Abstract
Objectives. Using composite endpoints and/or only first events in clinical research result in information loss and alternative statistical methods which incorporate recurrent event data exist. We compared information-loss under traditional analyses to alternative models. Design. We conducted a retrospective analysis of patients who underwent percutaneous coronary intervention (Jan2010-Dec2014) and constructed Cox models for a composite endpoint (readmission/death), a shared frailty model for recurrent events, and a joint frailty (JF) model to simultaneously account for recurrent and terminal events and evaluated the impact of heart failure (HF) on the outcome. Results. Among 4901 patients, 2047(41.8%) experienced a readmission or death within 1 year. Of those with recurrent events, 60% had ≥1 readmission and 6% had >4; a total of 121(2.5%) patients died during follow-up. The presence of HF conferred an adjusted Hazard ratio (HR) of 1.32 (95% CI: 1.18–1.47, p < .001) for the risk of composite endpoint (Cox model), 1.44 (95% CI: 1.36–1.52, p < .001) in the frailty model, and 1.34 (95% CI:1.22–1.46, p < .001) in the JF model. However, HF was not associated with death (HR 0.87, 95% CI: 0.52–1.48, p = .61) in the JF model. Conclusions. Using a composite endpoint and/or only the first event yields substantial loss of information, as many individuals endure >1 event. JF models reduce bias by simultaneously providing event-specific HRs for recurrent and terminal events.
Introduction
Disease is a dynamic process where events happen with differing intensities and patterns among individuals. Clinical research studies often assemble multiple clinically relevant outcomes into 1 composite end point and subjects are evaluated until the first event of interest in the composite occurs. The use of composite endpoints favors shorter follow-up, ease of analysis, and interpretation; however, they may also introduce bias as the components are associated. Composite endpoints are employed across different domains such as cardiology, nephrology, anesthesiology, respiratory and gastroenterology [Citation1]. Since patients with chronic disease frequently endure recurrent hospital admissions and are subject to different durations of follow-up, composite end points which stop at the first event do not appropriately measure the total burden of the disease. It is imperative to collect information on all events and employ the appropriate corresponding statistical methods. The dependent nature of the recurrent events needs to be considered when choosing the appropriate statistical methods [Citation2]. The Andersen–Gill (A-G) [Citation3], Wei–Lin–Weissfeld total time (WLW-TT) marginal model [Citation4], Prentice–Williams–Peterson gap time (PWP-GT) [Citation5] conditional model and frailty models are the most commonly employed statistical models for recurrent events, but have different assumptions. The A-G model assumes that events are independent with a common baseline hazard for every gap time; dependence and heterogeneity can be introduced via time-dependent covariates and robust standard errors. In the WLW-TT, a marginal model, preserves randomization by calculating the cumulative effect while considering the total time from randomization to every event. The WLW-TT assumes events are independent and uses robust standard errors to account for heterogeneity. In the PWP-GT model, a conditional model accounts for event dependence via stratification by event number. Frailty models introduce a random effect that accounts for unobserved heterogeneity in individuals. Shared frailty model with random effects is presumed to follow a gamma distribution with mean equal to one and unknown variance [Citation6,Citation7]. When a terminal event occurs along with recurrent events, a joint frailty (JF) model is recommended. The JF model accounts for event dependence and provides distinct hazard ratios for recurrent events and the terminal event [Citation8,Citation9]. In this study, we explore the magnitude of information lost when only the first event is considered and also compare the traditional methods to the JF model.
Methods
A retrospective study including patients who underwent a percutaneous coronary artery intervention (PCI) between 2010 and 2014 at the Baylor University Medical Center and the Baylor Heart and Vascular Hospital, Dallas, Texas was conducted. The information of the index PCI admission was obtained from the records captured in the American College of Cardiology CathPCI registry. Details on readmissions and death were obtained for one year following the index procedure. Details on readmissions were obtained from the Dallas-Fort Worth Hospital Council Foundation, which maintains a comprehensive Regional Master Patient Index (REMPI) database that captures data on patients admitted to different hospitals in the North Texas region [Citation10]. Readmissions with a cardiovascular (CV) principal diagnosis based on the International Classification of Diseases, Ninth Revision, Clinical Modification codes (acute myocardial infarction, sudden cardiac death, HF, stroke, cardiovascular procedure, cardiovascular hemorrhage, and other cardiovascular causes (a nonstroke intracranial hemorrhage, nonprocedural or nontraumatic vascular rupture (e.g. aortic aneurysm), or pulmonary hemorrhage from a pulmonary embolism) were included in analyses [Citation11]. The status (elective/emergent) of the PCI procedure was recorded from the catheterization records as noted by the interventionist. Emergent scheduling was defined as PCI conducted immediately to avoid morbidity/death. One-year mortality was updated using the Death Master File provided by the National Technical Information Service, as needed [Citation12]. Patients who died during the index admission were excluded. This study received Institutional Review Board approval with a waiver of informed consent.
Statistical analysis
Statistical analyses were conducted using STATA 14.2 and R 3.2.1. Chi-square/Fisher’s Exact tests were employed to compare proportions and Student’s t/Wilcoxon Rank sum tests were used to compare continuous variables as appropriate. A recurrent event was defined as a cardiovascular related readmission following the index event. The time-to-event analyses were conducted as follows:
Time to first event:
Cox proportional hazards survival analysis with a composite end point as the outcome, where the composite endpoint was either readmission or death;
Cox proportional hazards survival analysis with death as the outcome (readmissions ignored).
Recurrent events:
Shared frailty model where a frailty term was introduced to capture the heterogeneity among the individuals. Only recurrent readmissions were included and event of death was excluded for the analysis.
A joint frailty model where recurrent events and death were considered as distinct outcomes and analyzed specifically. The joint frailty model further identified the association between recurrent events and the terminal event.
Results
There were a total of 4901 patients who underwent PCI during the study period; 68.4% were male with a mean age of 63.9 ± 11.4 years. The comorbidities of hyperlipidemia, hypertension and diabetes were reported in 89, 86 and 40% of the patients, respectively. About 15% of the patients had underlying heart failure (HF) and 37% had an emergent PCI procedure.
Readmissions and death
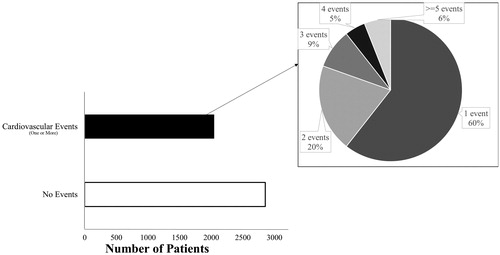
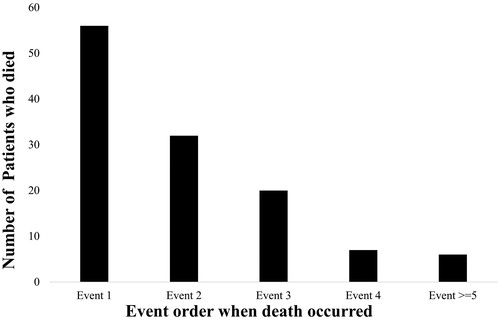
Among the 4901 patients, 2047 (41.8%) had recurrent events during the 1 year follow-up period. Patients with recurrent events were older with a median (range) age of 64.7 (31.2–94.6) years compared with those without recurrent events (63.8 (28.8–95.0), p = .01) at the time of PCI. The proportion of patients with comorbidities such as diabetes, cerebrovascular accident (CVA), peripheral vascular disease (PVD), chronic obstructive lung disease (COLD) and HF were higher among those who had a readmission/death (). Of those with recurrent events, 60% had at least one readmission and 6% had more than 4 readmissions (). Of the 2047 patients, 807 (40%) patients had more than 1 readmission and this additional information will be lost if only the first event is considered. A total of 121 (2.5%) patients died during the follow-up; 30 (24.8%) died without a preceding readmission. Of the remaining 91 patients, 28.6% (26/91), 35.2% (32/91), 15.3% (14/91) died after the first, second, and third readmissions, respectively ().
Table 1. Characteristics of patients with and without cardiovascular related events.
Estimates by the different models
Time to first event
The total number of records included in the time to first event analysis was 4901. Composite end point: A total of 2047 patients readmitted or died during the one year follow-up. Emergent scheduling, diabetes, HF, CVA, PVD and COLD were found to be associated with an increased risk of a composite end point. When considering only death as the outcome, a total of 121 patients had a terminal event during the follow-up of one year post PCI. Patients older than 63 years, emergency scheduling, diabetes, HF and COLD were associated with increased risk of death.
Recurrent event analysis
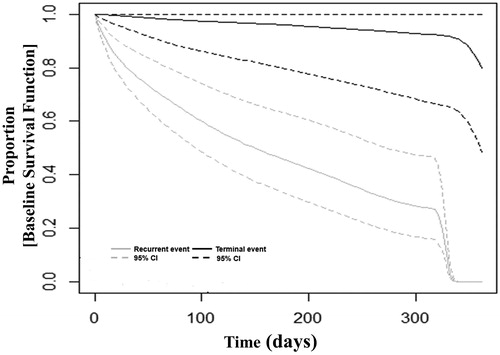
Information on readmissions of patients was included in this analysis, which increased the sample size to 8375. The shared frailty model included all the readmissions and revealed a significant frailty parameter capturing the presence of heterogeneity. A general JF model was then conducted where recurrent event and death were accounted as different events and event-specific hazard ratios were obtained for the outcomes of recurrent and terminal events following PCI. The JF model reiterated dependence between the recurrent events and also with the terminal event. displays the baseline survival function of a recurrent and a terminal event. describes the sample size, the underlying assumptions, and hazard ratios for various risk factors obtained from the different analytical approaches. The hazard ratios obtained by the Cox model for the outcome of a composite endpoint were similar to the HR for readmission by the JF model. For example, presence of HF conferred an adjusted HR of 1.32 (1.18–1.47), p < .001 for the risk of hospitalization or death in the Cox model, 1.34 (95%CI 1.22–1.46), p < .001 in the joint frailty model, and 1.44 (95% CI 1.36–1.52), p < .001 in the shared frailty model. However, HF was not associated with death (adj.HR 0.87, 95%CI: 0.52–1.48, p = .61) in the JF model.
Table 2. Adjusted hazard ratios by the different models.
Discussion
In this study, using a composite end point or considering the time to first event results in a substantial loss of follow-up information. We also found that employing a general JF model in the presence of a terminal event provides event-specific hazard ratios for recurrent events and the terminal event. The regression coefficients of the Cox model for the outcome of a composite endpoint were similar to that of the JF model for the outcome of readmissions. Further, the JF model included all the readmissions and was able to account for the terminal event.
In order to achieve adequate statistical power, composite endpoints combining several outcomes are frequently utilized. Composite end points are used not only in CV studies but also in renal, anesthesia, pulmonary disease and gastrointestinal disorders [Citation1]. Of the 40 clinical trials published in 2008, 73% were CV trials followed by nephrology (8%), gynecology (5%) and other specialties (15%) employed composite endpoints [Citation13]. Each component of a composite end point is considered equal important; when in reality, the CV components of stroke and myocardial infarction are limiting events and death is a more severe, terminal event. Death, when included with non-fatal events should not be compounded into a composite endpoint; it should be accounted for as a competing risk. A systematic review including PCI clinical trials stated that use of a standard major adverse cardiac event (MACE) composite end point should be avoided given the presence of heterogeneity among the subjects and variation in the trial hypothesis. In instances a composite endpoint is inevitable; the composite should be tailored to the hypothesis of the trial [Citation14]. The use of composite end points may include clinical outcomes of different organ systems. For example, The Aliskiren Trial in Type 2 Diabetes Using Cardio renal Endpoints (ALTITUDE) study employed a primary cardio renal composite outcome considering clinical events pertaining to cardiac and/or renal disease [Citation15]. The Canagliflozin Cardiovascular Assessment Study (CANVAS) program employed an organ-specific composite outcome to study the impact of the drug on the progression of cardiovascular and renal diseases [Citation16].
Considering time to the first event results in substantial loss of information. Analysis of the recurrent events in the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT) identified that approximately 40% of heart failure readmissions and cardiovascular deaths were ignored when only the time to first event was considered [Citation17]. Similarly, The Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM-Preserved) trial reported that only 54% of the event details were included during the time to first event analysis [Citation18]. The information lost under the ‘time to first event’ paradigm is highlighted by Anker and colleagues for various CV trials [Citation19]. A post hoc analysis of the CHARM-Preserved trial revealed that Canderstan offered greater benefits than originally thought after including readmissions data. They also reinforced that conducting an analysis including recurrent events increased the statistical power (>80%) compared to the first event analysis (56%) [Citation17]. The gains in statistical power from employing recurrent event analyses have been demonstrated by other studies [Citation20,Citation21]. Further, analyzing recurrent events provides a better sense of the true burden of the disease and aids the understanding of the disease progression. A randomized clinical trial employed the use of recurrent hospitalizations as a primary end point to avoid such loss of information [Citation22]. In our study, we found that 40% of follow-up information would have been lost if the primary analysis was only time until the first event, and only the first event was considered. Trials have considered counting the days lost due to hospitalization or death as the primary outcome in order to account for the readmissions [Citation23].
There are various statistical models employed to analyze recurrent events. As multiple events that occur within the same individual are dependent, appropriate methods must be employed [Citation3]. Frailty models consider the event times to be independent conditional on the random covariate which captures heterogeneity that may not be explained by other variables in the study [Citation8]. In instances where there is a terminal event in the presence of recurrent events, the dependence between the recurrent and terminal events is introduced in a JF model. The JF model provides distinct hazard ratios for the recurrent and the terminal events. This helps explain the effect of a variable on the different outcomes, as well as the burden of the study [Citation9]. In addition, employing the general JF model provides information on dependence between the recurrent events and also with the terminal event [Citation24]. The shared frailty model in our study showed that there was evidence of heterogeneity and dependence between events. Further, the JF model revealed that there was dependence between the recurrent events internally, as well as with the terminal event. With the indication of dependence between recurrent events and hospitalizations, it appears that readmissions provide an opportunity to postpone the terminal event. It has been recently stressed that reducing readmissions as part of the CMS financial penalty program did not help reduce death. In fact, death was kept at bay by being readmitted to the hospital [Citation25]. In our study, approximately 75% of the patients who died within one year had at least one preceding readmission, potentially providing an opportunity to avert death.
Limitations
Our study has the standard limitations of retrospective studies conducted using data collected in real world health systems. We were able to obtain a fairly comprehensive listing of hospital readmissions from the Dallas-Fort Worth Hospital Council Foundation, but it is limited to events occurring within the Dallas-Fort Worth area and as a result would not have captured a hospitalization outside of this catchment area. We had no confirmation of HF at baseline and the recurrent events were grouped with limited information on their individual diagnoses. In addition, the risk profiles of patients included in the analysis may have changed over the study period due to medications, additional procedures, or intercurrent illness.
Conclusions
Considering only the time to first event results in extensive loss of information. Including all readmissions increases the statistical power and helps evaluate the burden of disease. Use of a JF model provides detailed and more realistic effects of covariates such as baseline HF on the recurrent and the terminal events.
Disclosure statement
MS: Speakers Bureau: Lantheus Medical Imaging, Gilead Biosciences, On-X Life Technologies/CryoLife, Astra-Zeneca, Jaansen Pharmaceutical, Bristol-Myers-Squibb, Pfizer, Novartis, Sanofi-Aventis, Regeneron Pharmaceuticals. Consultant: On-X Life Technologies/CryoLife, Lantheus Medical Imaging, Gilead Biosciences, Astra-Zeneca, St Jude Medical; RS: Medtronic: Advisory Board, Global TAVR proctor; Boston Scientific: Advisory Board, Global TAVR proctor, Others: none to report.
Additional information
Funding
References
- Goldberg R, Gore JM, Barton B, et al. Individual and composite study endpoints: separating the wheat from the chaff. Am J Med. 2014;127:379–384.
- Glynn RJ, Buring JE. Ways of measuring rates of recurrent events. BMJ. 1996;312:364.
- Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann. Statist. 1982;10:1100–1120.
- Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073.
- Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika 1981;68:373–379.
- Therneau TM, Grambsch P. Modeling survival data. extending the cox model. New York (NY): Springer; 2000.
- Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44:324–333.
- Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, et al. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics. 2007;8:708–721.
- Rogers JK, Yaroshinsky A, Pocock SJ, et al. Analysis of recurrent events with an associated informative dropout time: application of the joint frailty model. Stat Med. 2016;35:2195–2205.
- DWHC Foundation [Internet]. Irwing (TX): DWHC; Available from: https://dfwhcfoundation.org/data/. Accessed 05 January 2018.
- Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). J Nucl Cardiol. 2015;22:1041–1144.
- National Technical Informatioin Service [Internet]. Alexandria (VA): NTIS; Available from: https://classic.ntis.gov/products/ssa-dmf/#. Accessed 05 January 2018.
- Gloria C, Lisa S, Steven W, et al. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ. 2010;341:c3920.
- Kip KE, Hollabaugh K, Marroquin OC, et al. The problem with composite end points in cardiovascular studies: the story of major adverse cardiac events and percutaneous coronary intervention. J Am Coll Cardiol. 2008;51:701–707.
- Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med. 2012;367:2204–2213.
- Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–334.
- Rogers JK, Kielhorn A, Borer JS, et al. Effect of ivabradine on numbers needed to treat for the prevention of recurrent hospitalizations in heart failure patients. Curr Med Res Opin. 2015;31:1903–1909.
- Rogers JK, Pocock SJ, McMurray JJ, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail. 2014;16:33–40.
- Anker SD, McMurray JJ. Time to move on from ‘time-to-first’: should all events be included in the analysis of clinical trials? Eur Heart J. 2012;33:2764–2765.
- Rondeau V. Statistical models for recurrent events and death: application to cancer events. Math Comput Model. 2010;52:949–955.
- Rogers JK, McMurray JJV, Pocock SJ, et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation. 2012;126:2317–2323.
- Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666.
- Cleland JG, Louis AA, Rigby AS, et al. Noninvasive home telemonitoring for patients with heart failure at high risk of re- current admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45:1654–1664.
- Król A, Mauguen A, Mazroui Y, et al. Tutorial in joint modeling and prediction: a statistical software for correlated longitudinal outcomes, recurrent events and a terminal event. J Stat Soft. 2017;81:1–52.
- Abdul-Aziz AA, Hayward RA, Aaronson KD, et al. Association between medicare hospital readmission penalties and 30-day combined excess readmission and mortality. JAMA Cardiol. 2017;2:200–203.