVEF 41–51%: 84.8, p = .02). There was a trend towards more AAD use at last follow-up in the LVEF <30% group but this did not reach statistical significance. VT freedom after start of AAD was approximately 25% and was not significant different between LVEF groups ().
Table 4. Clinical characteristics of patients undergoing one or multiple ablations.
Table 5A. VT Free survival after redo ablations.
Table 5B. VT Free survival after repeat procedures or start AAD (cumulative VT freedom) and all-cause mortality.
Mortality
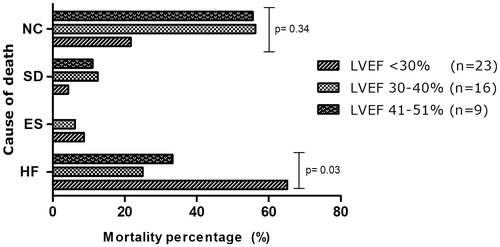
A total of 48 patients (33.6%) died during follow-up. The 30-day mortality rate was 7.0%. All-cause mortality rate was not significant different between LVEF subgroups (LVEF < 30%: 33.8%, LVEF 30-40%: 37.2%, LVEF 41–51%: 27.3%, p = .65) The distribution of cause of death in relation to LVEF is displayed in . The majority of deaths were related to progression of heart failure (45.8%) and this was significantly higher in LVEF < 30% subgroup (LVEF < 30%: 22.1%, LVEF 30–40%: 9.3, LVEF 41–51%: 9.1, p = .03). Three patients died during ES, none of these patients were in the LVEF 41–51% subgroup. Four patients died suddenly at home with unknown cause and cardiac rhythm derived from device registrations revealed asystole or pulseless electrical activity. When we excluded four patients with unknown cause of death, cardiac death (related to heart failure or arrhythmia) was significantly higher in LVEF < 30% subgroup (LVEF < 30%: 25.4%, LVEF 30–40%: 12.2%, LVEF 41–51%: 9.4%, p = .02). Non-cardiac causes of death were infection, ruptured aortic aneurysm, ischemic stroke, kidney failure and cancer (19 patients = 39.6%). This rate not significant different between all three LVEF subgroups (), but was significant higher in patients with LVEF < 30% compared to LVEF >30% (14/25 = 56% vs. 5/68 = 7.4%, p = .05). There was no significant difference between single vs repeat ablation groups in all-cause mortality (36.2% vs. 28.2%, log-rank p = .286) or cardiac death alone (35.2% vs. 28.2%, log-rank p = .116).
Discussion
In this retrospective study, the 2-year VT free survival was 56.6% after a single catheter ablation procedure in patients post-infarction recurrent VT. Almost two decades of intense study with the publication of a large number of single and multicenter series of non-selected patients have passed [Citation1,Citation2,Citation5]. Our data are consistent with a VT free survival of 57.2% in a retrospective study including a larger population of 1412 patients with comparable patient characteristics [Citation6]. The inclusion period was also similar (2004–2015). The evolvement of ablation techniques, procedural endpoints and experience of local operating electrophysiologists over the past decade was also comparable to previous reports. Procedural strategies have significantly changed over the past decade after publication of multiple studies [Citation12]; for example core isolation [Citation13] box lesions and targeting clinical VT or the entire substrate [Citation3]. In this study, patients were included over a time-course of 10 years, compromising detailed explanation of used ablation techniques in older records from patients ablated before 2010. Previous reports demonstrated that both endo- and epicardial homogenization of the scar significantly increases freedom from VA, compared to endocardial ablation alone [Citation4]. Epicardial ablation could only be performed in 4 patients (2.8%) in our population, while operators reported suspicion of epicardial substrate in 13.3% of cases. The latter is line with previously reported incidence of approximately 15–30% critical epicardial substrates in patients with ischemic cardiomyopathy [Citation14]. Reason for low amount of epicardial ablations performed could be the high incidence of previous CABG (46.2%) in our population. Previous studies with similar successrates after ablation described similar low amount of epicardial ablations [Citation6,Citation15]. In only 22.4% VT was hemodynamically tolerated mappable during procedure in our study, this is in accordance with a presumed ∼70% hemodynamically unstable VTs [Citation9]. Noninducibility with programmed ventricular stimulation has been used as the end point for catheter ablation of scar-related VT with inconsistent results. In several studies, noninducibility of VT post-ablation was independently associated with lower mortality and VT recurrence [Citation6–8,Citation16–18], while others found this endpoint to be a poor predictor [Citation14,Citation19,Citation20]. In this study, noninducibility was tested in only half of the study population (54.9%). Interestingly, there was a significant increase in noninduciblity testing in patients ablated in the last 5 years compared to the first. Also, when noninducibiltiy was tested, patients treated in the last 5 years more often reached complete success compared to the first 5 years. However, this did not result in a significant different in VT free survival rates between first and last 5 years. The majority of electrophysiologists performing the ablation procedures were present during the entire duration of 10 year. Complication rates were similar to previous studies with 6–8% procedure related complications [Citation9,Citation21].
Outcome in different LVEF subgroups
This study provides additional information about outcome of ablation in different LVEF subgroups. The likelihood of achieving VT free survival after a single ablation procedure was highest among patients with mild LV dysfunction (LVEF >40%) compared to patients with LVEF < 30%. Time to VT recurrence was also significant shorter in LVEF <30% subgroup. However, there was a significant higher number of patients with significant VT burden reduction (>50% reduction of VT events post-ablation compared to pre-ablation) in lower LVEF subgroups. Furthermore, increase in VT burden only occurred in the LVEF <30% subgroup. Whether this indicates a possible pro-arrhythmogenic effect of extensive ablation or represents worsening of heart-failure remains unknown. Proportion substrate mapping compared to substrate mapping with additional mapping during VT was not significant different between LVEF subgroups. Procedural success was not significant different between LVEF subgroups. As previously reported, noninducibility was only tested in half of the population, therefore statements about acute procedural success should be made very carefully. There was a trend towards larger portion of ‘not tested’ for inducibility in lower LVEF subgroups, mainly due to hemodynamic intolerance of ventricular pacing or VT in this subgroup with the risk of hemodynamic collapse.
In multivariate analysis, LVEF <30% (vs 41–51%) remained an independent predictor of VT recurrence. Patients with low LVEF had significant more anteroseptal infarction locations and higher number of inducible VT morphologies pre-ablation compared to less severe LV dysfunction. We hypothesize that these patients have a larger and more complex arrhythmic substrate and may have compromised elimination of all possible arrhythmogenic pathways. Multiple inducible VT morphologies have been pointed out repeatedly as a predictor of arrhythmia recurrence in long-term follow-up [Citation19,Citation21,Citation22]. Scar extension measured with MRI was a predictor of VT recurrence after endocardial ablation in patients with post-infarction recurrent VT [Citation23]. Unfortunately, MRI was not available for data analysis in our study. In our experience, procedures sometimes were prematurely ended because of occurrence of spontaneous arrhythmia due to acute decompensated heart failure with pulmonary congestion and hemodynamic instability and therefore did not reach the aimed acute procedural endpoints. Advanced stage of heart failure which enhances the susceptibility to spontaneous VT may constitute another reasonable explanation for higher VT recurrence rate in patients with severe LVEF dysfunction. Both recurrent VT and LVEF have been described as predictors of early mortality (<30 days) [Citation24] and late all-cause mortality after catheter ablation [Citation7,Citation9] It is not yet confirmed that lower mortality is indeed due to a lower VT recurrence and that VT recurrence may constitute a marker rather than a cause of increased mortality. Our mortality rate is similar to previously reported rates. All-cause mortality was not significant higher in patients with severe LV dysfunction, as would be expected. This may be explained by the significant higher non-cardiac death rate in patients with LVEF < 30% compared to LVEF >30%. Focusing on cardiac death due to heath failure or arrhythmia, death rates were significant higher in patients with severe LV dysfunction.
Repeat ablation procedures
Previous studies described that repeat catheter ablation procedures can be performed safely and are not substantially worse than those for patients undergoing a first ablation attempt [Citation22,Citation25]. Long-term mortality was worse for patients undergoing multiple ablations compared to single ablations if VT recurred [Citation25]. Patients free of recurrent VT, whether with one or multiple procedures, could simply have represented a less sick population with a less complex or less extensive substrate. In this study, patients referred for repeat ablations (27.1%) represented a sicker population with significant higher frequency of LV aneurysms and electrical storms, compared to patient with a single ablation. In the general study population, redo ablation resulted in approximately 30–40% gain of VT freedom and 60% recurrence () This is in line with previous reports of comparable success rates after multiple ablations [Citation22]. In LVEF subgroups, achievement of VT freedom appears to be higher after repeat ablation in LVEF > 40% compared to LVEF <40%. Larger study populations are necessary to confirm this statement. Repeat ablation is often a matter of shared decision making between patient and physician with different aspects to take into consideration; age, co-morbidity, high VT burden, re-enforcement of the previous ablation procedure or epicardial approach. Current data showed that patients achieving VT freedom after one or repeat procedures is significant lower when there is severe LV dysfunction. We encourage to aim for quality of life improvement with a substantial VT burden reduction rather than complete VT freedom in these patients. In contrast, patients with mild LV dysfunction are highly likely to achieve VT freedom with either one or repeat ablation procedures.
Limitations
The importance of missing data regarding details of the ablation procedure and lack of a predefined ablation protocol and endpoints due to the retrospective design and inclusion period of 10 years with poor preserved patient records in some cases, are an important limitation. Likewise, we could not report detailed specifications of the arrhythmia in the event of an ES (VF, monomorphic/polymorphic VT). There is a possibility that patients with spontaneous termination of slow VT episodes without symptoms are missed during follow-up, this may bias our outcome results. The impact of the learning curve and the evolution in the available technology on the outcome was not evaluated. The discrepancy between the low number of epicardial ablations performed and reported percentage of cases with a high suspicion of epicardial substrate may indicate incomplete ablation of substrate and is considered a potential bias of ablation outcome. Total revascularization in half of the patients, may have also biased our outcome. Another limitation of our study is lack of serial visualization of scar burden using advanced imaging techniques such as MRI to support our hypothesis that incomplete substrate ablation limits favorable outcome in patients with an extensive myocardial infarction scar burden and severe LV dysfunction. The study represents a population with an advanced stage of LV remodeling and impaired systolic function, hence the outcomes in patients ablated in an earlier stage of the disease may be different from these presented in the current study.
Conclusion
This retrospective single center study presents long-term VT free survival after catheter ablation in patients with post-infarction recurrent VT. The likelihood of achieving VT freedom after a single ablation procedure was highest in mild LV dysfunction (LVEF >40%). Detailed evaluation of different LVEF subgroups revealed higher frequency of anteroseptal infarction in low LVEF subgroups and higher number of inducible VT morphologies during ablation procedure, indicating an extensive and complex substrate. The role of repeat ablation needs to be investigated in a prospective study to determine whether this can reduce mortality in patients with VT recurrence. In future studies, success rates after single and multiple ablation efforts should be reported with respect to different LVEF subgroups with distinct procedural endpoints and focus on VT burden reduction rather than VT free survival.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Liang JJ, Santangeli P, Callans DJ. Long-term outcomes of ventricular tachycardia ablation in different types of structural heart disease. Arrhythm Electrophysiol Rev. 2015;4:177–183.
- Bricenõ DF, Romero J, Villablanca PA, et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace. 2018;20:104–115.
- Di Biase L, Burkhardt JD, Lakkireddy D, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy the VISTA randomized multicenter trial. J Am Coll Cardiol. 2015;66:2872–2882.
- Di Biase L, Santangeli P, Burkhardt DJ, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:132–141.
- Marchlinski FE, Callans DJ, Gottlieb CD, et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288–1296.
- Siontis KC, Kim HM, Stevenson WG, et al. Prognostic impact of the timing of recurrence of infarct-related ventricular tachycardia after catheter ablation. Circ Arrhythmia Electrophysiol. 2016;9:pii: e004432.
- Sauer WH, Zado E, Gerstenfeld EP, et al. Incidence and predictors of mortality following ablation of ventricular tachycardia in patients with an implantable cardioverter-defibrillator. Hear Rhythm. 2010;50:227–234.
- Tilz RR, Lin T, Eckardt L, et al. Ablation outcomes and predictors of mortality following catheter ablation for ventricular tachycardia: data from the german multicenter ablation registry. J Am Heart Assoc. 2018;7:pii: e007045.
- Tung R, Vaseghi M, Frankel DS, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an international VT ablation center collaborative group study. Hear Rhythm. 2015;12:1997–2007.
- Vergara P, Tzou WS, Tung R, et al. Predictive score for identifying survival and recurrence risk profiles in patients undergoing ventricular tachycardia ablation. Circ Arrhythmia Electrophysiol. 2018;11:e006730.
- Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2015;20:f249–f253.
- Santangeli P, Frankel DS, Marchlinski FE. End points for ablation of scar-related ventricular tachycardia. Circ Arrhythmia Electrophysiol. 2014;7:949–960.
- Tzou WS, Frankel DS, Hegeman T, et al. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythmia Electrophysiol. 2015;8:353–361.
- Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction the multicenter thermocool ventricular tachycardia ablation trial. Circulation 2008;16:2773–2782.
- Dinov B, Fiedler L, Schönbauer R, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014;129:728–736.
- Ghanbari H, Baser K, Yokokawa M, et al. Noninducibility in postinfarction ventricular tachycardia as an end point for ventricular tachycardia ablation and its effects on outcomes. Circ Arrhythm Electrophysiol. 2014;7:677.
- Yokokawa M, Kim HM, Baser K, et al. Predictive value of programmed ventricular stimulation after catheter ablation of post-infarction ventricular tachycardia. J Am Coll Cardiol. 2015;65:1954–1959.
- Essebag V, Joza J, Doucette S, et al. Prognostic value of non-inducibility on outcomes of ventricular tachycardia ablation: a ventricular tachycardia ablation vs enhanced drug therapy in structural heart disease (VANISH) substudy. Can J Cardiol. 2017;33:S17–S18.
- Tung R, Josephson ME, Reddy V, et al. Influence of clinical and procedural predictors on ventricular tachycardia ablation outcomes: an analysis from the substrate mapping and ablation in sinus rhythm to halt ventricular tachycardia trial (SMASH-VT). J Cardiovasc Electrophysiol. 2010;21:799–803.
- Della Bella P, De Ponti R, Uriarte JAS, et al. Catheter ablation and antiarrhythmic drugs for haemodynamically tolerated post-infarction ventricular tachycardia: long-term outcome in relation to acute electrophysiological findings. Eur Heart J. 2002;23:414–424.
- Calkins H, Epstein A, Packer D, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000;35:1905–1914.
- Kosmidou I, Inada K, Seiler J, et al. Role of repeat procedures for catheter ablation of postinfarction ventricular tachycardia. Hear Rhythm. 2011;8:1516–1522.
- Ávila P, Pérez-David E, Izquierdo M, et al. Scar extension measured by magnetic resonance-based signal intensity mapping predicts ventricular tachycardia recurrence after substrate ablation in patients with previous myocardial infarction. JACC Clin Electrophysiol. 2015;1:353–365.
- Santangeli P, Frankel DS, Tung R, et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J Am Coll Cardiol. 2017;69:2105–2115.
- Tzou WS, Tung R, Frankel DS, et al. Outcomes after repeat ablation of ventricular tachycardia in structural heart disease: an analysis from the International VT Ablation Center Collaborative Group. Hear Rhythm. 2017;14:991–997.